Hesitancy to Receive the Second COVID-19 Vaccine Booster Dose among Older Adults in Hong Kong: A Random Telephone Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
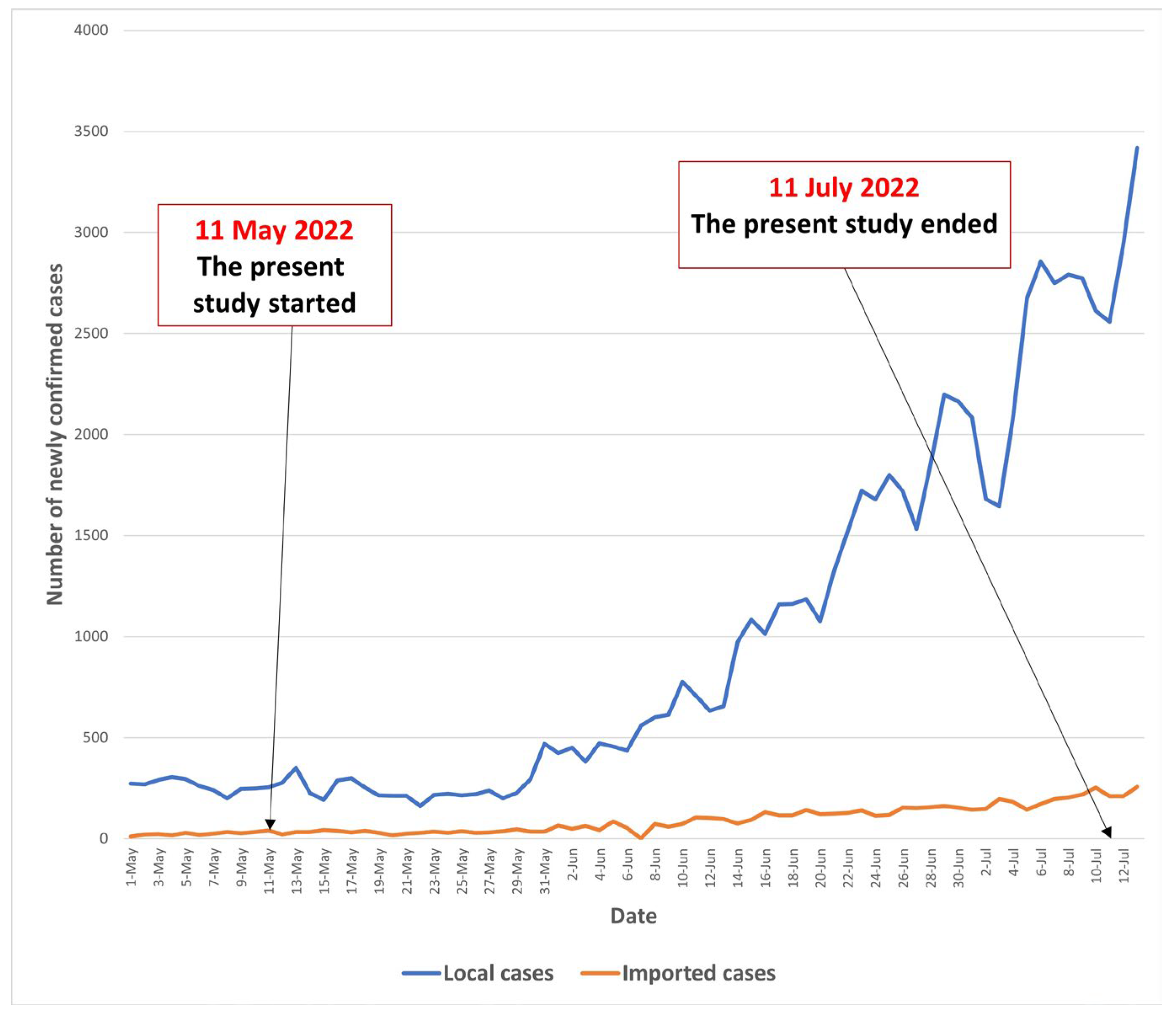
2.2. Participants and Data Collection
2.3. Measures
2.3.1. Design of the Questionnaire
2.3.2. Background Characteristics
2.3.3. COVID-19 Vaccination Uptake and Hesitancy to Receive the Second COVID-19 Vaccine Booster Dose
2.3.4. Perceptions Related to COVID-19, Second COVID-19 Vaccine Booster Dose and Vaccine Fatigue
2.3.5. Interpersonal Level Variables
2.4. Sample Size Planning
2.5. Statistical Analyses
3. Results
3.1. Background Characteristics of the Participants
3.2. Second COVID-19 Vaccine Booster Dose Uptake and Vaccine Hesitancy to Receive the Second COVID-19 Vaccine Booster Dose
3.3. Perceptions Related to COVID-19 and the Second COVID-19 Vaccine Booster Dose
3.4. Factors Associated with Hesitancy to Receive the Second COVID-19 Vaccine Booster Dose
- Half of the participants (52.4%) were hesitant to receive the second COVID-19 vaccine booster dose.
- Perceived benefits, cues to action, and perceived self-efficacy of receiving the second booster dose were associated with lower vaccine hesitancy.
- Perceived barriers and vaccine fatigue (tired of receiving repeated COVID-19 vaccination) were associated with higher vaccine hesitancy.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus COVID-19 Dashboard. Available online: https://covid19.who.int (accessed on 19 January 2023).
- Kang, S.-J.; Jung, S.I. Age-Related Morbidity and Mortality among Patients with COVID-19. Infect. Chemother. 2020, 52, 154–164. [Google Scholar] [CrossRef]
- Government of Hong Kong SAR. Latest Situation of Novel Coronavirus Infection in Hong Kong. Available online: https://www.chp.gov.hk/files/pdf/local_situation_covid19_tc.pdf (accessed on 19 December 2022).
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ-Br. Med. J. 2021, 373, n1088. [Google Scholar]
- Hyams, C.; Marlow, R.; Maseko, Z.; King, J.; Ward, L.; Fox, K.; Heath, R.; Tuner, A.; Friedrich, Z.; Morrison, L.; et al. Effec-tiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: A test-negative, case-control study. Lancet Infect Dis. 2021, 21, 1539–1548. [Google Scholar]
- Mazagatos, C.; Monge, S.; Olmedo, C.; Vega, L.; Gallego, P.; Martin-Merino, E.; Sierra, M.J.; Limia, A.; Larrauri, A. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53 2020 to 13 2021. Eurosurveillance 2021, 26, 2100452. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.; Huppert, A.; O’Brien, K.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 2022, 22, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Patalon, T.; Gazit, S.; Pitzer, V.E.; Prunas, O.; Warren, J.L.; Weinberger, D.M. Odds of Testing Positive for SARS-CoV-2 Following Receipt of 3 vs 2 Doses of the BNT162b2 mRNA Vaccine. JAMA Intern. Med. 2022, 182, 179–184. [Google Scholar]
- Jantarabenjakul, W.; Sodsai, P.; Chantasrisawad, N.; Jitsatja, A.; Ninwattana, S.; Thippamom, N.; Ruenjaiman, V.; Tan, C.W.; Pradit, R.; Sophonphan, V.; et al. Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers. Vaccines 2022, 10, 639. [Google Scholar]
- Grewal, R.; Kitchen, S.A.; Nguyen, L.; Buchan, S.A.; Wilson, S.E.; Costa, A.P.; Kwong, J.C. Effectiveness of a fourth dose of COVID-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: Test negative design study. BMJ 2022, 378, e071502. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022, 386, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Sergienko, R.; Friger, M.; Peretz, A.; Beckenstein, T.; Yaron, S.; Netzer, D.; Hammerman, A. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat. Med. 2022, 28, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Saciuk, Y.; Perez, G.; Peretz, A.; Pitzer, V.; Patalon, T. Short Term, Relative Effectiveness of Four Doses Compared to Three Dose of the BNT162b2 Vaccine in Israel. BMJ 2022, 377, e071113. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Levy, M.E.; Gaglani, M.; Irving, S.A.; Stockwell, M.; Dascomb, K.; DeSilva, M.B.; Reese, S.E.; Liao, I.C.; Ong, Y.C.; et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA Vaccine Doses among Immunocompetent Adults during Periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 Sublineages Predominated—VISION Network, 10 States, December 2021–June 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 931–939. [Google Scholar]
- Magen, O.; Waxman, J.G.; Makov-Assif, M.; Vered, R.; Dicker, D.; Hernán, M.A.; Lipsitch, M.; Reis, B.Y.; Balicer, R.D.; Dagan, N. Fourth Dose of BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2022, 386, 1603–1614. [Google Scholar] [CrossRef]
- Yechezkel, M.; Mofaz, M.; Painsky, A.; Patalon, T.; Gazit, S.; Shmueli, E.; Yamin, D. Safety of the fourth COVID-19 BNT162b2 mRNA (second booster) vaccine: A prospective and retrospective cohort study. Lancet Respir. Med. 2022, 11, 139–150. [Google Scholar] [CrossRef]
- Hause, A.M.; Baggs, J.; Marquez, P.; Abara, W.E.; Baumblatt, J.; Blanc, P.G.; Su, J.R.; Hugueley, B.; Parker, C.; Myers, T.R.; et al. Safety Monitoring of COVID-19 mRNA Vaccine Second Booster Doses among Adults Aged ≥ 50 Years—United States, March 29, 2022–July 10, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 971–976. [Google Scholar]
- World Health Organisation. Good Practice Statement on the Use of Second Booster Doses for COVID-19 Vaccines. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-good-practice-statement-second-booster (accessed on 19 December 2022).
- Centers for Disease Control and Prevention. CDC Strengthens Recommendations and Expands Eligibility for COVID-19 Booster Shots. Available online: https://www.cdc.gov/media/releases/2022/s0519-covid-booster-acip.html (accessed on 19 December 2022).
- Department of Health and Aged Care. Clinical Recommendations for COVID-19 Vaccines. Available online: https://www.health.gov.au/our-work/covid-19-vaccines/advice-for-providers/clinical-guidance/clinical-recommendations (accessed on 19 December 2022).
- European Medicines Agency. ECDC and EMA Update Recommendations on Additional Booster Doses of mRNA COVID-19 Vaccines. Available online: https://www.ema.europa.eu/en/news/ecdc-ema-update-recommendations-additional-booster-doses-mrna-covid-19-vaccines (accessed on 19 December 2022).
- Public Health Agencies of Canada. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): Initial Guidance on a Second Booster Dose of COVID-19 Vaccines in Canada. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/naci-guidance-second-booster-dose-covid-19-vaccines.pdf (accessed on 19 December 2022).
- Singapore Ministry of Health. FAQs—Booster Doses. Available online: https://www.moh.gov.sg/covid-19/vaccination/faqs-on-getting-vaccinated#secondbooster (accessed on 19 December 2022).
- The Government of Hong Kong SAR. Fourth Dose COVID-19 Vaccination Arrangements for Persons Aged 60 or Above. Available online: https://www.info.gov.hk/gia/general/202204/08/P2022040800458.htm (accessed on 19 December 2022).
- The Government of Hong Kong SAR. COVID-19 Vaccination Arrangements for Children Aged Six Months or Above and for Persons Aged from 50 to 59 Receiving Fourth Dose. Available online: https://www.info.gov.hk/gia/general/202208/02/P2022080200699.htm (accessed on 19 December 2022).
- Miao, Y.D.; Li, Y.; Zhang, W.L.; Wu, J.; Gu, J.Q.; Wang, M.Y.; Wei, W.; Ye, B.Z.; Miao, C.Y.; Tarimo, C.S.; et al. The Psychological Experience of COVID-19 Vaccination and Its Impact on the Willingness to Receive Booster Vaccines among the Chinese Population: Evidence from a National Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 5464. [Google Scholar]
- Qin, C.; Yan, W.; Tao, L.; Liu, M.; Liu, J. The Association between Risk Perception and Hesitancy toward the Booster Dose of COVID-19 Vaccine among People Aged 60 Years and Older in China. Vaccines 2022, 10, 1112. [Google Scholar] [CrossRef]
- Lai, X.; Zhu, H.; Wang, J.; Huang, Y.; Jing, R.; Lyu, Y.; Zhang, H.; Feng, H.; Guo, J.; Fang, H. Public Perceptions and Acceptance of COVID-19 Booster Vaccination in China: A Cross-Sectional Study. Vaccines 2021, 9, 1461. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.Y.; Wang, R.T.; Tao, L.Y.; Liu, M.; Liu, J. Acceptance of a Third Dose of COVID-19 Vaccine and Associated Factors in China Based on Health Belief Model: A National Cross-Sectional Study. Vaccines 2022, 10, 89. [Google Scholar] [PubMed]
- Galanis, P.; Vraka, I.; Katsiroumpa, A.; Siskou, O.; Konstantakopoulou, O.; Katsoulas, T.; Mariolis-Sapsakos, T.; Kaitelidou, D. Predictors of Willingness of the General Public to Receive a Second COVID-19 Booster Dose or a New COVID-19 Vaccine: A Cross-Sectional Study in Greece. Vaccines 2022, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P.; Vraka, I.; Katsiroumpa, A.; Siskou, O.; Konstantakopoulou, O.; Katsoulas, T.; Mariolis-Sapsakos, T.; Kaitelidou, D. Predictors of second COVID-19 booster dose or new COVID-19 vaccine hesitancy among nurses: A cross-sectional study. J. Clin. Nurs. 2022. [Google Scholar] [CrossRef]
- Su, Z.H.; Cheshmehzangi, A.L.; McDonnell, D.; da Veiga, C.P.; Xiang, Y.T. Mind the “Vaccine Fatigue”. Front. Immunol. 2022, 13, 904971. [Google Scholar] [CrossRef]
- Abbas, S.; Abbas, B.; Amir, S.; Wajahat, M. Evaluation of adverse effects with COVID-19 vaccination in Pakistan. Pak. J. Med. Sci. 2021, 37, 1959–1964. [Google Scholar] [CrossRef]
- Zhang, K.C.; Fang, Y.; Cao, H.; Chen, H.; Hu, T.; Chen, Y.; Zhou, X.; Wang, Z. Behavioral Intention to Receive a COVID-19 Vaccination among Chinese Factory Workers: Cross-sectional Online Survey. J. Med. Internet Res. 2021, 23, e24673. [Google Scholar] [CrossRef]
- Singh, A.; Lai, A.H.Y.; Wang, J.; Asim, S.; Chan, P.S.-F.; Wang, Z.; Yeoh, E.K. Multilevel Determinants of COVID-19 Vaccine Uptake among South Asian Ethnic Minorities in Hong Kong: Cross-sectional Web-Based Survey. JMIR Public Health Surveill. 2021, 7, e31707. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Y.; Yu, F.-Y.; Chan, P.S.-F.; Chen, S.; Sun, F. Facilitators and Barriers to Take up a COVID-19 Vaccine Booster Dose among Community-Dwelling Older Adults in Hong Kong: A Population-Based Random Telephone Survey. Vaccines 2022, 10, 966. [Google Scholar] [CrossRef]
- Wang, Z.X.; Fang, Y.; Dong, W.; Lau, M.; Mo, P.K.H. Illness representations on pneumonia and pneumococcal vaccination uptake among community-living Chinese people with high-risk conditions aged ≥65 years—A population-based study. Hum. Vaccines Immunother. 2021, 17, 1455–1462. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Y.; Yu, F.-Y.; Chan, P.S.-F.; Chen, S. Governmental Incentives, Satisfaction with Health Promotional Materials, and COVID-19 Vaccination Uptake among Community-Dwelling Older Adults in Hong Kong: A Random Telephone Survey. Vaccines 2022, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Fang, Y.; Ip, M.; Lau, M.; Lau, J.T.F. Facilitators and barriers to completing recommended doses of pneu-mococcal vaccination among community-living individuals aged ≥65 years in Hong Kong—A population-based study. Hum. Vaccines Immunother. 2021, 17, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Cheung, J.K.; Wu, P.; Ni, M.Y.; Cowling, B.J.; Liao, Q. Temporal changes in factors associated with COVID-19 vaccine hesitancy and uptake among adults in Hong Kong: Serial cross-sectional surveys. Lancet Reg. Health 2022, 23, 100441. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, S.; Zhang, K.; Chen, S.; Chan, P.S.-F.; Fang, Y.; Cao, H.; Chen, H.; Hu, T.; Chen, Y.; et al. Changes in Parents’ COVID-19 Vaccine Hesitancy for Children Aged 3–17 Years before and after the Rollout of the National Childhood COVID-19 Vaccination Program in China: Repeated Cross-Sectional Surveys. Vaccines 2022, 10, 1478. [Google Scholar] [CrossRef]
- Pan, Y.; Xin, M.; Zhang, C.; Dong, W.; Fang, Y.; Wu, W.; Li, M.; Pang, J.; Zheng, Z.; Wang, Z.; et al. Associations of Mental Health and Personal Preventive Measure Compliance with Exposure to COVID-19 Information during Work Resumption Following the COVID-19 Outbreak in China: Cross-Sectional Survey Study. J. Med. Internet Res. 2020, 22, e22596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, Y.; Chan, P.S.-F.; Yu, F.Y.; Sun, F. The Changes in Levels and Barriers of Physical Activity Among Community-Dwelling Older Adults during and after the Fifth Wave of COVID-19 Outbreak in Hong Kong: Repeated Random Telephone Surveys. JMIR Aging 2023, 6, e42223. [Google Scholar] [CrossRef] [PubMed]
- Hong Kong SAR Government. Elderly Employment Status. Available online: https://www.hkeconomy.gov.hk/tc/pdf/el/el-2019-10c.pdf (accessed on 21 January 2023).
- Society for Community Organization. Various Protections for Working Elders and Retirement Intentions Research. Available online: https://soco.org.hk/wp-content/uploads/2022/05/Study-on-the-Protection-and-Retirement-Intentions-of-the-Working-Grassroots-Elderly-1-MAY-2022.pdf (accessed on 21 January 2023).
- Hong Kong Census and Statistics Department. Population By-Census: Thematic Report: Older Persons. Available online: https://www.statistics.gov.hk/pub/B11201052016XXXXB0100.pdf (accessed on 21 January 2023).
| Characteristics | N | % |
|---|---|---|
| Sociodemographic characteristics | ||
| Age, years | ||
| 65–69 | 182 | 49.2 |
| 70–74 | 125 | 33.8 |
| 75 or above | 63 | 17.0 |
| Gender | ||
| Male | 145 | 39.2 |
| Female | 225 | 60.8 |
| Relationship status | ||
| Currently single | 94 | 25.4 |
| Married or cohabited with a partner | 276 | 74.6 |
| Education level | ||
| Primary or below | 157 | 42.4 |
| Secondary | 173 | 46.8 |
| Tertiary or above | 40 | 10.8 |
| Current employment status | ||
| Unemployed/retired/housewife | 319 | 86.2 |
| Full-time/part-time | 51 | 13.8 |
| Monthly household income, HK$ (US$) | ||
| <20,000 (2580) | 273 | 73.8 |
| 20,000 (2580) or above | 49 | 13.2 |
| Refuse to disclose | 48 | 13.0 |
| Receiving Comprehensive Social Security Assistance (CSSA) | ||
| No | 197 | 53.2 |
| Yes | 173 | 46.8 |
| Living alone | ||
| No | 304 | 82.2 |
| Yes | 66 | 17.8 |
| Health conditions | ||
| Presence of chronic conditions, yes | ||
| Hypertension | 173 | 46.8 |
| Chronic cardiovascular diseases | 40 | 10.8 |
| Chronic lung diseases | 6 | 1.6 |
| Chronic liver diseases | 8 | 2.2 |
| Chronic kidney diseases | 2 | 0.5 |
| Diabetes Mellitus | 70 | 18.9 |
| Any of the above | 223 | 60.3 |
| History of COVID-19 infection | ||
| No | 276 | 74.6 |
| Yes | 94 | 25.4 |
| History of seasonal influenza vaccination | ||
| No | 125 | 33.8 |
| Yes | 245 | 66.2 |
| History of pneumococcal vaccination | ||
| No | 264 | 71.4 |
| Yes | 106 | 28.6 |
| Characteristics | N | % |
|---|---|---|
| COVID-19 vaccination uptake | ||
| Number of doses of COVID-19 vaccination | ||
| 0 | 16 | 4.3 |
| 1 | 13 | 3.5 |
| 2 | 123 | 33.2 |
| 3 | 205 | 55.4 |
| 4 | 13 | 3.5 |
| Types of second COVID-19 booster dose (among 13 participants who had received the second booster dose) | ||
| CoronaVac | 5 | 38.5 |
| Comirnaty | 8 | 61.5 |
| Side-effects of the second COVID-19 vaccine booster dose (among 13 participants who had received the second booster dose) | ||
| Not at all | 6 | 46.2 |
| Very mild | 3 | 23.1 |
| Mild | 3 | 23.1 |
| Moderate | 0 | 0.0 |
| Severe | 0 | 0.0 |
| Very severe | 1 | 7.7 |
| Likelihood to receive the second COVID-19 booster dose in the future 6 months (among 357 participants who had not received the second booster dose) | ||
| Very unlikely/unlikely/neutral | 187 | 52.4 |
| Likely/very likely | 170 | 47.6 |
| Perceptions related to COVID-19 and the second booster dose | ||
| Perceived susceptibility to COVID-19, high/very high | ||
| Chance of COVID-19 infection | 14 | 3.8 |
| Chance of having another wave of COVID-19 outbreak in Hong Kong | 89 | 24.0 |
| Perceived Susceptibility Scale 1 Mean (SD) | 5.3 | (1.3) |
| Median (IQR) | 5.0 | (4, 6) |
| Perceived severity of COVID-19, high | ||
| Chance of having severe illness caused by COVID-19 | 78 | 21.1 |
| Chance of having financial difficulties caused by COVID-19 | 39 | 10.5 |
| Chance of transmitting COVID-19 to family members | 122 | 33.0 |
| Perceived Severity Scale 2 Mean (SD) | 5.3 | (1.7) |
| Median (IQR) | 5.0 | (4, 6) |
| Perceived benefit of the second COVID-19 vaccine booster dose, agree | ||
| Receiving a second booster dose can maintain your antibody level and strengthen the protection against COVID-19 | 219 | 59.2 |
| A second booster dose is highly effective in protecting from the Omicron variant | 177 | 47.8 |
| A second booster dose is highly effective in preventing severe consequences of COVID-19 | 273 | 73.8 |
| Perceived Benefit Scale 3 Mean (SD) | 7.5 | (1.6) |
| Median (IQR) | 8.0 | (6, 9) |
| Perceived barrier to receive the second COVID-19 vaccine booster dose, agree | ||
| The protection offered by the second booster dose is weaker among people with older age | 61 | 16.5 |
| The level of side effects of the second booster dose is more severe among people with older age | 63 | 17.0 |
| The duration of protection offered by the second booster dose is shorter among people with older age | 74 | 20.0 |
| Presence of chronic diseases would decrease the protection of the second booster dose | 126 | 34.1 |
| Perceived Barrier Scale 4 Mean (SD) | 7.3 | (2.4) |
| Median (IQR) | 8.0 | (5, 9) |
| Cue to action related to the second COVID-19 vaccine booster dose, agree | ||
| Your family doctors would suggest you to receive the second booster dose | 100 | 27.0 |
| Your family members would suggest you to receive the second booster dose | 162 | 43.8 |
| Cue to Action Scale 5 Mean (SD) | 4.6 | (0.9) |
| Median (IQR) | 4.0 | (4, 5) |
| Perceived self-efficacy to take up the second COVID-19 vaccine booster dose, agree | ||
| You are confident to receive the second booster dose | 321 | 86.8 |
| Item score, mean (SD) | 2.8 | 0.5 |
| Median (IQR) | 3.0 | (3, 3) |
| Tired of receiving repeated COVID-19 vaccination (vaccine fatigue), agree | 201 | 54.3 |
| Item score, mean (SD) | 2.1 | (1.0) |
| Median (IQR) | 3.0 | (1, 3) |
| Frequency of exposure to the following contents on TV, radio, newspaper and Internet in the past month | ||
| People infected with COVID-19 after receiving three doses of COVID-19 vaccines, sometimes/always | 234 | 63.3 |
| Item score, mean (SD) | 2.7 | (1.0) |
| Median (IQR) | 3.0 | (2, 3) |
| People recovered from COVID-19 without seeking medical consultation, sometimes/always | 179 | 62.2 |
| Item score, mean (SD) | 2.7 | (1.0) |
| Median (IQR) | 3.0 | (2, 3) |
| Thoughtful consideration about the veracity of COVID-19-specific information, sometimes/always | 198 | 53.5 |
| Item score, mean (SD) | 2.5 | (1.1) |
| Median (IQR) | 3.0 | (2, 4) |
| Characteristics | OR (95%CI) | p Values |
|---|---|---|
| Sociodemographic characteristics | ||
| Age, years | ||
| 65–69 | 1.0 | |
| 70–74 | 0.93 (0.59, 1.47) | 0.76 |
| 75 or above | 1.15 (0.65, 2.04) | 0.64 |
| Gender | ||
| Male | 1.0 | |
| Female | 1.92 (1.26, 2.94) | 0.002 |
| Relationship status | ||
| Currently single | 1.0 | |
| Married or cohabited with a partner | 0.82 (0.51, 1.31) | 0.41 |
| Education level | ||
| Primary or below | 1.0 | |
| Secondary | 0.71 (0.46, 1.09) | 0.12 |
| Tertiary or above | 0.58 (0.29, 1.17) | 0.13 |
| Current employment status | ||
| Unemployed/retired/housewife | 1.0 | |
| Full-time/part-time | 0.65 (0.35, 1.18) | 0.15 |
| Monthly household income, HK$ (US$) | ||
| <20,000 (2580) | 1.0 | |
| 20,000 (2580) or above | 0.55 (0.30, 1.03) | 0.06 |
| Refuse to disclose | 0.67 (0.36, 1.26) | 0.21 |
| Receiving Comprehensive Social Security Assistance (CSSA) | ||
| No | 1.0 | |
| Yes | 0.61 (0.28, 1.34) | 0.22 |
| Living alone | ||
| No | 1.0 | |
| Yes | 1.64 (0.96, 2.83) | 0.07 |
| Health conditions | ||
| Presence of any chronic conditions | ||
| No | 1.0 | |
| Yes | 0.89 (0.58, 1.34) | 0.57 |
| History of COVID-19 infection | ||
| No | 1.0 | |
| Yes | 1.37 (0.86, 2.19) | 0.19 |
| History of seasonal influenza vaccination | ||
| No | 1.0 | |
| Yes | 0.68 (0.44, 1.06) | 0.09 |
| History of pneumococcal vaccination | ||
| No | 1.0 | |
| Yes | 0.79 (0.50, 1.23) | 0.29 |
| OR (95%CI) | p Values | AOR (95%CI) | p Values | |
|---|---|---|---|---|
| Perceptions related to COVID-19 and the second booster dose | ||||
| Perceived Susceptibility Scale | 0.97 (0.83, 1.14) | 0.74 | 0.94 (0.80, 1.11) | 0.49 |
| Perceived Severity Scale | 1.00 (0.89, 1.12) | 0.95 | 0.99 (0.88, 1.11) | 0.83 |
| Perceived Benefit Scale | 0.52 (0.44, 0.61) | <0.001 | 0.50 (0.42, 0.60) | <0.001 |
| Perceived Barrier Scale | 1.21 (1.11, 1.32) | <0.001 | 1.23 (1.12, 1.34) | <0.001 |
| Cue to Action Scale | 0.41 (0.31, 0.53) | <0.001 | 0.39 (0.30, 0.52) | <0.001 |
| Perceived self-efficacy | 0.37 (0.21, 0.64) | <0.001 | 0.37 (0.21, 0.66) | 0.001 |
| Tired of receiving repeated COVID-19 vaccination (vaccine fatigue) | 1.97 (1.58, 2.46) | <0.001 | 1.90 (1.52, 2.38) | <0.001 |
| Frequency of exposure to the following contents on TV, radio, newspaper and Internet in the past month | ||||
| People infected with COVID-19 after receiving three doses of COVID-19 vaccines | 1.05 (0.85, 1.28) | 0.67 | 1.02 (0.83, 1.53) | 0.83 |
| People recovered from COVID-19 without seeking medical consultation | 0.95 (0.78, 1.17) | 0.95 | 0.92 (0.74, 1.13) | 0.41 |
| Thoughtful consideration about the veracity of COVID-19-specific information | 0.94 (0.78, 1.13) | 0.51 | 0.91 (0.76, 1.10) | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, P.S.-f.; Lee, M.L.-t.; Fang, Y.; Yu, F.-y.; Ye, D.; Chen, S.; Kawuki, J.; Liang, X.; Wang, Z. Hesitancy to Receive the Second COVID-19 Vaccine Booster Dose among Older Adults in Hong Kong: A Random Telephone Survey. Vaccines 2023, 11, 392. https://doi.org/10.3390/vaccines11020392
Chan PS-f, Lee ML-t, Fang Y, Yu F-y, Ye D, Chen S, Kawuki J, Liang X, Wang Z. Hesitancy to Receive the Second COVID-19 Vaccine Booster Dose among Older Adults in Hong Kong: A Random Telephone Survey. Vaccines. 2023; 11(2):392. https://doi.org/10.3390/vaccines11020392
Chicago/Turabian StyleChan, Paul Shing-fong, Marco Lok-tin Lee, Yuan Fang, Fuk-yuen Yu, Danhua Ye, Siyu Chen, Joseph Kawuki, Xue Liang, and Zixin Wang. 2023. "Hesitancy to Receive the Second COVID-19 Vaccine Booster Dose among Older Adults in Hong Kong: A Random Telephone Survey" Vaccines 11, no. 2: 392. https://doi.org/10.3390/vaccines11020392
APA StyleChan, P. S.-f., Lee, M. L.-t., Fang, Y., Yu, F.-y., Ye, D., Chen, S., Kawuki, J., Liang, X., & Wang, Z. (2023). Hesitancy to Receive the Second COVID-19 Vaccine Booster Dose among Older Adults in Hong Kong: A Random Telephone Survey. Vaccines, 11(2), 392. https://doi.org/10.3390/vaccines11020392









