In Vitro Expansion of Vδ1+ T Cells from Cord Blood by Using Artificial Antigen-Presenting Cells and Anti-CD3 Antibody
Abstract
1. Introduction
2. Materials and Methods
2.1. Cord Blood and Peripheral Bloods and Cell Lines
2.2. Cord Blood γδ T-Cell Expansion
2.3. Vγ9Vδ2 T-Cell Expansion
2.4. Flow Cytometer Assay
2.5. Cytotoxicity Assay
2.6. Secretome Analysis
2.7. Statistics
3. Results
3.1. Effect of Anti-CD3 Antibody Stimulation on γδ T-Cell Subtypes’ Proliferation
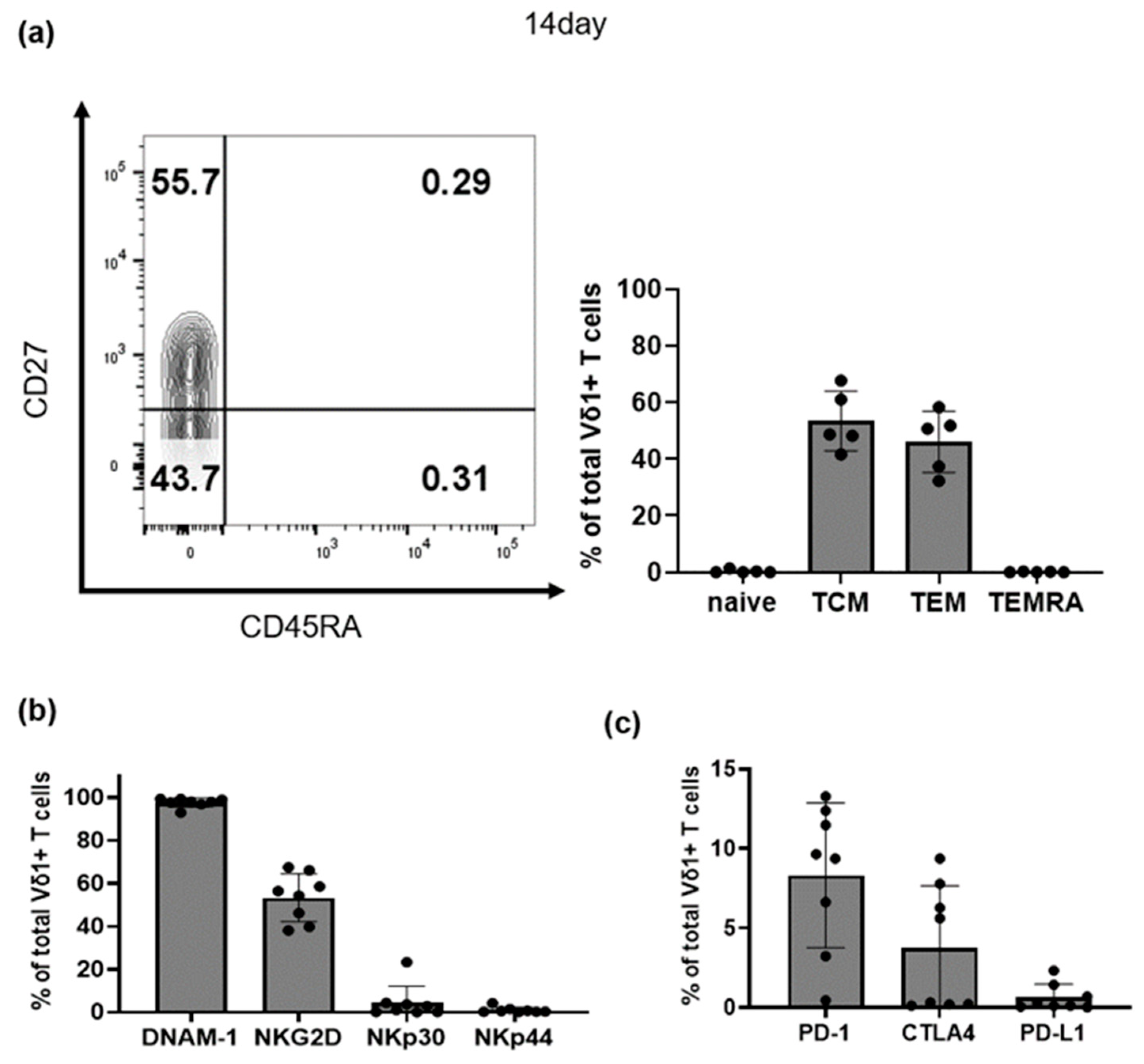
3.2. Characteristics of Selectively Expanded Vδ1+ T Cells
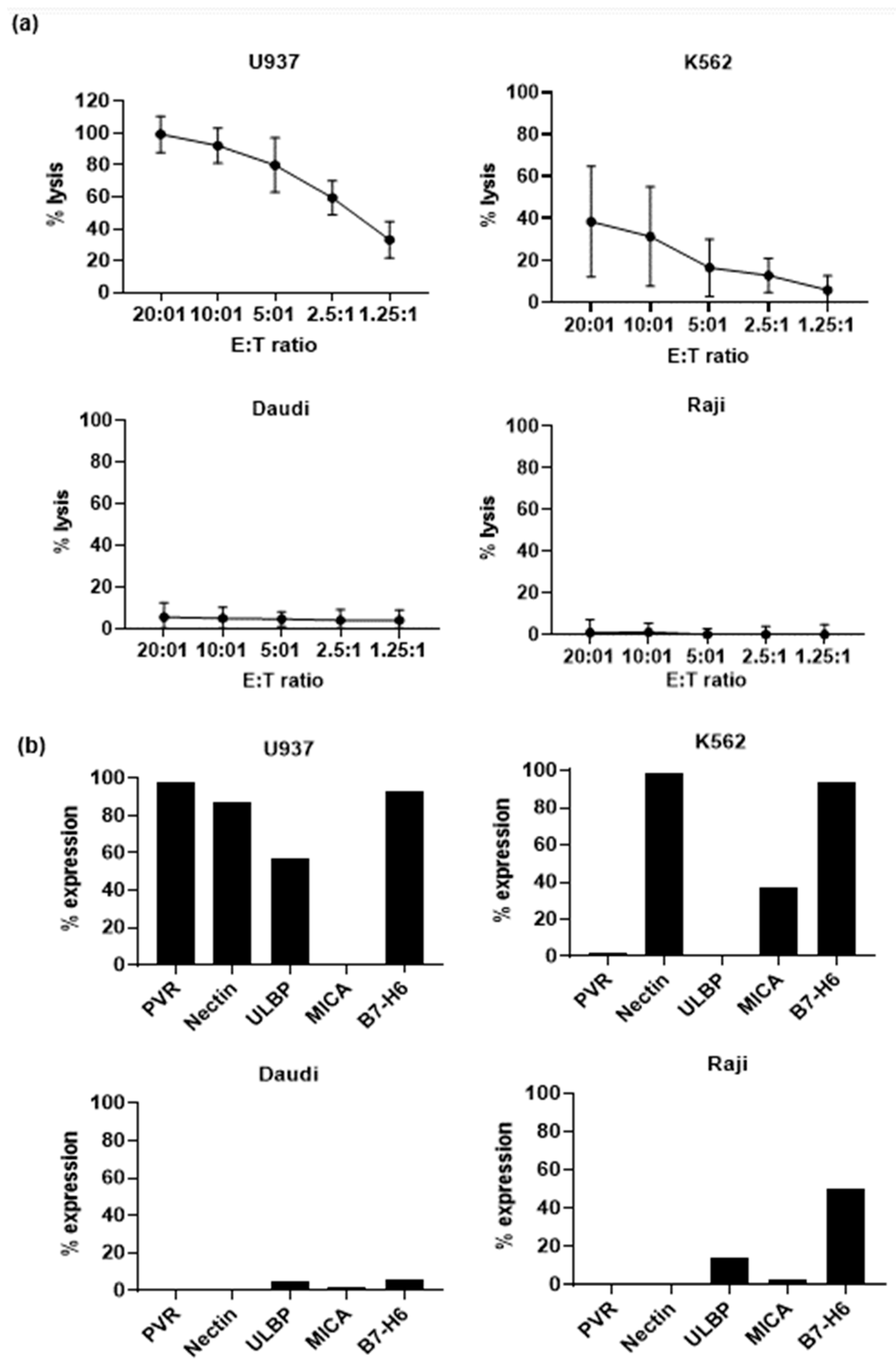
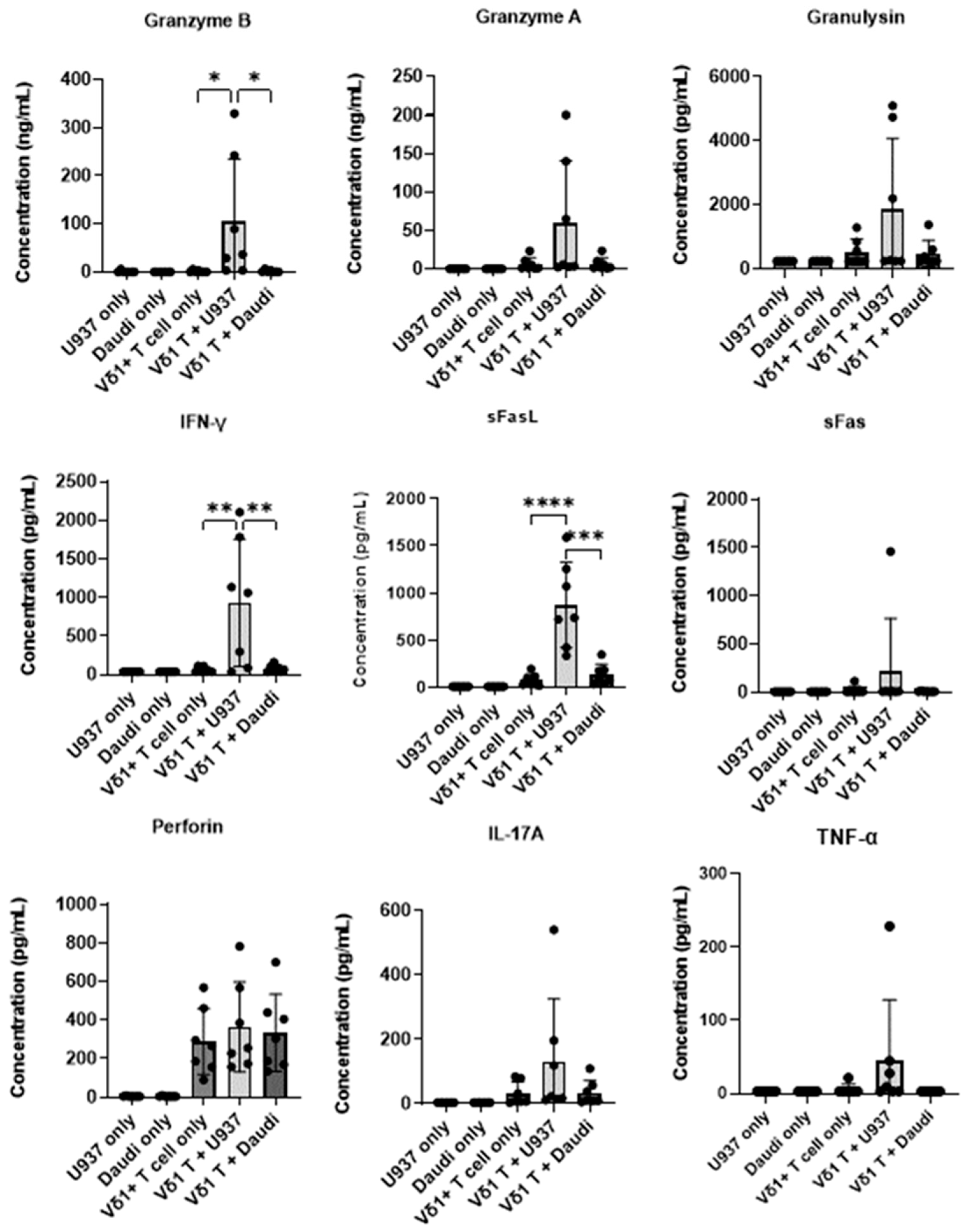
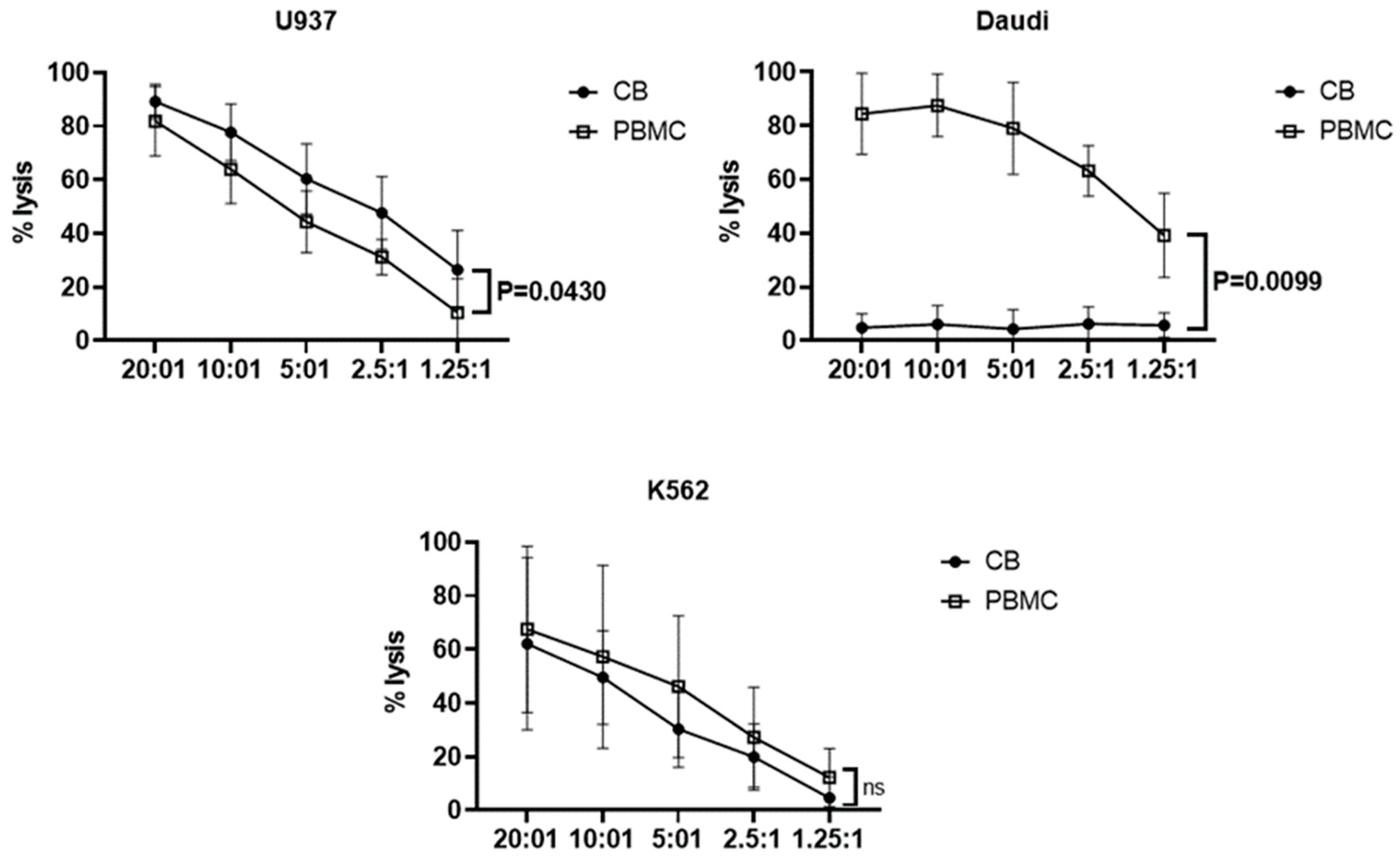
3.3. Effector Functions of Vδ1+ T Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (gammadelta) T cells: Friend or foe in cancer development? J. Transl. Med. 2018, 16, 3. [Google Scholar] [CrossRef]
- Carding, S.R.; Egan, P.J. Gammadelta T cells: Functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002, 2, 336–345. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Pang, L.; Xie, G.; Welte, T.; Saxena, V.; Wicker, J.; Mann, B.; Soong, L.; Barrett, A.; et al. The co-stimulatory effects of MyD88-dependent Toll-like receptor signaling on activation of murine gam-madelta T cells. PLoS ONE 2014, 9, e108156. [Google Scholar]
- Wang, L.; Kamath, A.; Das, H.; Li, L.; Bukowski, J.F. Antibacterial effect of human Vγ2Vδ2 T cells in vivo. J. Clin. Investig. 2001, 108, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Mizuno, Y.; Takaki, K.; Takada, H.; Akeda, H.; Aoki, T.; Nagata, M.; Ueda, K.; Matsuzaki, G.; Yoshikai, Y. Predominant activation and expansion of V gamma 9-bearing gamma delta T cells in vivo as well as in vitro in Salmonella infection. J. Clin. Investig. 1992, 90, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Born, W.K.; O’Brien, R.L. Antigen-restricted gammadelta T-cell receptors? Arch. Immunol. Ther. Exp. 2009, 57, 129–135. [Google Scholar] [CrossRef]
- Perera, M.K.; Carter, R.; Goonewardene, R.; Mendis, K.N. Transient increase in circulating gamma/delta T cells during Plasmodium vivax malarial paroxysms. J. Exp. Med. 1994, 179, 311–315. [Google Scholar] [CrossRef]
- Dechanet, J. Implication of gamma-delta T cells in the human immune response to cytomegalovirus. J. Clin. Virol. 1999, 12, 137. [Google Scholar] [CrossRef]
- Kabelitz, D.; Wesch, D. Role of gamma delta T-lymphocytes in HIV infection. Eur. J. Med. Res. 2001, 6, 169–174. [Google Scholar]
- Kabelitz, D.; Kalyan, S.; Oberg, H.H.; Wesch, D. Human Vdelta2 versus non-Vdelta2 gammadelta T cells in antitumor immunity. Oncoimmunology 2013, 2, e23304. [Google Scholar] [CrossRef] [PubMed]
- Holtmeier, W.; Kabelitz, D. gammadelta T cells link innate and adaptive immune responses. Chem. Immunol. Allergy 2005, 86, 151–183. [Google Scholar]
- Siegers, G.M.; Lamb, L.S., Jr. Cytotoxic and regulatory properties of circulating Vdelta1+ gammadelta T cells: A new player on the cell therapy field? Mol. Ther. 2014, 22, 1416–1422. [Google Scholar] [CrossRef]
- Deniger, D.C.; Moyes, J.S.; Cooper, L.J.N. Clinical Applications of Gamma Delta T Cells with Multivalent Immunity. Front. Immunol. 2014, 5, 636. [Google Scholar] [CrossRef]
- Nagamine, I.; Yamaguchi, Y.; Ohara, M.; Ikeda, T.; Okada, M. Induction of gamma delta T cells using zoledronate plus interleukin-2 in patients with metastatic cancer. Hiroshima J. Med. Sci. 2009, 58, 37–44. [Google Scholar]
- Thompson, K.; Roelofs, A.J.; Jauhiainen, M.; Mönkkönen, H.; Mönkkönen, J.; Rogers, M.J. Activation of γδ T cells by bisphosphonates. Adv. Exp. Med. Biol. 2010, 658, 11–20. [Google Scholar]
- Zhou, J.; Kang, N.; Cui, L.; Ba, D.; He, W. Anti-gammadelta TCR antibody-expanded gammadelta T cells: A better choice for the adoptive immunotherapy of lymphoid malignancies. Cell Mol. Immunol. 2012, 9, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.; Correia, D.V.; Fernandes-Platzgummer, A.; da Silva, C.L.; da Silva, M.G.; Anjos, D.R.; Silva-Santos, B. Delta One T Cells for Immunotherapy of Chronic Lymphocytic Leukemia: Clinical-Grade Expan-sion/Differentiation and Preclinical Proof of ConceptDelta One T Cells for Adoptive Immunotherapy of CLL. Clin. Cancer Res. 2016, 22, 5795–5804. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Ortolani, R.; Veneri, D.; Raffaelli, R.; Peroni, D.; Pigozzi, R.; Colombatti, M.; Vella, A. Lymphocyte phenotypic subsets in umbilical cord blood compared to peripheral blood from related mothers. Cytom. Part B Clin. Cytom. 2011, 80B, 248–253. [Google Scholar] [CrossRef]
- Berglund, S.; Gaballa, A.; Sawaisorn, P.; Sundberg, B.; Uhlin, M. Expansion of Gammadelta T Cells from Cord Blood: A Therapeutical Possibility. Stem. Cells Int. 2018, 2018, 8529104. [Google Scholar] [CrossRef] [PubMed]
- Vermijlen, D.; Ellis, P.; Langford, C.; Klein, A.; Engel, R.; Willimann, K.; Jomaa, H.; Hayday, A.C.; Eberl, M. Distinct cytokine-driven responses of activated blood gammadelta T cells: Insights into unconventional T cell pleiotropy. J. Immunol. 2007, 178, 4304–4314. [Google Scholar] [CrossRef]
- Pitard, V.; Roumanes, D.; Lafarge, X.; Couzi, L.; Garrigue, I.; Lafon, M.E.; Merville, P.; Moreau, J.F.; Déchanet-Merville, J. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 2008, 112, 1317–1324. [Google Scholar] [CrossRef]
- Lafarge, X.; Merville, P.; Cazin, M.C.; Bergé, F.; Potaux, L.; Moreau, J.F.; Déchanet-Merville, J. Cytomegalovirus Infection in Transplant Recipients Resolves When Circulating γδ T Lymphocytes Expand, Suggesting a Protective Antiviral Role. J. Infect. Dis. 2001, 184, 533–541. [Google Scholar] [CrossRef]
- Placido, R.; Auricchio, G.; Gabriele, I.; Galli, E.; Brunetti, E.; Colizzi, V.; Battistini, L.; Mancino, G. Characterization of the immune response of human cord-blood derived gamma/delta T cells to stimulation with aminobisphosphonate compounds. Int. J. Immunopathol. Pharmacol. 2011, 24, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kalyan, S.; Kabelitz, D. Defining the nature of human gammadelta T cells: A biographical sketch of the highly empathetic. Cell Mol. Immunol. 2013, 10, 21–29. [Google Scholar] [CrossRef]
- Dimova, T.; Brouwer, M.; Gosselin, F.; Tassignon, J.; Leo, O.; Donner, C.; Marchant, A.; Vermijlen, D. Effector Vgamma9Vdelta2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc. Natl. Acad. Sci. USA 2015, 112, E556–E565. [Google Scholar] [CrossRef] [PubMed]
- Cairo, C.; Sagnia, B.; Cappelli, G.; Colizzi, V.; Leke, R.G.; Leke, R.J.; Pauza, C.D. Human cord blood gammadelta T cells expressing public Vgamma2 chains dominate the response to bisphosphonate plus interleukin-15. Immunology 2013, 138, 346–360. [Google Scholar] [CrossRef]
- Deniger, D.C.; Maiti, S.N.; Mi, T.; Switzer, K.C.; Ramachandran, V.; Hurton, L.V.; Ang, S.; Olivares, S.; Rabinovich, B.A.; Huls, M.H.; et al. Activating and Propagating Polyclonal Gamma Delta T Cells with Broad Specificity for Malignancies. Clin. Cancer Res. 2014, 20, 5708–5719. [Google Scholar] [CrossRef]
- Cho, H.W.; Kim, S.Y.; Sohn, D.H.; Lee, M.J.; Park, M.Y.; Sohn, H.J.; Cho, H.I.; Kim, T.G. Triple costimulation via CD80, 4-1BB, and CD83 ligand elicits the long-term growth of Vgamma9Vdelta2 T cells in low levels of IL-2. J. Leukoc. Biol. 2016, 99, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, Y.; Hur, G.; Lee, S.E.; Cho, H.I.; Sohn, H.J.; Cho, B.S.; Kim, H.J.; Kim, T.G. Gammadelta T cells cultured with artificial antigen-presenting cells and IL-2 show long-term proliferation and enhanced effector functions compared with gammadelta T cells cultured with only IL-2 after stimulation with zoledronic acid. Cytotherapy 2021, 23, 908–917. [Google Scholar] [CrossRef]
- Hyun, S.-J.; Sohn, H.-J.; Lee, H.-J.; Lee, S.-D.; Kim, S.; Sohn, D.-H.; Hong, C.-H.; Choi, H.; Cho, H.-I.; Kim, T.-G. Comprehensive Analysis of Cytomegalovirus pp65 Antigen-Specific CD8+ T Cell Responses According to Human Leukocyte Antigen Class I Allotypes and Intraindividual Dominance. Front. Immunol. 2017, 8, 1591. [Google Scholar] [CrossRef]
- Sauce, D.; Almeida, J.R.; Larsen, M.; Haro, L.; Autran, B.; Freeman, G.J.; Appay, V. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. Aids 2007, 21, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Morath, A.; Schamel, W.W. alphabeta and gammadelta T cell receptors: Similar but different. J. Leukoc. Biol. 2020, 107, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Dopfer, E.P.; Hartl, F.A.; Oberg, H.H.; Siegers, G.M.; Yousefi, O.S.; Kock, S.; Fiala, G.J.; Garcillán, B.; Sandstrom, A.; Alarcón, B.; et al. The CD3 conformational change in the gammadelta T cell receptor is not triggered by antigens but can be enforced to enhance tumor killing. Cell Rep. 2014, 7, 1704–1715. [Google Scholar] [CrossRef]
- Dokouhaki, P.; Han, M.; Joe, B.; Li, M.; Johnston, M.R.; Tsao, M.S.; Zhang, L. Adoptive immunotherapy of cancer using ex vivo expanded human gammadelta T cells: A new approach. Cancer Lett. 2010, 297, 126–136. [Google Scholar] [CrossRef]
- Janssen, O.; Wesselborg, S.; Heckl-Ostreicher, B.; Pechhold, K.; Bender, A.; Schondelmaier, S.; Moldenhauer, G.; Kabelitz, D. T cell receptor/CD3-signaling induces death by apoptosis in human T cell receptor gamma delta + T cells. J. Immunol. 1991, 146, 35–39. [Google Scholar] [CrossRef]
- Narazaki, H.; Watari, E.; Shimizu, M.; Owaki, A.; Das, H.; Fukunaga, Y.; Takahashi, H.; Sugita, M. Perforin-dependent killing of tumor cells by Vγ1Vδ1-bearing T-cells. Immunol. Lett. 2003, 86, 113–119. [Google Scholar] [CrossRef]
- Di Lorenzo, B.; Simões, A.E.; Caiado, F.; Tieppo, P.; Correia, D.V.; Carvalho, T.; da Silva, M.G.; Déchanet-Merville, J.; Schumacher, T.N.; Prinz, I.; et al. Broad Cytotoxic Targeting of Acute Myeloid Leukemia by Polyclonal Delta One T Cells AML Targeting by Cytotoxic Delta One T Cells. Cancer Immunol. Res. 2019, 7, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.V.; Fogli, M.; Hudspeth, K.; da Silva, M.G.; Mavilio, D.; Silva-Santos, B. Differentiation of human peripheral blood Vdelta1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 2011, 118, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef]
- Yazdanifar, M.; Barbarito, G.; Bertaina, A.; Airoldi, I. γδ T cells: The ideal tool for cancer immunotherapy. Cells 2020, 9, 1305. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.T.; Jin, C.; Sarikonda, G.; Wang, H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: Discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007, 215, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Fayen, J.D.; Tykocinski, M.L. The expansion of human gammadelta T cells in response to Daudi cells requires the partici-pation of CD4+ T cells. Immunology 1999, 97, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hur, G.; Choi, H.; Lee, Y.; Sohn, H.-J.; Kim, S.-Y.; Kim, T.-G. In Vitro Expansion of Vδ1+ T Cells from Cord Blood by Using Artificial Antigen-Presenting Cells and Anti-CD3 Antibody. Vaccines 2023, 11, 406. https://doi.org/10.3390/vaccines11020406
Hur G, Choi H, Lee Y, Sohn H-J, Kim S-Y, Kim T-G. In Vitro Expansion of Vδ1+ T Cells from Cord Blood by Using Artificial Antigen-Presenting Cells and Anti-CD3 Antibody. Vaccines. 2023; 11(2):406. https://doi.org/10.3390/vaccines11020406
Chicago/Turabian StyleHur, Gaeun, Haeyoun Choi, Yunkyeong Lee, Hyun-Jung Sohn, Su-Yeon Kim, and Tai-Gyu Kim. 2023. "In Vitro Expansion of Vδ1+ T Cells from Cord Blood by Using Artificial Antigen-Presenting Cells and Anti-CD3 Antibody" Vaccines 11, no. 2: 406. https://doi.org/10.3390/vaccines11020406
APA StyleHur, G., Choi, H., Lee, Y., Sohn, H.-J., Kim, S.-Y., & Kim, T.-G. (2023). In Vitro Expansion of Vδ1+ T Cells from Cord Blood by Using Artificial Antigen-Presenting Cells and Anti-CD3 Antibody. Vaccines, 11(2), 406. https://doi.org/10.3390/vaccines11020406



