The Use of an Adjuvant System Improves Innate and Adaptive Immune Response When Associated with a Leishmania (Viannia) braziliensis Antigen in a Vaccine Candidate against L. (Leishmania) infantum Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mice and Skin Sensitization
2.3. Cell Recruitment Profile and Differential Leukocyte Counts
2.4. Chemokines and Cytokines Measurement
2.5. Mice Immunization and Challenge
2.6. Leishmania (V.) braziliensis Antigen
2.7. Parasites and Soluble Leishmania (L.) infantum Antigen
2.8. Lymphocytes Proliferation Assay and Intracellular Cytokines Staining
2.9. Central and Effector Memory T Cell Phenotypes
2.10. Parasite Load in Mice Spleen and Liver
2.11. Statistical Analysis
3. Results
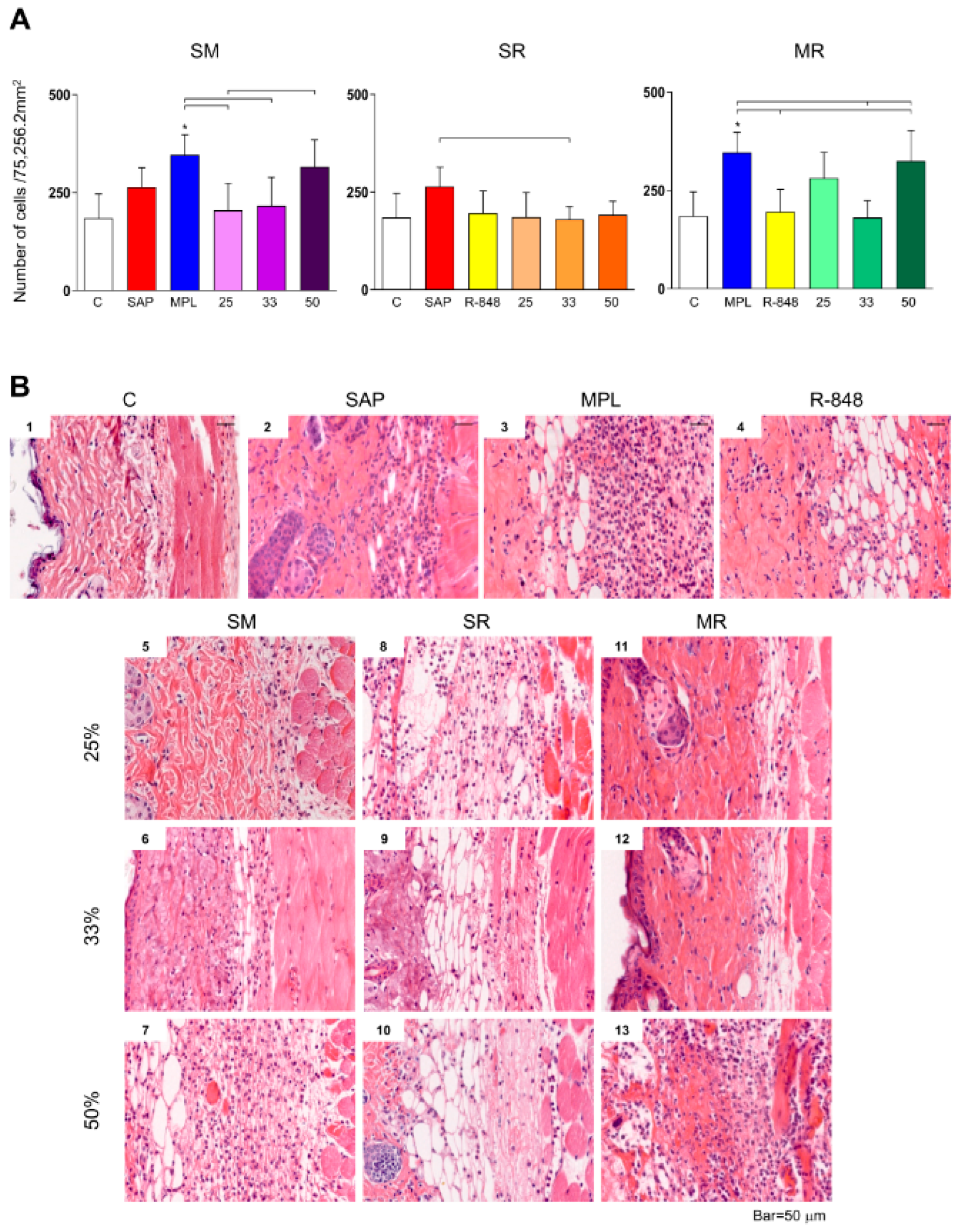
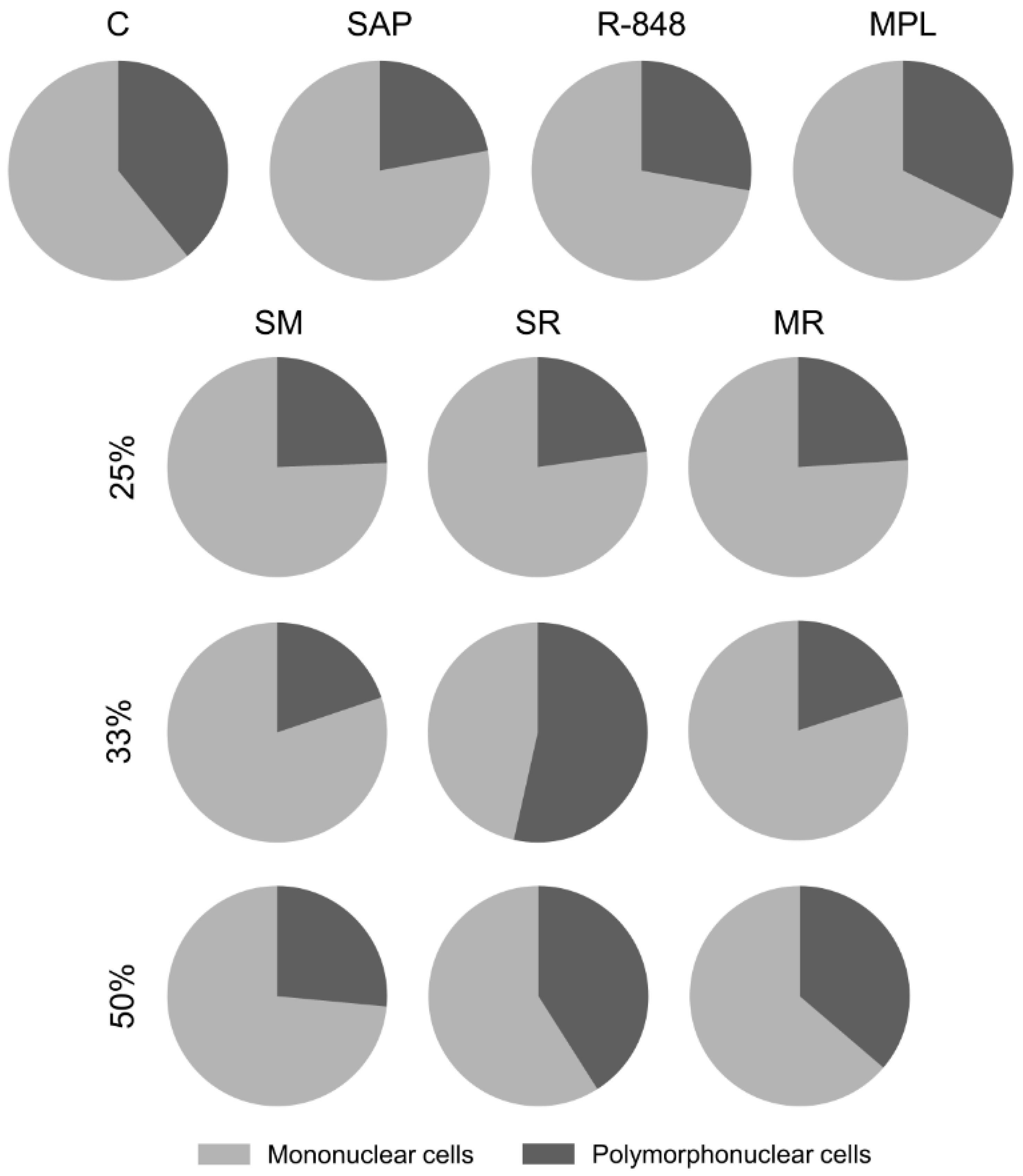
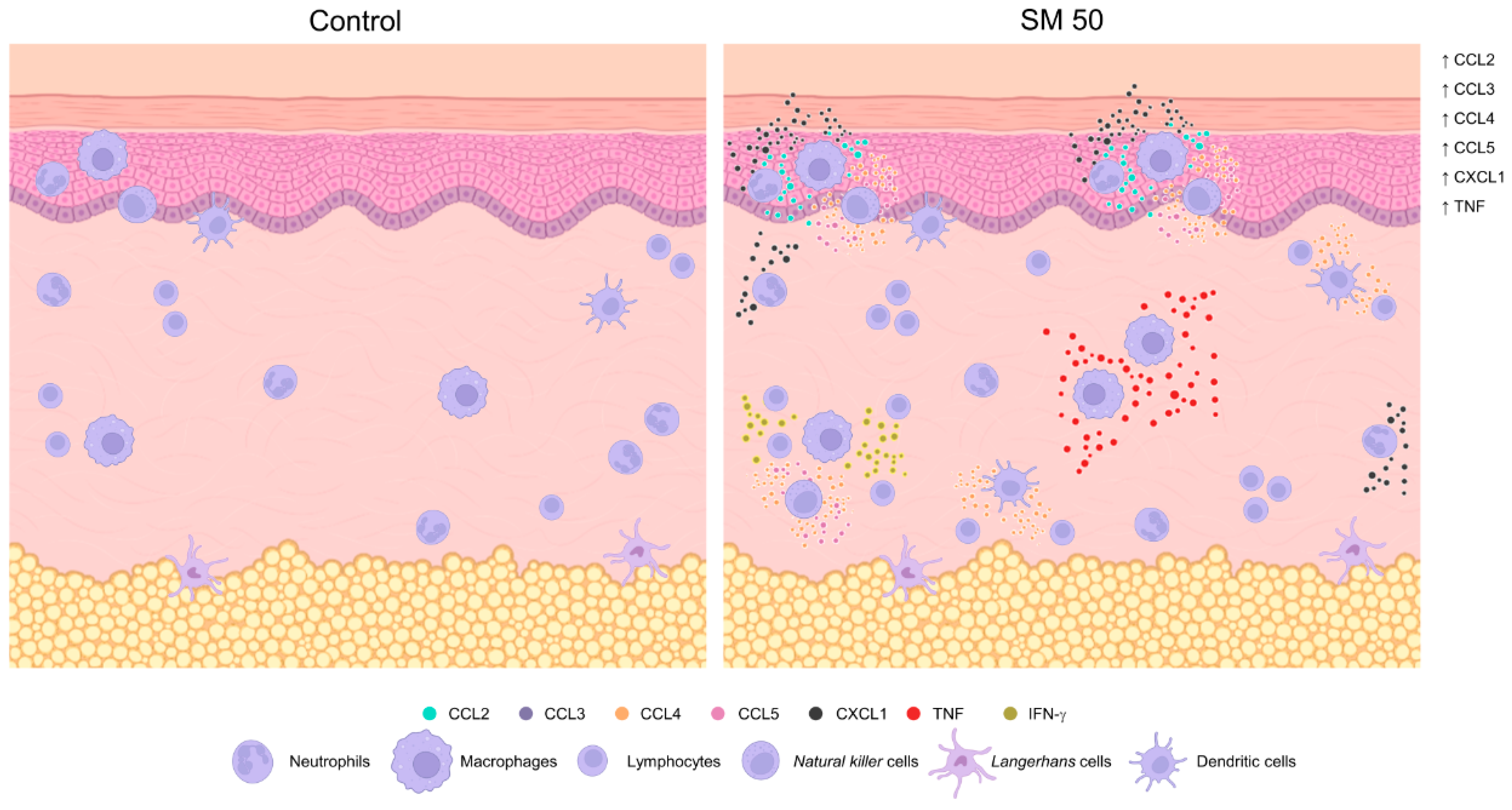
3.1. Inflammatory Cells Infiltrating in Mouse Skin
3.2. Sensitization with the SR Adjuvant System Promotes a Polymorphonuclear Cells Influx to the Inoculum Site
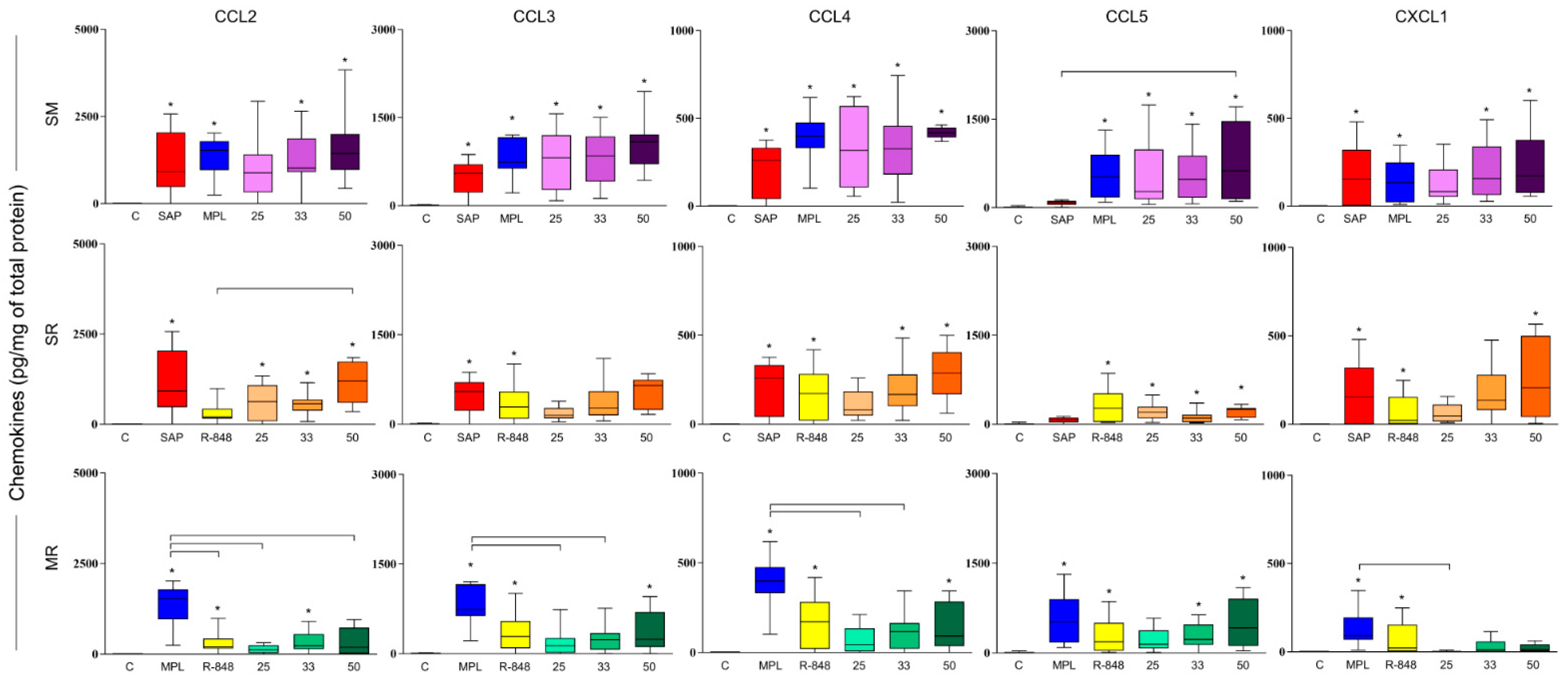
3.3. SM Adjuvant System-Induced Chemokine Production in Mouse Skin
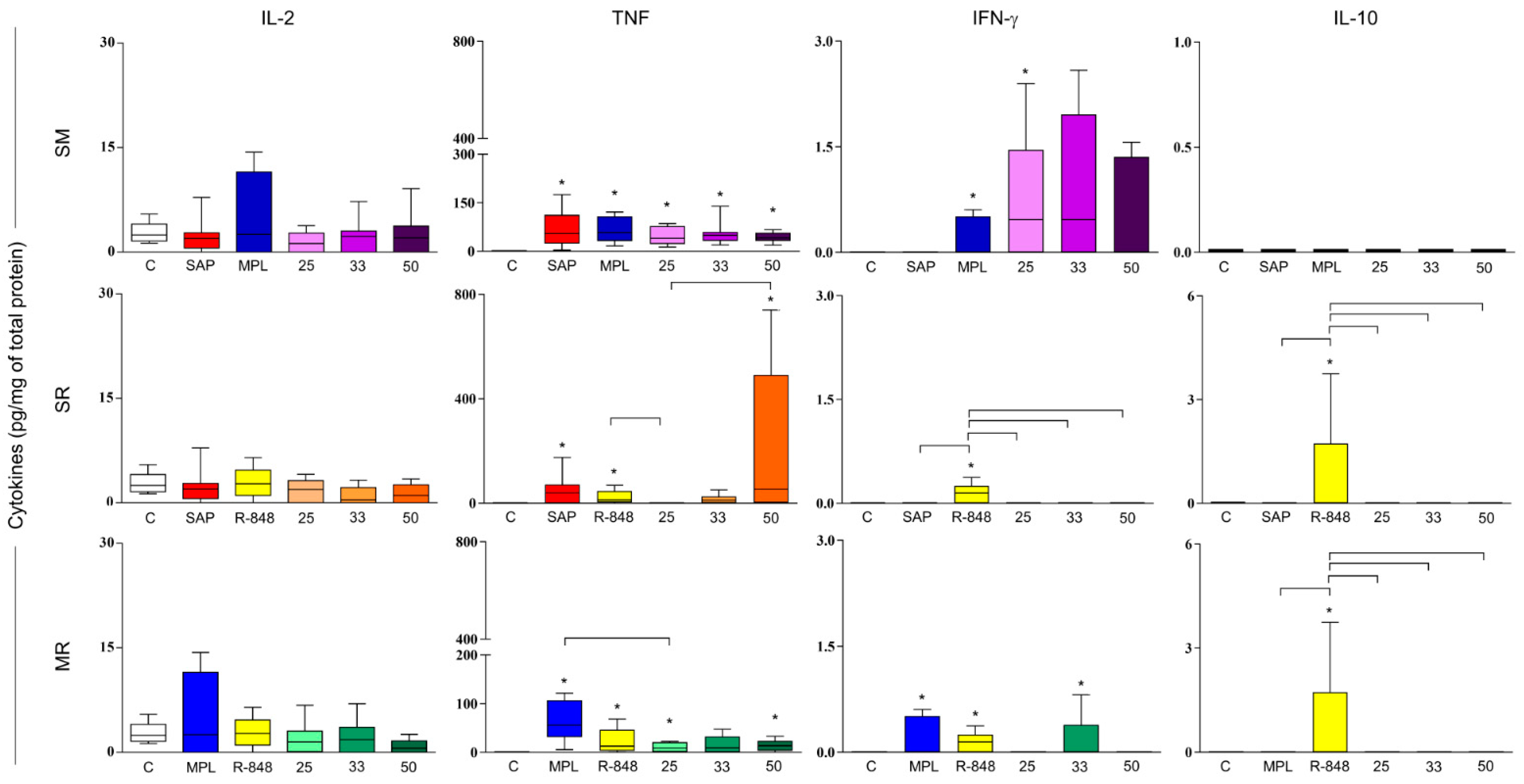
3.4. Sensitization with Adjuvant Systems Led to Pro-Inflammatory Microenvironment in Mouse Skin
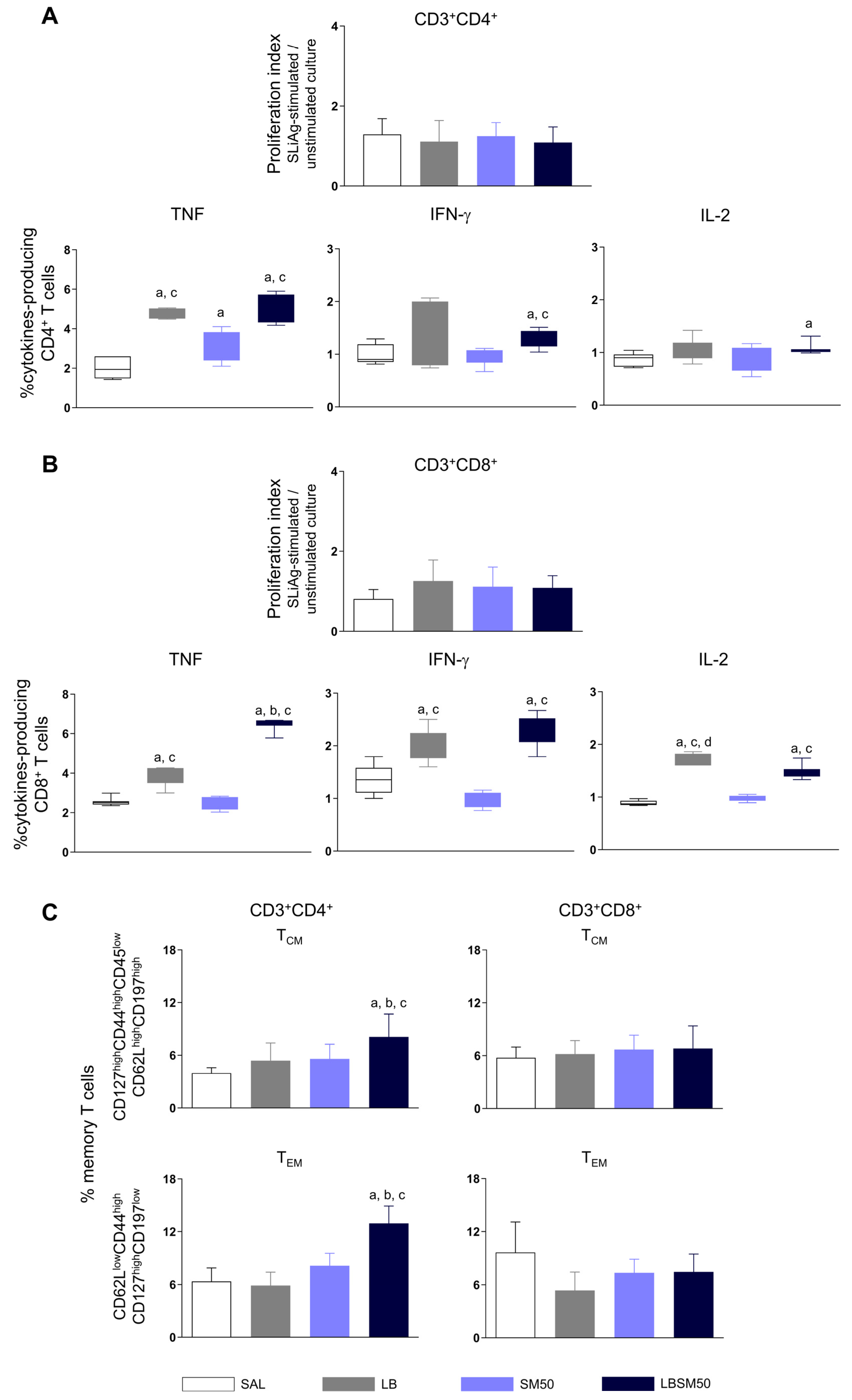
3.5. Immunization with LBSM50 Stimulated Expressive Pro-Inflammatory Cytokines Production
3.6. Immunization with LBSM50 Induced Differentiation in Memory CD4+ T cells
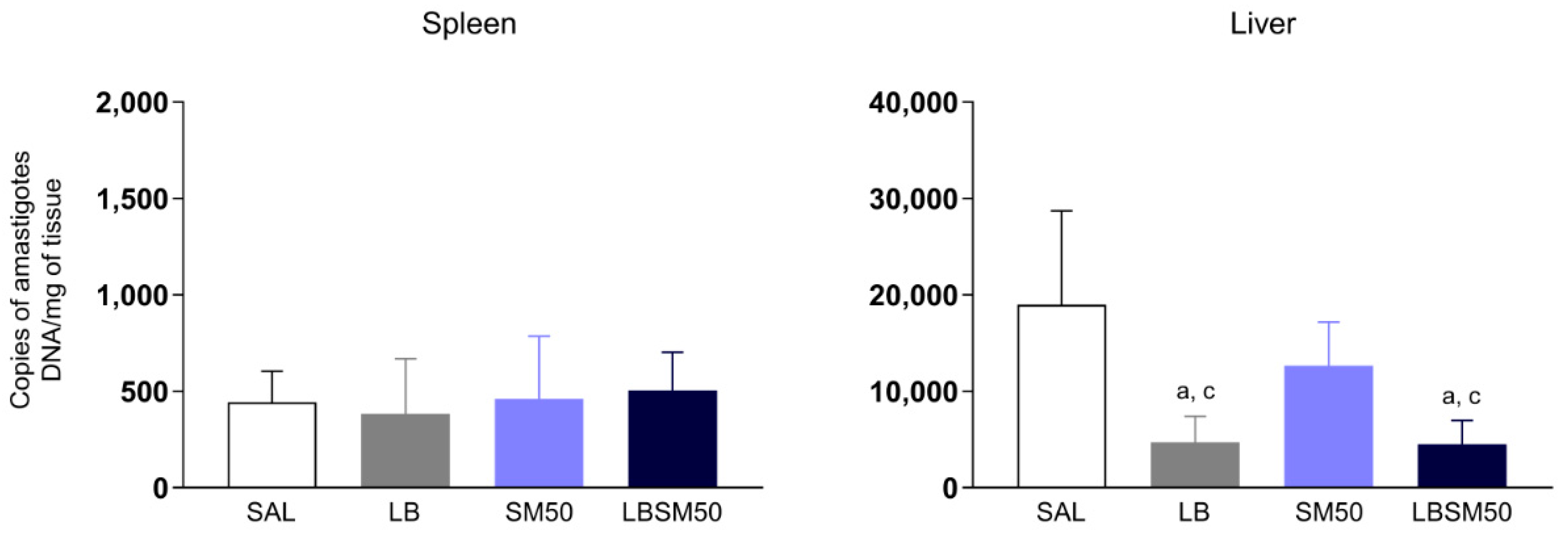
3.7. Immunization with LBSM50 Elicited Partial Protection against Leishmania Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bottazzi, M.E.; Hotez, P.J. “Running the Gauntlet”: Formidable challenges in advancing neglected tropical diseases vaccines from development through licensure, and a “Call to Action”. Hum. Vaccines Immunother. 2019, 15, 2235. Available online: https://pubmed.ncbi.nlm.nih.gov/31180271/ (accessed on 26 December 2022). [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupeze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, R.; Aouizerate, J.; Cadusseau, J.; Yara, S.; Authier, F. Aluminum adjuvants of vaccines injected into the muscle: Normal fate, pathology and associated disease. Morphologie 2016, 100, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Integrating Neglected Tropical Diseases into Global Health and Development: Fourth WHO Report on Neglected Tropical Diseases. Available online: https://apps.who.int/iris/handle/10665/255011 (accessed on 26 December 2022).
- Rostamian, M.; Niknam, H.M. Evaluation of the adjuvant effect of agonists of toll-like receptor 4 and 7/8 in a vaccine against leishmaniasis in BALB/c mice. Mol. Immunol. 2017, 91, 202–208. Available online: https://pubmed.ncbi.nlm.nih.gov/28963929/ (accessed on 26 December 2022). [CrossRef] [PubMed]
- Moafi, M.; Rezvan, H.; Sherkat, R.; Taleban, R. Leishmania Vaccines Entered in Clinical Trials: A Review of Literature. Int. J. Prev. Med. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Vitoriano-Souza, J.; Moreira, N.D.D.; Teixeira-Carvalho, A.; Carneiro, C.M.; Siqueira, F.A.M.; Vieira, P.M.D.A.; Giunchetti, R.C.; Moura, S.A.D.L.; Fujiwara, R.T.; Melo, M.N.; et al. Cell Recruitment and Cytokines in Skin Mice Sensitized with the Vaccine Adjuvants: Saponin, Incomplete Freund’s Adjuvant, and Monophosphoryl Lipid A. PLoS ONE 2012, 7, e40745. [Google Scholar] [CrossRef]
- Sun, H.X.; Xie, Y.; Ye, Y.P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. Available online: https://pubmed.ncbi.nlm.nih.gov/19208455/ (accessed on 26 December 2022). [CrossRef]
- Vitoriano-Souza, J.; Mathias, F.A.S.; das Dores Moreira, N.; de Oliveira Aguiar-Soares, R.D.; de Abreu Vieira, P.M.; Teixeira-Carvalho, A.; Carneiro, C.M.; Giunchetti, R.C.; de Brito, R.C.F.; Fujiwara, R.T.; et al. Effect on cellular recruitment and the innate immune response by combining saponin, monophosphoryl lipid-A and Incomplete Freund’s Adjuvant with Leishmania (Viannia) braziliensis antigens for a vaccine formulation. Vaccine 2019, 37, 7269–7279. Available online: https://pubmed.ncbi.nlm.nih.gov/31575491/ (accessed on 26 December 2022). [CrossRef]
- Pulendran, B.S.; Arunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. Available online: https://pubmed.ncbi.nlm.nih.gov/33824489/ (accessed on 26 December 2022). [CrossRef]
- Garçon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccin. Immunother. 2017, 13, 19–33. Available online: https://pubmed.ncbi.nlm.nih.gov/27636098/ (accessed on 26 December 2022). [CrossRef]
- Garçon, N.; Da Silva, F.T. Development and Evaluation of AS04, a Novel and Improved Adjuvant System Containing 3-O-Desacyl-4′- Monophosphoryl Lipid A and Aluminum Salt. In Immunopotentiators in Modern Vaccines; Academic Press: Cambridge, MA, USA, 2017; pp. 287–309. [Google Scholar]
- Garçon, N.; Heppner, D.G.; Cohen, J. Development of RTS,S/AS02: A purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev. Vaccines 2003, 2, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Ahmad, M.Z.; Syed, R.; Alsenaidy, M.A.; Albraiki, S.A. Lignin nanoparticles as a promising vaccine adjuvant and delivery system for ovalbumin. Int. J. Biol. Macromol. 2020, 163, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- McKay, P.F.; King, D.F.L.; Mann, J.; Barinaga, G.; Carter, D.; Shattock, R.J. TLR4 and TLR7/8 Adjuvant Combinations Generate Different Vaccine Antigen-Specific Immune Outcomes in Minipigs when Administered via the ID or IN Routes. PLoS ONE 2016, 11, e0148984. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Van Mechelen, M.; Wettendorff, M. Development and evaluation of AS04, a novel and improved adjuvant system containing MPL and aluminum salt. Immunopotentiators Mod. Vaccines 2006, 161–177. [Google Scholar] [CrossRef]
- Tritto, E.; Mosca, F.; De Gregorio, E. Mechanism of action of licensed vaccine adjuvants. Vaccine 2009, 27, 3331–3334. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.C.; Coulter, A.R. Adjuvants—A classification and review of their modes of action. Vaccine 1997, 15, 248–256. Available online: https://pubmed.ncbi.nlm.nih.gov/9139482/ (accessed on 26 December 2022).
- Liu, G.; Anderson, C.; Scaltreto, H.; Barbon, J.; Kensil, C.R. QS-21 structure/function studies: Effect of acylation on adjuvant activity. Vaccine 2002, 20, 2808–2815. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. Available online: https://pubmed.ncbi.nlm.nih.gov/15325725/ (accessed on 26 December 2022). [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. Available online: https://www.nature.com/articles/nm.3409 (accessed on 26 December 2022).
- Baldrick, P.; Richardson, D.; Woroniecki, S.R.; Lees, B. Pollinex® Quattro Ragweed: Safety evaluation of a new allergy vaccine adjuvanted with monophosphoryl lipid A (MPL®) for the treatment of ragweed pollen allergy. J. Appl. Toxicol. 2007, 27, 399–409. [Google Scholar] [CrossRef]
- Kazmin, D.; Nakaya, H.I.; Lee, E.K.; Johnson, M.J.; van der Most, R.; Berg, R.A.V.D.; Ballou, W.R.; Jongert, E.; Wille-Reece, U.; Ockenhouse, C.; et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc. Natl. Acad. Sci. USA 2017, 114, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Matlashewski, G. Immunization with a Toll-Like Receptor 7 and/or 8 Agonist Vaccine Adjuvant Increases Protective Immunity against Leishmania major in BALB/c Mice. Infect. Immun. 2008, 76, 3777–3783. Available online: https://pubmed.ncbi.nlm.nih.gov/18474642/ (accessed on 26 December 2022). [CrossRef] [PubMed]
- Vasilakos, J.P.; Tomai, M.A. The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev. Vaccines 2013, 12, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Tomai, M.A.; Miller, R.L.; Lipson, K.E.; Kieper, W.C.; Zarraga, I.E.; Vasilakos, J.P. Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev. Vaccines 2007, 6, 835–847. [Google Scholar] [CrossRef]
- Craft, N.; Birnbaum, R.; Quanquin, N.; Erfe, M.C.B.; Quant, C.; Haskell, J.; Bruhn, K.W. Topical Resiquimod Protects against Visceral Infection with Leishmania infantum chagasi in Mice. Clin. Vaccine Immunol. 2014, 21, 1314–1322. [Google Scholar] [CrossRef]
- Mathias, F.A.S.; de Oliveira Cardoso, J.M.; Reis, L.E.S.; de Souza, J.V.; das Dores Moreira, N.; Di Paschoale Ostolin, T.L.V.; de Brito, R.C.F.; de Oliveira Aguiar Soares, R.D.; de Abreu Vieira, P.M.; Carneiro, C.M.; et al. Cell Immune Response in Mice Skin Stimulated with Different Adjuvants by Intradermal Route. J. Vaccines Immunol. Immunopathol. 2022, 7, 170. [Google Scholar]
- Giunchetti, R.C.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Roatt, B.M.; de Oliveira Aguiar-Soares, R.D.; de Souza, J.V.; das Dores Moreira, N.; Malaquias, L.C.C.; Castro, L.L.M.; et al. Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs. Vaccine 2007, 25, 7674–7686. [Google Scholar] [CrossRef]
- Reis, A.B.; Teixeira-Carvalho, A.; Vale, A.M.; Marques, M.J.; Giunchetti, R.C.; Mayrink, W.; Guerra, L.L.; Andrade, R.A.; Corrêa-Oliveira, R.; Martins-Filho, O.A. Isotype patterns of immunoglobulins: Hallmarks for clinical status and tissue parasite density in brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Veter Immunol. Immunopathol. 2006, 112, 102–116. [Google Scholar] [CrossRef]
- Brito, R.C.F.D.; Ruiz, J.C.; Cardoso, J.M.d.O.; Ostolin, T.L.V.D.P.; Reis, L.E.S.; Mathias, F.A.S.; Aguiar-Soares, R.D.d.O.; Roatt, B.M.; Corrêa-Oliveira, R.; Resende, D.d.M.; et al. Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis. Vaccines 2020, 8, 252. Available online: https://www.mdpi.com/2076-393X/8/2/252/htm (accessed on 26 December 2022). [CrossRef]
- De Brito, R.C.F.; de Oliveira Cardoso, J.M.; Reis, L.E.S.; Mathias, F.A.S.; de Oliveira Aguiar-Soares, R.D.; Teixeira-Carvalho, A.; Roatt, B.M.; Corrêa-Oliveira, R.; Ruiz, J.C.; de Melo Resende, D.; et al. Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice. Vaccines 2019, 7, 162. Available online: https://www.mdpi.com/2076-393X/7/4/162/htm (accessed on 26 December 2022).
- Reis, L.E.S.; de Brito, R.C.F.; Cardoso, J.M.D.O.; Mathias, F.A.S.; Soares, R.D.O.A.; Carneiro, C.M.; Vieira, P.M.D.A.; Ramos, G.S.; Frézard, F.J.G.; Roatt, B.M.; et al. Mixed Formulation of Conventional and Pegylated Meglumine Antimoniate-Containing Liposomes Reduces Inflammatory Process and Parasite Burden in Leishmania infantum-Infected BALB/c Mice. Antimicrob. Agents Chemother. 2017, 61, e00962-17. [Google Scholar] [CrossRef] [PubMed]
- Dendouga, N.; Fochesato, M.; Lockman, L.; Mossman, S.; Giannini, S.L. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine 2012, 30, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Matlashewski, G. Loss of virulence in Leishmania donovani deficient in an amastigote-specific protein, A2. Proc. Natl. Acad. Sci. USA 1997, 94, 8807–8811. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.E. Immunological concepts of vaccine adjuvant activity. Curr. Opin. Immunol. 2000, 12, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Richmond, A. Chemokine Regulation of Neutrophil Infiltration of Skin Wounds. Adv. Wound Care 2015, 4, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.P.; Nicholls, A.J.; Wong, C.H.Y. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551. Available online: https://pubmed.ncbi.nlm.nih.gov/29387942/ (accessed on 27 December 2022). [CrossRef]
- Abbadie, C.; Lindia, J.A.; Cumiskey, A.M.; Peterson, L.B.; Mudgett, J.S.; Bayne, E.K.; DeMartino, J.A.; MacIntyre, D.E.; Forrest, M.J. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Natl. Acad. Sci. USA 2003, 100, 7947–7952. [Google Scholar] [CrossRef]
- Golec, M.; Lemieszek, M.; Skórska, C.; Sitkowska, J.; Zwoliński, J.; Mackiewicz, B.; Góra-Florek, A.; Milanowski, J.; Dutkiewicz, J. Cathelicidin related antimicrobial peptide, laminin, Toll-like receptors and chemokines levels in experimental hypersensitivity pneumonitis in mice. Pathol. Biol. 2015, 63, 130–135. [Google Scholar] [CrossRef]
- Andrews, K.; Abdelsamed, H.; Yi, A.-K.; Miller, M.A.; Fitzpatrick, E.A. TLR2 Regulates Neutrophil Recruitment and Cytokine Production with Minor Contributions from TLR9 during Hypersensitivity Pneumonitis. PLoS ONE 2013, 8, e73143. [Google Scholar] [CrossRef]
- Mentkowski, K.I.; Euscher, L.M.; Patel, A.; Alevriadou, B.R.; Lang, J.K. Monocyte recruitment and fate specification after myocardial infarction. Am. J. Physiol. Physiol. 2020, 319, C797–C806. [Google Scholar] [CrossRef]
- De la Fuente López, M.; Landskron, G.; Parada, D.; Dubois-Camacho, K.; Simian, D.; Martinez, M.; Romero, D.; Roa, J.C.; Chahuán, I.; Gutiérrez, R.; et al. The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumour. Biol. 2018, 40, 1010428318810059. Available online: https://pubmed.ncbi.nlm.nih.gov/30419802/ (accessed on 30 January 2023). [CrossRef]
- Hussen, J.; Frank, C.; Düvel, A.; Koy, M.; Schuberth, H.-J. The chemokine CCL5 induces selective migration of bovine classical monocytes and drives their differentiation into LPS-hyporesponsive macrophages in vitro. Dev. Comp. Immunol. 2014, 47, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kanno, E.; Kawakami, K.; Tanno, H.; Suzuki, A.; Sato, N.; Masaki, A.; Imamura, A.; Takagi, N.; Miura, T.; Yamamoto, H.; et al. Contribution of CARD9-mediated signalling to wound healing in skin. Exp. Dermatol. 2017, 26, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Li, T.; Wang, Q.; Qian, J.; Lu, Y.; Zhang, M.; Bi, E.; Yang, M.; Reu, F.; et al. Chemokines CCL2, 3, 14 stimulate macrophage bone marrow homing, proliferation, and polarization in multiple myeloma. Oncotarget 2015, 6, 24218–24229. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. Available online: https://pubmed.ncbi.nlm.nih.gov/24892271/ (accessed on 27 December 2022). [CrossRef] [PubMed]
- Lombardi, V.; Van Overtvelt, L.; Horiot, S.; Moingeon, P. Human Dendritic Cells Stimulated via TLR7 and/or TLR8 Induce the Sequential Production of Il-10, IFN-γ, and IL-17A by Naive CD4+ T Cells. J. Immunol. 2009, 182, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Peine, K.J.; Gupta, G.; Brackman, D.J.; Papenfuss, T.L.; Ainslie, K.M.; Satoskar, A.R.; Bachelder, E.M. Liposomal resiquimod for the treatment of Leishmania donovani infection. J. Antimicrob. Chemother. 2014, 69, 168–175. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Collignon, C.; Bourguignon, P.; Wouters, S.; Fierens, K.; Fochesato, M.; Dendouga, N.; Langlet, C.; Malissen, B.; Lambrecht, B.N.; et al. Enhancement of Adaptive Immunity by the Human Vaccine Adjuvant AS01 Depends on Activated Dendritic Cells. J. Immunol. 2014, 193, 1920–1930. [Google Scholar] [CrossRef]
- Coccia, M.; Collignon, C.; Hervé, C.; Chalon, A.; Welsby, I.; Detienne, S.; van Helden, M.J.; Dutta, S.; Genito, C.J.; Waters, N.C.; et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. npj Vaccines 2017, 2, 25. [Google Scholar] [CrossRef]
- Elikaee, S.; Mohebali, M.; Rezaei, S.; Eslami, H.; Khamesipour, A.; Keshavarz, H.; Eshraghian, M.R. Leishmania major p27 gene knockout as a novel live attenuated vaccine candidate: Protective immunity and efficacy evaluation against cutaneous and visceral leishmaniasis in BALB/c mice. Vaccine 2019, 37, 3221–3228. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Dey, R.; Gannavaram, S.; Solanki, S.; Salotra, P.; Nakhasi, H.L. Generation of growth arrested Leishmania amastigotes: A tool to develop live attenuated vaccine candidates against visceral leishmaniasis. Vaccine 2014, 32, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, J.A.; Gannavaram, S.; Santiago, H.D.C.; Selvapandiyan, A.; Souza, D.M.; Passos, L.S.A.; de Mendonça, L.Z.; Lemos-Giunchetti, D.D.S.; Ricci, N.D.; Bartholomeu, D.C.; et al. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine 2015, 33, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Vitoriano-Souza, J.; Reis, A.B.; Moreira, N.D.; Giunchetti, R.C.; Correa-Oliveira, R.; Carneiro, C.M. Kinetics of cell migration to the dermis and hypodermis in dogs vaccinated with antigenic compounds of Leishmania braziliensis plus saponin. Vaccine 2008, 26, 3922–3931. [Google Scholar] [CrossRef] [PubMed]
- das Dores Moreira, N.; Giunchetti, R.C.; Carneiro, C.M.; Vitoriano-Souza, J.; Roatt, B.M.; Malaquias, L.C.C.; Corrêa-Oliveira, R.; Reis, A.B. Histological study of cell migration in the dermis of hamsters after immunisation with two different vaccines against visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 418–424. Available online: https://pubmed.ncbi.nlm.nih.gov/19147234/ (accessed on 26 December 2022). [CrossRef] [PubMed]
- Agallou, M.; Margaroni, M.; Kotsakis, S.D.; Karagouni, E. A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania infantum. Vaccines 2020, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gomes, G.; Rodrigues, A.; Teixeira, F.; Carreira, J.; Alexandre-Pires, G.; Carvalho, S.; Santos-Mateus, D.; Martins, C.; Vale-Gato, I.; Marques, C.; et al. Immunization with the Leishmania infantum recombinant cyclophilin protein 1 confers partial protection to subsequent parasite infection and generates specific memory T cells. Vaccine 2014, 32, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bhattacharya, P.; Dagur, P.K.; Karmakar, S.; Ismail, N.; Joshi, A.B.; Akue, A.D.; KuKuruga, M.; McCoy, J.P.; Dey, R.; et al. Live Attenuated Leishmania donovani Centrin Gene–Deleted Parasites Induce IL-23–Dependent IL-17–Protective Immune Response against Visceral Leishmaniasis in a Murine Model. J. Immunol. 2018, 200, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. Available online: https://pubmed.ncbi.nlm.nih.gov/24336101/ (accessed on 26 December 2022). [CrossRef]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. Available online: https://pubmed.ncbi.nlm.nih.gov/10537110/ (accessed on 26 December 2022).
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.B.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. Available online: https://www.nature.com/articles/nm1592 (accessed on 26 December 2022). [CrossRef]
- Mutwiri, G.; Gerdts, V.; Van Drunen Littel-Van Den Hurk, S.; Auray, G.; Eng, N.; Garlapati, S.; Babiuk, L.A.; Potter, A. Combination adjuvants: The next generation of adjuvants? Expert Rev. Vaccines 2011, 10, 95–107. Available online: https://pubmed.ncbi.nlm.nih.gov/21162624/ (accessed on 27 December 2022). [CrossRef] [PubMed]
- Rodrigues, A.; Claro, M.; Alexandre-Pires, G.; Santos-Mateus, D.; Martins, C.; Valério-Bolas, A.; Rafael-Fernandes, M.; Pereira, M.; da Fonseca, I.P.; Tomás, A.; et al. Leishmania infantum antigens modulate memory cell subsets of liver resident T lymphocyte. Immunobiology 2017, 222, 409–422. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Couto, L.; Ribeiro-Romão, R.P.; Saavedra, A.F.; Souza, B.L.D.S.C.; Moreira, O.C.; Gomes-Silva, A.; Rossi-Bergmann, B.; Da-Cruz, A.M.; Pinto, E.F. Intranasal Vaccination with Leishmanial Antigens Protects Golden Hamsters (Mesocricetus auratus) Against Leishmania (Viannia) braziliensis Infection. PLoS Neglected Trop. Dis. 2015, 9, e3439. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.F.; Cortezia, M.D.M.; Rossi-Bergmann, B. Interferon-gamma-inducing oral vaccination with Leishmania amazonensis antigens protects BALB/c and C57BL/6 mice against cutaneous leishmaniasis. Vaccine 2003, 21, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathias, F.A.S.; Ostolin, T.L.V.D.P.; Reis, L.E.S.; Cardoso, J.M.d.O.; De Brito, R.C.F.; Aguiar Soares, R.D.d.O.; Roatt, B.M.; Vieira, P.M.d.A.; Reis, A.B. The Use of an Adjuvant System Improves Innate and Adaptive Immune Response When Associated with a Leishmania (Viannia) braziliensis Antigen in a Vaccine Candidate against L. (Leishmania) infantum Infection. Vaccines 2023, 11, 395. https://doi.org/10.3390/vaccines11020395
Mathias FAS, Ostolin TLVDP, Reis LES, Cardoso JMdO, De Brito RCF, Aguiar Soares RDdO, Roatt BM, Vieira PMdA, Reis AB. The Use of an Adjuvant System Improves Innate and Adaptive Immune Response When Associated with a Leishmania (Viannia) braziliensis Antigen in a Vaccine Candidate against L. (Leishmania) infantum Infection. Vaccines. 2023; 11(2):395. https://doi.org/10.3390/vaccines11020395
Chicago/Turabian StyleMathias, Fernando Augusto Siqueira, Thais Lopes Valentim Di Paschoale Ostolin, Levi Eduardo Soares Reis, Jamille Mirelle de Oliveira Cardoso, Rory Cristiane Fortes De Brito, Rodrigo Dian de Oliveira Aguiar Soares, Bruno Mendes Roatt, Paula Melo de Abreu Vieira, and Alexandre Barbosa Reis. 2023. "The Use of an Adjuvant System Improves Innate and Adaptive Immune Response When Associated with a Leishmania (Viannia) braziliensis Antigen in a Vaccine Candidate against L. (Leishmania) infantum Infection" Vaccines 11, no. 2: 395. https://doi.org/10.3390/vaccines11020395
APA StyleMathias, F. A. S., Ostolin, T. L. V. D. P., Reis, L. E. S., Cardoso, J. M. d. O., De Brito, R. C. F., Aguiar Soares, R. D. d. O., Roatt, B. M., Vieira, P. M. d. A., & Reis, A. B. (2023). The Use of an Adjuvant System Improves Innate and Adaptive Immune Response When Associated with a Leishmania (Viannia) braziliensis Antigen in a Vaccine Candidate against L. (Leishmania) infantum Infection. Vaccines, 11(2), 395. https://doi.org/10.3390/vaccines11020395






