COVID-19 Incidence and Vaccine Effectiveness in University Staff, 1 March 2020–2 April 2022
Abstract
:1. Background
2. Materials & Methods
2.1. Ethical Considerations
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
- Pre-vaccination era: 1 March 2020–26 December 2020;
- Post-vaccination era, before the spread of the Omicron variant: 27 December 2020–30 November 2021;
- During the Omicron transmission period: 1 December 2021–2 April 2022.
3. Results
4. Discussion
4.1. Key Findings
4.2. Interpretation of the Findings
4.3. Vaccine Effectiveness
4.4. Generalizability
4.5. Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piapan, L.; De Michieli, P.; Ronchese, F.; Rui, F.; Peresson, M.; Segat, L.; D’Agaro, P.; Negro, C.; Bovenzi, M.; Larese Filon, F. COVID-19 outbreaks in hospital workers during the first COVID-19 wave. Occup. Med. 2022, 72, 110–117. [Google Scholar] [CrossRef]
- Piapan, L.; De Michieli, P.; Ronchese, F.; Rui, F.; Mauro, M.; Peresson, M.; Segat, L.; D’Agaro, P.; Negro, C.; Bovenzi, M.; et al. COVID-19 outbreak in healthcare workers in hospitals in Trieste, North-east Italy. J. Hosp. Infect. 2020, 106, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.; Negro, C.; Cegolon, L.; Larese Filon, F. Risk of Vaccine Breakthrough SARS-CoV-2 Infection and Associated Factors in Healthcare Workers of Trieste Teaching Hospitals (North-Eastern Italy). Viruses 2022, 14, 336. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Rui, F.; Ronchese, F.; De Michieli, P.; Negro, C. Incidence of COVID-19 infection in hospital workers from 1 March 2020 to 31 May 2021 routinely tested, before and after vaccination with BNT162B2. Sci. Rep. 2022, 12, 2533. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Ronchese, F.; Ricci, F.; Negro, C.; Laese-Filon, F. SARS-CoV-2 Infection in Health Care Workers of Trieste (North-Eastern Italy), 1 October 2020–7 February 2022: Occupational Risk and the Impact of the Omicron Variant. Viruses 2022, 14, 1663. [Google Scholar] [CrossRef]
- Cegolon, L.; Negro, C.; Mastrangelo, G.; Larese Filon, F. Primary SARS-CoV-2 Infections, Re-infections and Vaccine Effectiveness during the Omicron Transmission Period in Healthcare Workers of Trieste and Gorizia (Northeast Italy), 1 December 2021–31 May 2022. Viruses 2022, 14, 2688. [Google Scholar] [CrossRef]
- Wong, J.; Cummings, K.J.; Gibb, K.; Rodriguez, A.; Heinzerling, A.; Vergara, X.P. Risk factors for COVID-19 among Californians working outside the home. J. Ind. Med. 2023, 66, 233–242. [Google Scholar] [CrossRef]
- Fadel, M.; Gilbert, F.; Legeay, C.; Dubée, V.; Esquirol, Y.; Verdun-Esquer, C.; Dinh, A.; Sembajwe, G.; Goldberg, M.; Roquelaure, Y.; et al. Mat-O-Covid investigators. Association between COVID-19 infection and work exposure assessed by the Mat-O-Covid job exposure matrix in the CONSTANCES cohort. Occup. Environ. Med. 2022, 79, 782–789. [Google Scholar] [CrossRef]
- Marinaccio, A.; Boccuni, F.; Rondinone, B.M.; Brusco, A.; D’Amario, S.; Iavicoli, S. Occupational factors in the COVID-19 pandemic in Italy: Compensation claims applications support establishing an occupational surveillance system. Occup. Environ. Med. 2020, 77, 818–821. [Google Scholar] [CrossRef]
- Verbeeck, J.; Vandersmissen, G.; Peeters, J.; Klamer, S.; Hancart, S.; Lernout, T.; Dewatripont, M.; Godderis, L.; Molenberghs, G. Confirmed COVID-19 Cases per Economic Activity during Autumn Wave in Belgium. Int. J. Environ. Res. Public Health 2021, 18, 12489. [Google Scholar] [CrossRef]
- The Jobs at Risk Index (JARI). Available online: https://autonomy.work/portfolio/jari/#1585055386778-0066374b-191a (accessed on 18 February 2023).
- Rhodes, S.; Wilkinson, J.; Pearce, N.; Mueller, W.; Cherrie, M.; Stocking, K.; Gittins, M.; Katikireddi, S.V.; Tongeren, M.V. Occupational differences in SARS-CoV-2 infection: Analysis of the UK ONS COVID-19 infection survey. J. Epidemiol. Community Health 2022, 76, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Wiryasaputra, R.; Huang, C.Y.; Kristiani, E.; Liu, P.Y.; Yeh, T.K.; Yang, C.T. Review of an intelligent indoor environment monitoring and management system for COVID-19 risk mitigation. Front. Public Health 2023, 10, 1022055. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Rigó, M.; Formazin, M.; Liebers, F.; Latza, U.; Castell, S.; Jöckel, K.H.; Greiser, K.H.; Michels, K.B.; Krause, G.; et al. Occupation and SARS-CoV-2 infection risk among 108 960 workers during the first pandemic wave in Germany. Scand. J. Work Environ. Health 2022, 48, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.; Nygård, K.; Methi, F.; Vold, L.; Telle, K. Occupational risk of COVID-19 in the first versus second epidemic wave in Norway, 2020. Eurosurveillance 2021, 26, 2001875. [Google Scholar] [CrossRef] [PubMed]
- Möhner, M.; Wolik, A. Differences in COVID-19 Risk between Occupational Groups and Employment Sectors in Germany. Dtsch. Arztebl. Int. 2020, 117, 641–642. [Google Scholar] [CrossRef]
- Stringhini, S.; Zaballa, M.E.; Pullen, N.; de Mestral, C.; Perez-Saez, J.; Dumont, R.; Picazio, A.; Pennacchio, F.; Dibner, Y.; Yerly, S.; et al. Large variation in anti-SARS-CoV-2 antibody prevalence among essential workers in Geneva, Switzerland. Nat. Commun. 2021, 12, 3455. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Cegolon, L.; Mastrangelo, G.; Emanuelli, E.; Camerotto, R.; Spinato, G.; Frezza, D. Early negativization of SARS-CoV-2 infection by nasal spray of seawater plus additives: The RENAISSANCE open-label controlled clinical trial. Pharmaceutics 2022, 14, 2502. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Clinical Characteristics of COVID-19. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/clinical (accessed on 1 February 2023).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, A.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Mastrangelo, G.; Bellizzi, S.; LArese Filon, F.; SAlata, C. Supporting the Aspecific Physiological Defenses of Upper Airways against Emerging SARS-CoV-2 Variants. Pathogens 2023, 12, 211. [Google Scholar] [CrossRef]
- Pires, L.; Wilson, B.C.; Bremner, R.; Lang, A.; Larouche, J.; McDonald, R.; Pearson, J.D.; Trcka, D.; Wrana, J.; Wu, J.; et al. Translational feasibility and efficacy of nasal photodynamic disinfection of SARS-CoV-2. Sci. Rep. 2022, 12, 14438. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Sheward, D.J.; Kim, C.; Ehling, R.A.; Pankow, A.; Dopico, X.C.; Dyrdak, R.; Martin, D.P.; Reddy, S.T.; Dillner, J.; Hedestam, G.B.K.; et al. Neutralisation sensitivity of the SARS-CoV-2 Omicron (B.1.1.529) variant: A cross-sectional study. Lancet Infect. Dis. 2022, 22, 813–820. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Furuse, Y.; Sando, E.; Tsuchiya, N.; Miyahara, R.; Yasuda, I.; Ko, Y.K.; Saito, M.; Morimoto, K.; Imamura, T.; Shobugawa, Y.; et al. Clusters of Coronavirus Disease in Communities.; Japan.; January–April 2020. Emerg. Infect. Dis. 2020, 26, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.P.; McCaffrey, K.; Friedrichs, M.; LaCross, N.; Lewis, N.M.; Sage, K.; Barbeau, B.; Vilven, D.; Rose, C.; Braby, S.; et al. Racial and Ethnic Disparities among COVID-19 Cases in Workplace Outbreaks by Industry Sector—Utah, 6 March–5 June 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1133–1138. [Google Scholar] [CrossRef]
- Murti, M.; Achonu, C.; Smith, B.T.; Brown, K.A.; Kim, J.H.; Johnson, J.; Ravindran, S.; Buchan, S.A. COVID-19 Workplace Outbreaks by Industry Sector and Their Associated Household Transmission, Ontario, Canada, January to June, 2020. J. Occup. Environ. Med. 2021, 63, 574–580. [Google Scholar] [CrossRef]
- Middleton, J.; Reintjes, R.; Lopes, H. Meat plants—A new front line in the COVID-19 pandemic. BMJ 2020, 370, m2716. [Google Scholar] [CrossRef]
- Methi, F.; Telle, K.; Magnusson, K. COVID-19 infection among bartenders and waiters before and after pub lockdown. Occup. Environ. Med. 2022, 79, 46–48. [Google Scholar] [CrossRef]
- Kripattanapong, S.; Jitpeera, C.; Wongsanuphat, S.; Issarasongkhram, M.; Mungaomklang, A.; Suphanchaimat, R. Clusters of coronavirus disease (COVID-19) in pubs, bars and nightclubs in Bangkok, 2020. OSIR 2020, 13, 146–153. [Google Scholar]
- De Matteis, S.; Cencedda, V.; Pilia, I.; Cocco, P. COVID-19 incidence in a cohort of public transport workers. Med. Lav. 2022, 113, e2022039. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Salata, C.; Piccoli, E.; Juarez, V.; Palu’, G.; Mastrangelo, G.; Calistri, A. In vitro antiviral activity of hypothiocyanite against A/H1N1/2009 pandemic influenza virus. Int. J. Hyg. Environ. Health 2014, 217, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L. Investigating hypothiocyanite against SARS-CoV-2. Int. J. Hyg. Environ. Health 2020, 227, 113520. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Javanbakht, M.; Mastrangelo, G. Nasal disinfection for the prevention and control of COVID-19: A scoping review on potential chemo-preventive agents. Int. J. Hyg. Environ. Health 2020, 230, 113605. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Mirandola, M.; Salaris, C.; Salvati, M.V.; Mastrangelo, G.; Salata, C. Hypothiocyanite and Hypothiocyanite/Lactoferrin Mixture Exhibit Virucidal Activity In Vitro against SARS-CoV-2. Pathogens 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- van Tongeren, M.; Rhodes, S.; Pearce, N. Occupation and SARS-CoV-2 infection risk among workers during the first pandemic wave in Germany: Potential for bias. Scand. J. Work Environ. Health 2022, 48, 586–587. [Google Scholar] [CrossRef]

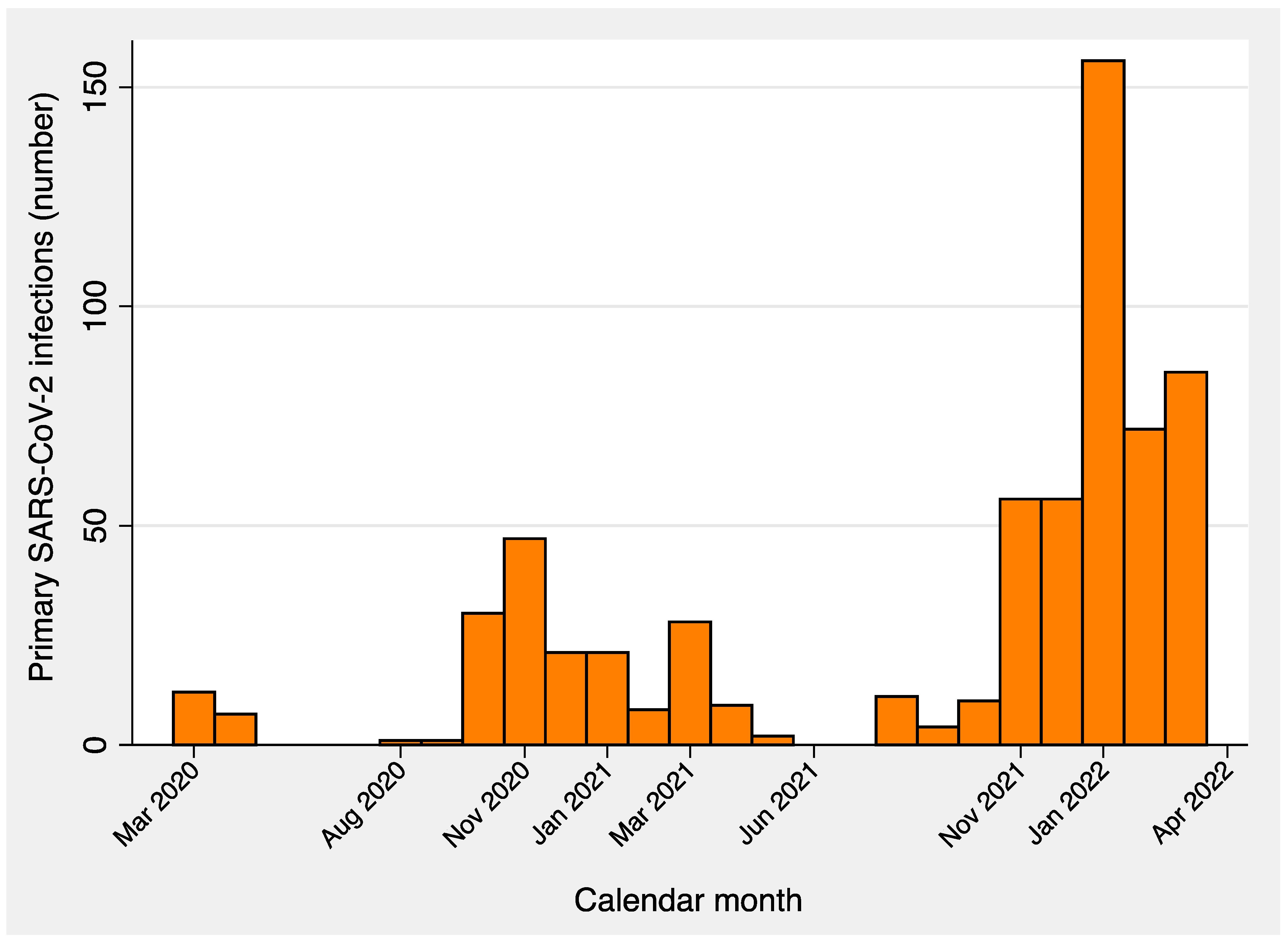
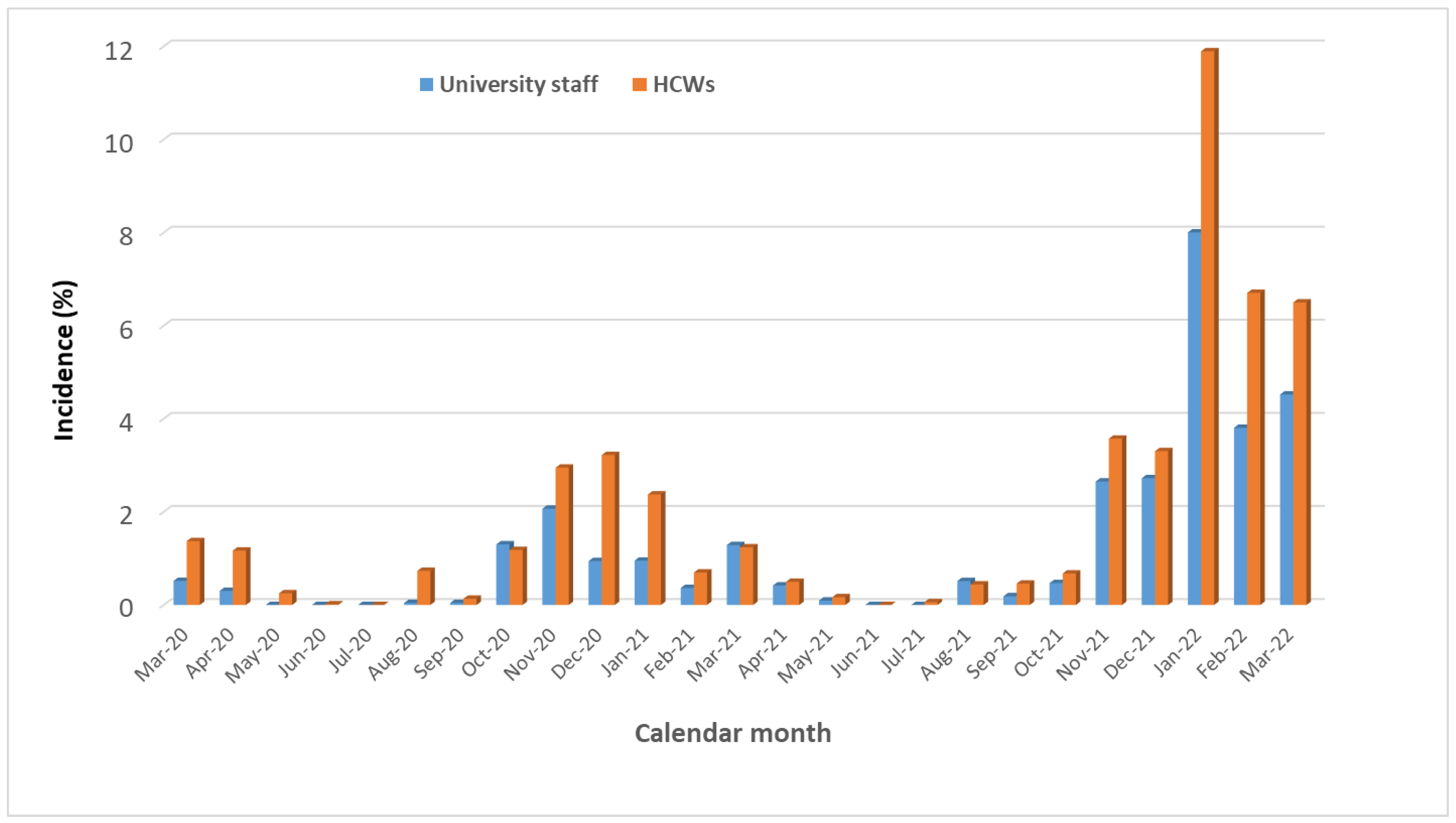
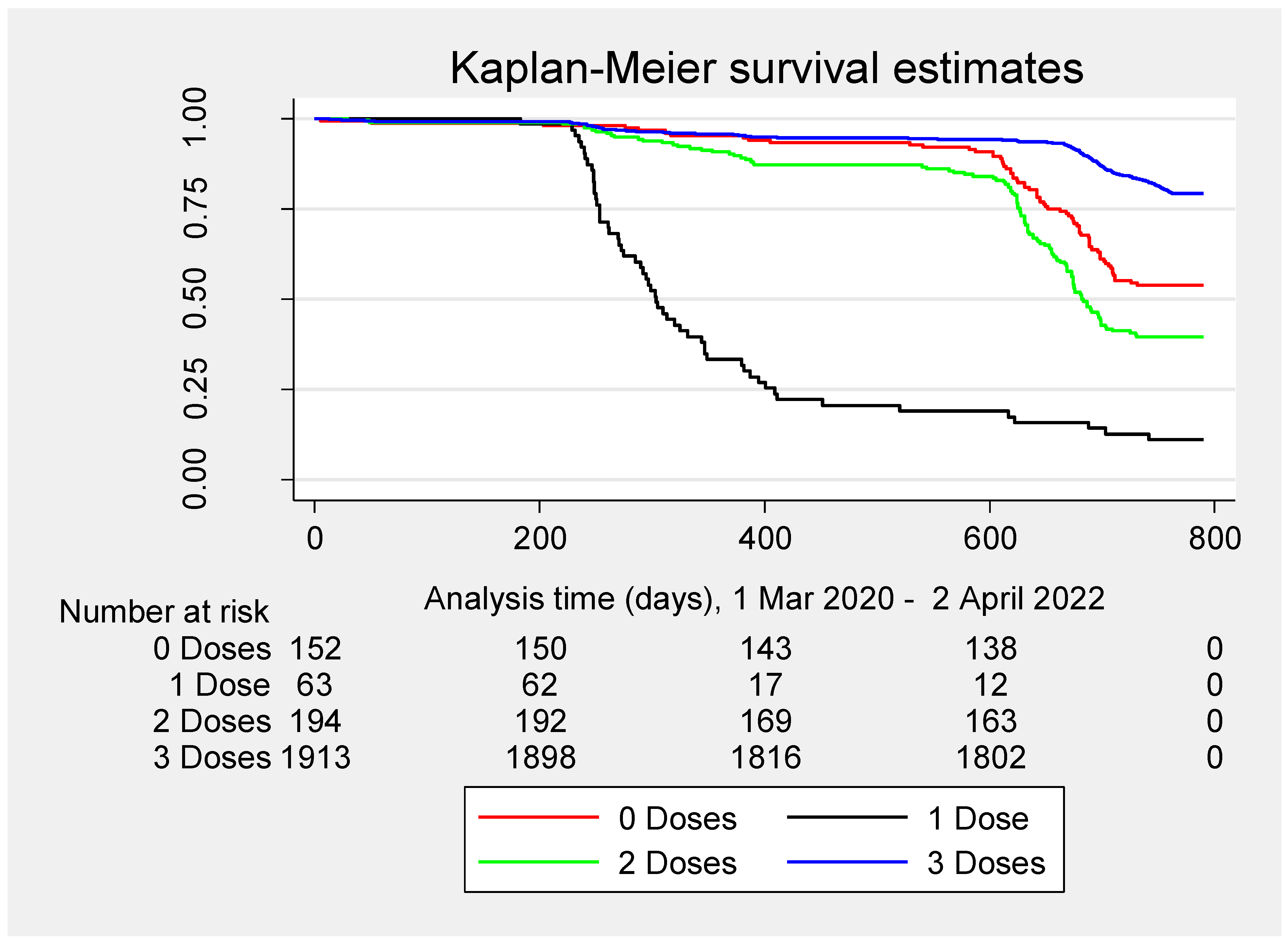
| Terms | Total | Primary SARS-CoV-2 Infections | p-Value | |||
|---|---|---|---|---|---|---|
| Negative | Positive | |||||
| Total (row %) | 2323 (100) | 1686 (72.6) | 637 (27.4) | |||
| Sex | Females | 1140 (49.1) | 827 (49.1) | 313 (49.1) | 0.971 | |
| Males | 1183 (50.9) | 859 (50.9) | 324 (50.8) | |||
| Age (years) | Mean ± SD | 47.9 ± 13.9 | 48.5 ± 14.0 | 44.9 ± 13.3 | <0.001 | |
| <41 | 766 (33.0) | 516 (30.6) | 250 (39.3) | <0.001 | ||
| 41–55 | 759 (32.7) | 537 (31.9) | 222 (34.9) | |||
| 56+ | 798 (34.5) | 633 (37.5) | 165 (25.9) | |||
| Occupation | Administrative Clerks | 493 (21.2) | 394 (23.4) | 144 (22.6) | <0.001 | |
| Academic staff | 493 (21.2) | 388 (23.1) | 105 (16.5) | |||
| PhD students | 643 (27.7) | 500 (29.7) | 143 (22.5) | |||
| Postgraduate specialist medical trainees | 213 (9.2) | 113 (6.7) | 100 (15.7) | |||
| Short-term contract | Healthcare sector | 376 (16.2) | 254 (15.1) | 122 (19.2) | ||
| Other | 60 (2.6) | 37 (2.2) | 23 (3.6) | |||
| Department | Administrative & technical support | 595 (25.6) | 440 (26.10) | 155 (24.3) | <0.001 | |
| Physics | 100 (4.3) | 75 (4.5) | 25 (3.9) | |||
| Engineering & Architecture | 193 (8.3) | 148 (8.8) | 45 (7.1) | |||
| Mathematics & Geosciences | 125 (5.4) | 97 (5.8) | 28 (4.4) | |||
| Chemical & Pharmaceutical Sciences | 64 (2.8) | 50 (3.0) | 14 (2.2) | |||
| Life Sciences | 168 (7.2) | 134 (8.0) | 34 (5.3) | |||
| Economics, Business & Statistics | 66 (2.8) | 54 (3.2) | 12 (1.9) | |||
| Law, Language & Interpreting | 75 (3.2) | 62 (3.7) | 13 (2.0) | |||
| Political & Social Sciences | 34 (1.5) | 29 (1.7) | 5 (0.8) | |||
| Human Sciences | 108 (4.7) | 87 (5.2) | 21 (3.30) | |||
| Medical, Surgical & Health Sciences | 724 (31.2) | 467 (27.1) | 257 (40.4) | |||
| Not specified | 71 (3.1) | 43 (2.6) | 28 (4.4) | |||
| N. doses of COVID-19 vaccine (M: 19) | 0 | 152 (6.5) | 82 (4.9) | 70 (11.0) | <0.001 | |
| 1 | 63 (2.7) | 7 (0.42) | 56 (8.8) | |||
| 2 | 194 (8.4) | 77 (4.6) | 117 (18.5) | |||
| 3 | 1913 (82.4) | 1520 (90.2) | 394 (61.9) | |||
| 4 | 1 | 1 | 0 | |||
| Total number of swab tests | Mean ± SD | 12.6 ± 15.8 | 10.1 ± 14.6 | 19.21 ± 17.0 | <0.001 | |
| Median (IQR) | 4 (1; 23) | 3 (1; 15) | 12 (4; 33) | <0.001 | ||
| 0 | 323 (13.9) | 323 | 0 | <0.001 | ||
| 1–2 | 504 (21.7) | 464 (27.5) | 40 (6.3) | |||
| 3–5 | 504 (21.7) | 328 (19.5) | 176 (27.6) | |||
| 6–26 | 506 (21.8) | 304 (18.0) | 202 (31.7) | |||
| 27+ | 486 (20.9) | 267 (15.8) | 219 (34.4) | |||
| First Vaccine dose (M:19) | Comirnaty | 1127 (52.2) | 781 (48.9) | 346 (61.7) | <0.001 | |
| Spikevax | 107 (5.0) | 62 (3.9) | 45 (8.0) | |||
| Vaxzevria | 913 (42.3) | 746 (46.7) | 167 (29.8) | |||
| Jannsen (N = 9)/Nuvaxovid (N = 2) | 11 (0.5) | 8 (0.5) | 3 (0.5) | |||
| Second Vaccine dose (M:19) | Comirnaty | 1130 (54.0) | 803 (50.6) | 327 (64.9) | <0.001 | |
| Spikevax | 94 (4.5) | 63 (4.0) | 31 (6.2) | |||
| Vaxzevria | 865 (41.4) | 719 (45.3) | 146 (29.0) | |||
| Nuvaxovid | 3 (0.1) | 3 (0.2) | 0 | |||
| Third Vaccine dose (M:19) | Comirnaty | 1087 (47.2) | 805 (53.2) | 282 (71.6) | <0.001 | |
| Spikevax | 819 (35.6) | 707 (46.7) | 112 (28.4) | |||
| Nuvaxovid | 1 | 1 (0.1) | 0 | |||
| Booster dose | Heterologous * | 812 (43.6) | 705 (47.5) | 107 (29.1) | <0.001 | |
| Homologous ** | 1052 (56.4) | 778 (52.5) | 274 (71.9) | |||
| Calendar Month | Vaccine Type | 0 Doses | 1 Dose | 2 Doses | 3 Doses | 4 Doses |
|---|---|---|---|---|---|---|
| 27 Dec 2020–31 Jan 2021 | Cumulative uptake | 1275 (54.9) | 613 (26.4) | 435 (18.7) | 0 | 0 |
| Comirnaty | 608 | 433 | ||||
| Unknown * | 5 | 2 | ||||
| 1–28 February 2021 | Cumulative uptake | 1106 (47.6) | 606 (26.1) | 611 (26.3) | 0 | 0 |
| Comirnaty | 79 | 173 | ||||
| Spikevax | 1 | 0 | ||||
| Vaxzevria | 520 | 0 | ||||
| Unknown * | 4 | 3 | ||||
| 1–31 March 2021 | Cumulative uptake | 686 (29.5) | 938 (40.4) | 699 (30.1) | 0 | 0 |
| Comirnaty | 39 | 82 | ||||
| Spikevax | 1 | 1 | ||||
| Vaxzevria | 380 | 0 | ||||
| Unknown * | 0 | 5 | ||||
| 1–30 April 2021 | Cumulative uptake | 620 (26.7) | 959 (41.3) | 744 (32.0) | 0 | 0 |
| Comirnaty | 59 | 44 | ||||
| Spikevax | 1 | 1 | ||||
| Vaxzevria | 6 | 0 | ||||
| 1–31 May 2021 | Cumulative uptake | 491 (21.1) | 239 (10.3) | 1593 (68.6) | 0 | 0 |
| Comirnaty | 85 | 65 | ||||
| Spikevax | 31 | 0 | ||||
| Vaxzevria | 7 | 784 | ||||
| Jannsen | 4 | 0 | ||||
| Unknown * | 1 | 0 | ||||
| 1–30 June 2021 | Cumulative uptake | 315 (13.6) | 248 (10.7) | 1760 (75.8) | 0 | 0 |
| Comirnaty | 154 | 68 | ||||
| Spikevax | 17 | 27 | ||||
| Vaxzevria | 0 | 72 | ||||
| Jannsen | 4 | 0 | ||||
| Unknown * | 1 | 0 | ||||
| 1–31 July 2021 | Cumulative uptake | 297 (12.8) | 87 (3.7) | 1939 (83.5) | 0 | 0 |
| Comirnaty | 16 | 153 | ||||
| Spikevax | 2 | 18 | ||||
| Vaxzevria | 0 | 8 | ||||
| 1–31 August 2021 | Cumulative uptake | 219 (9.4) | 128 (5.5) | 1976 (85.1) | 0 | 0 |
| Comirnaty | 50 | 28 | ||||
| Spikevax | 28 | 6 | ||||
| Vaxzevria | 0 | 8 | ||||
| Unknown * | 1 | 2 | ||||
| 1–30 September 2021 | Cumulative uptake | 188 (8.1) | 97 (4.2) | 2035 (87.6) | 2 (0.1) | 0 |
| Comirnaty | 27 | 43 | 1 | |||
| Spikevax | 1 | 15 | 0 | |||
| Jannsen | 1 | 0 | 0 | |||
| Unknown * | 1 | 3 | 1 | |||
| 1–31 October 2021 | Cumulative uptake | 179 (7.7) | 87 (3.7) | 1873 (80.6) | 184 (7.9) | 0 |
| Comirnaty | 5 | 17 | 181 | |||
| Spikevax | 3 | 1 | 0 | |||
| 1–30 November 2021 | Cumulative uptake | 171 (7.4) | 87 (3.7) | 1408 (60.6) | 657 (28.3) | 0 |
| Comirnaty | 1 | 5 | 437 | |||
| Spikevax | 5 | 1 | 34 | |||
| Unknown * | 0 | 0 | 2 | |||
| 1–31 December 2021 | Cumulative uptake | 168 (7.2) | 85 (3.7) | 501 (21.6) | 1569 (67.5) | 0 |
| Comirnaty | 0 | 0 | 375 | |||
| Spikevax | 5 | 3 | 536 | |||
| Vaxzevria | 0 | 3 | 0 | |||
| Unknown * | 0 | 1 | 1 | |||
| 1–31 January 2022 | Cumulative uptake | 155 (6.7) | 86 (3.7) | 260 (11.2) | 1822 (78.4) | 0 |
| Comirnaty | 2 | 1 | 17 | |||
| Spikevax | 12 | 12 | 234 | |||
| Unknown * | 0 | 0 | 2 | |||
| 1–28 February 2022 | Cumulative uptake | 154 (6.6) | 71 (3.1) | 210 (9.0) | 1888 (81.3) | 0 |
| Comirnaty | 2 | 9 | 53 | |||
| Spikevax | 0 | 8 | 13 | |||
| 1 Mar 2022–2 Apr 2022 | Cumulative uptake | 152 (6.5) | 63 (2.7) | 194 (8.4) | 1913 (82.4) | 1 |
| Comirnaty | 0 | 6 | 23 | 0 | ||
| Spikevax | 0 | 1 | 2 | 0 | ||
| Nuvaxovid | 2 | 3 | 1 | 0 | ||
| Unknown * | 0 | 0 | 0 | 1 |
| SARS-CoV-2 Infections | Total (N = 637) | ||
|---|---|---|---|
| COVID-19 wave | I (7 Mar 2020–31 May 2020) | 19 (3.0) | |
| II (1 Jun 2020–30 Sep 2020) | 2 (0.3) | ||
| III (1 Oct 2020–31 Dec 2020) | 98 (15.4) | ||
| IVa (1 Jan 2021–31 Mar 2021) | 57 (9.0) | ||
| IVb (1 Apr 2021–30 Sep 2021) | 26 (4.1) | ||
| V (1 Oct 2021–30 Nov 2021) | 66 (10.4) | ||
| VI (1 Dec 2021–2 April 2022) | 369 (57.9) | ||
| Pandemic era | Pre-vaccination (1 Mar 2020–09 Jan 2021) | 129 (20.3) | |
| Pre-Omicron (10 Jan 2021–30 Nov 2021) | 139 (21.8) | ||
| Omicron (1 Dec 2021–2 Apr 2022) | 369 (57.9) | ||
| COVID-19 vaccination | Before vaccination | 222 (34.9) | |
| Between 1st and 2nd dose | 14 + days since 1st vaccine dose | 15 (2.4) | |
| <14 days since 1st vaccine dose | 14 (2.2) | ||
| Between 2nd and 3rd dose | 7 + days since 2nd vaccine dose | 110 (17.3) | |
| <7 days since 2nd vaccine dose | 0 | ||
| Between 3rd and 4th dose | 7 + days since 3rd vaccine dose | 267 (41.9) | |
| <7 days since 3rd vaccine dose | 9 (1.4) | ||
| Re-infections | 28 (4.4) | ||
| Terms | Cases (Number) | Person-Days | Cases × 10,000 p-d | |
|---|---|---|---|---|
| Entire study period (1 Mar 2020–2 Apr 2022) | All workers | 637 | 1,690,346 | 3.77 |
| Fully unvaccinated | 70 | 106,876.53 | 6.55 | |
| Entire vaccination era (27 Dec 2020–2 Apr 2022) | 14 + days since 1st dose | 7 | 9690.47 | 7.22 |
| 7 + days since 2nd dose | 89 | 118,917.79 | 7.48 | |
| 7 + days since 3rd dose | 276 | 1,395,087 | 1.98 | |
| Pre-Omicron wave (4 Sept 2021–30 Nov 2021) | 7 + days since homologous booster * | 3 | 616,488 | 0.05 |
| 7 + days since heterologous booster ** | 0 | 556,950 | 0 | |
| Omicron wave (1 Dec 2021–2 April 2022) | 7 + days since homologous booster * | 180 | 741,625.06 | 2.43 |
| 7 + days since heterologous booster ** | 89 | 620,232.51 | 1.43 | |
| Terms | Multivariable Cox Regression aHR (95% CI) | ||||
|---|---|---|---|---|---|
| MODEL 1 (1815 obs.) | MODEL 2 (1791 obs.) | MODEL 3 (2053 obs.) | |||
| Sex | Female | Reference | Reference | Reference | |
| Male | 1.24 (0.87; 1.77) | 1.23 (0.82; 1.85) | 1.09 (0.88; 1.34) | ||
| Age (years) | <41 | Reference | Reference | Reference | |
| 41–55 | 1.00 (0.56; 1.76) | 2.59 (1.39; 4.83) | 0.97 (0.72; 1.32) | ||
| 56+ | 1.07 (0.61; 1.87) | 1.48 (0.75; 2.92) | 0.63 (0.45; 0.87) | ||
| Occupation | Clerks | Reference | Reference | Reference | |
| Academic staff | 1.51 (0.63; 3.61) | 0.85 (0.34; 2.14) | 0.79 (0.48; 1.28) | ||
| PhD students | 1.32 (0.59; 3.93) | 0.73 (0.31; 1.71) | 0.66 (0.42; 1.02) | ||
| Postgraduate medical trainees | 2.67 (0.88; 8.06) | 2.87 (0.77; 10.65) | 2.16 (1.04; 4.48) | ||
| Short-term contractors | Health sector | 1.13 (0.42; 3.01) | 1.34 (0.44; 4.02) | 1.51 (0.77; 2.96) | |
| Other | 3.958 | 2.318 | 0.41 (0.13; 1.23) | ||
| Department | Administrative & technical support | Reference | Reference | Reference | |
| Physics | 0.50 (0.13; 1.90) | 1.94 (0.66; 5.64) | 1.49 (0.77; 2.86) | ||
| Engineering and Architecture | 0.52 (0.18; 1.47) | 0.48 (0.14; 1.66) | 1.45 (0.88; 2.40) | ||
| Mathematics and Geosciences | 0.54 (0.16; 1.75) | 0.89 (0.26; 3.04) | 1.44 (0.80; 2.58) | ||
| Chemical and Pharmaceutical Sciences | 0.26 (0.03; 2.09) | 1.08 (0.29; 3.97) | 1.28 (0.60; 2.70) | ||
| Life Sciences | 0.97 (0.39; 2.43) | 0.50 (0.13; 2.02) | 1.10 (0.60; 2.01) | ||
| Economics, Business and Statistics | 1.78−20 | 0.90 (0.25; 3.31) | 0.84 (0.36; 1.97) | ||
| Law, Language and Interpreting Studies | 0.84 (0.25; 2.82) | 0.40 (0.05; 3.25) | 1.06 (0.48; 2.35) | ||
| Political and Social Sciences | 0.83 (0.17; 4.06) | 0.73 (0.14; 3.78) | 0.26 (0.04; 1.98) | ||
| Human Sciences | 0.60 (0.18; 2.04) | 2.39−16 | 1.30 (0.67; 2.51) | ||
| Medical, Surgical, Health Sciences | 1.67 (0.69; 4.02) | 1.09 (0.39; 3.05) | 1.05 (0.56; 1.98) | ||
| Not specified | 3.73−9 (1.03−9; 1.35−8) | 7.00−9 (1.52−9; 3.22−8) | 3.99 (1.54; 10.32) | ||
| N. doses of COVID-19 vaccine | 0 | Reference | Reference | ||
| 1 | 1.86 (0.62; 5.56) | 0.89 (0.27; 2.92) | |||
| 2 | 1.53 (0.90; 2.60) | 1.50 (0.98; 2.29) | |||
| 3 | 0.10 (0.06; 0.16) | 0.37 (0.27; 0.52) | |||
| Terms | Crude Risk | Adjusted Risk | ||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Sex + Age | Sex + Age + Job Task | Sex + Age + Dpt | Sex + Age + Job Task + Dpt | ||||
| HR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |||
| Booster dose | Heterologous | Reference | Reference | Reference | Reference | Reference | Reference | |
| Homologous | 1.76 (1.37; 2.27) | 1.76 (1.36; 2.27) | 1.54 (1.18; 2.02) | 0.91 (0.64; 1.29) | 0.87 (0.61: 1.24) | 0.92 (0.62; 1.35) | ||
| Age (years) | <41 | Reference | Reference | Reference | Reference | |||
| 56+ | 0.49 (0.35; 0.67) | 0.64 (0.43; 0.94) | 0.51 (0.36; 0.70) | 0.66 (0.44; 0.98) | ||||
| Job task | Administrative clerks | Reference | Reference | |||||
| Postgraduate medical trainees | 3.49 (2.05; 5.92) | 3.11 (1.28; 7.52) | ||||||
| Contractors in health sector | 2.09 (1.33; 3.28) | |||||||
| Dpt | Administrative/technical | Reference | ||||||
| Medical/Surgical/Health | 2.41 (1.63; 3.58) | |||||||
| Not specified | 2.21 (1.16; 4.19) | 5.26 1.76; 15.70) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegolon, L.; Negro, C.; Pesce, M.; Filon, F.L. COVID-19 Incidence and Vaccine Effectiveness in University Staff, 1 March 2020–2 April 2022. Vaccines 2023, 11, 483. https://doi.org/10.3390/vaccines11020483
Cegolon L, Negro C, Pesce M, Filon FL. COVID-19 Incidence and Vaccine Effectiveness in University Staff, 1 March 2020–2 April 2022. Vaccines. 2023; 11(2):483. https://doi.org/10.3390/vaccines11020483
Chicago/Turabian StyleCegolon, Luca, Corrado Negro, Marco Pesce, and Francesca Larese Filon. 2023. "COVID-19 Incidence and Vaccine Effectiveness in University Staff, 1 March 2020–2 April 2022" Vaccines 11, no. 2: 483. https://doi.org/10.3390/vaccines11020483
APA StyleCegolon, L., Negro, C., Pesce, M., & Filon, F. L. (2023). COVID-19 Incidence and Vaccine Effectiveness in University Staff, 1 March 2020–2 April 2022. Vaccines, 11(2), 483. https://doi.org/10.3390/vaccines11020483







