Long-Term Immunological Memory of SARS-CoV-2 Is Present in Patients with Primary Antibody Deficiencies for up to a Year after Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Antibody Response to SARS-CoV-2
2.3. T-Cell Response to SARS-CoV-2
2.4. Lymphocyte Isolation and Flow Cytometry
2.5. Clinical and Immunologic Phenotyping of CVID Patients
2.6. Data Analysis
3. Results
3.1. Subjects
3.2. Vaccination Status and Previous SARS-CoV-2 Infection
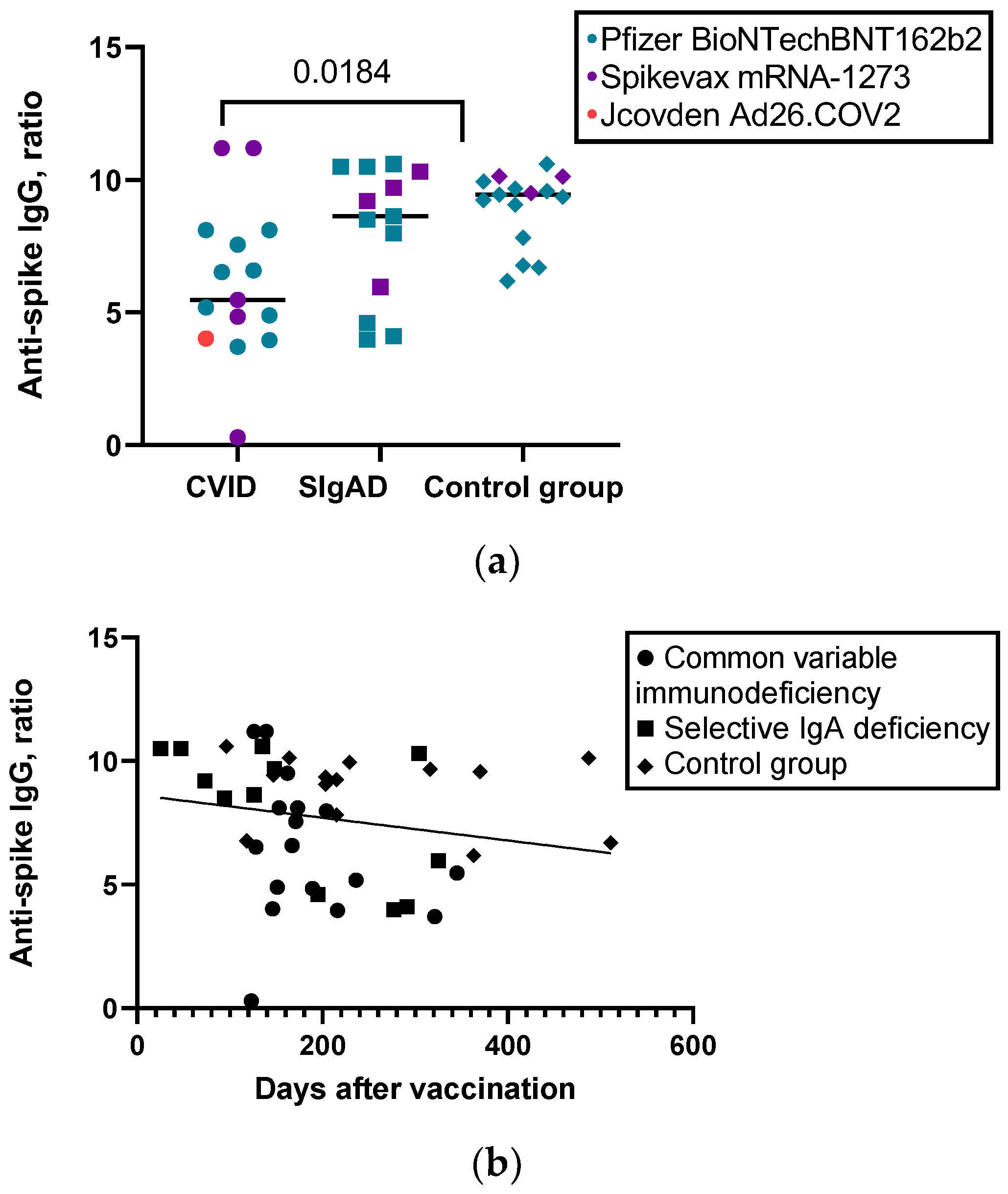
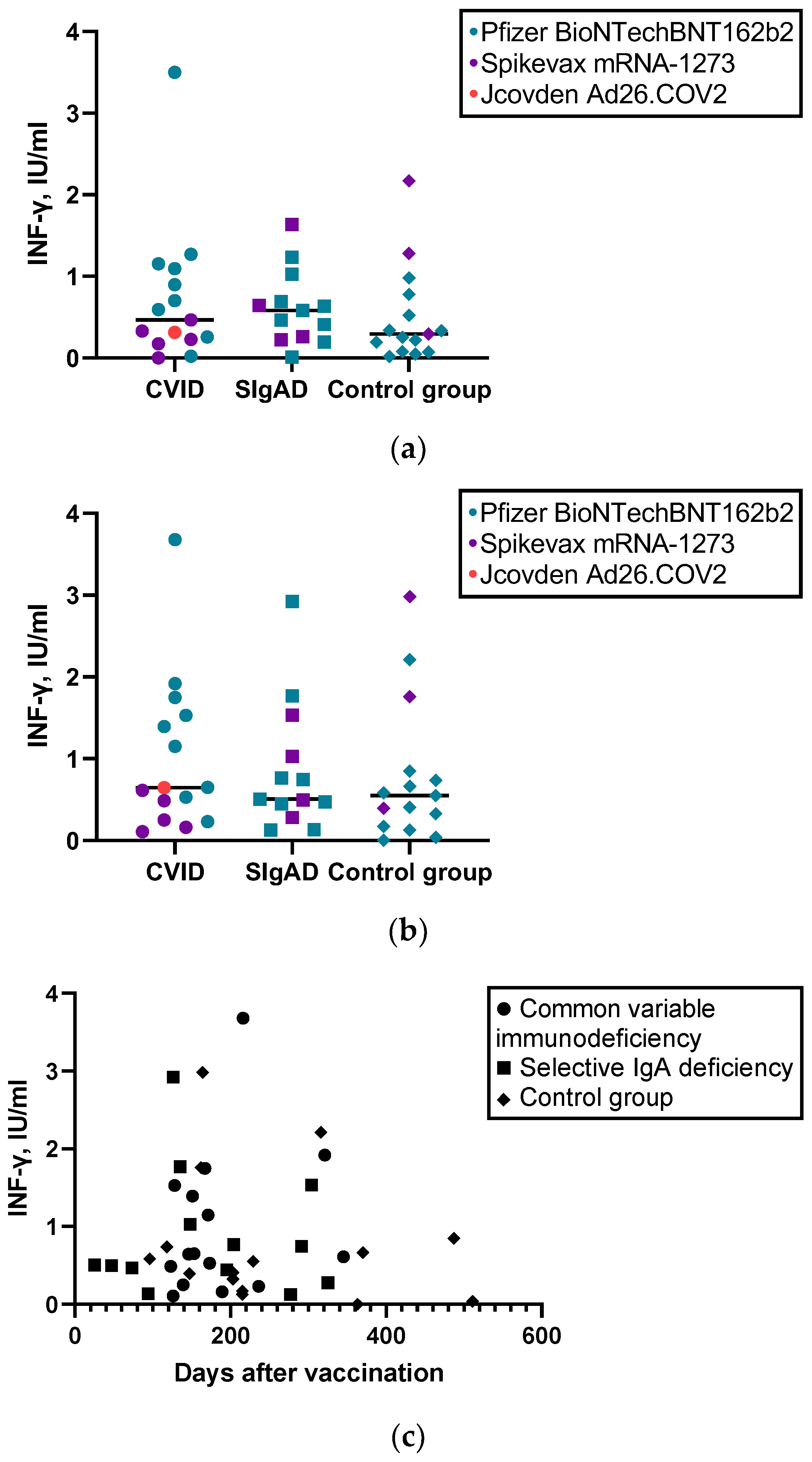
3.3. Humoral and Cellular Response to SARS-CoV-2 Vaccine
3.4. Immunological Memory to SARS-CoV-2 and Demographic/Clinical/Immunologic Phenotyping Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gathmann, B.; Grimbacher, B.; Beauté, J.; Dudoit, Y.; Mahlaoui, N.; Fischer, A.; Knerr, V.; Kindle, G.; Micol, R.; Benslama, L.; et al. The European Internet-Based Patient and Research Database for Primary Immunodeficiencies: Results 2006–2008. In Proceedings of the Clinical and Experimental Immunology; Oxford University Press: Oxford, UK, 2009; Volume 157. [Google Scholar]
- Yazdani, R.; Azizi, G.; Abolhassani, H.; Aghamohammadi, A. Selective IgA Deficiency: Epidemiology, Pathogenesis, Clinical Phenotype, Diagnosis, Prognosis and Management. Scand. J. Immunol. 2017, 85, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.S.J.; Bosco, J.J.; Aui, P.M.; Stirling, R.G.; Cameron, P.U.; Chatelier, J.; Hore-Lacy, F.; O’Hehir, R.E.; van Zelm, M.C. Predominantly Antibody-Deficient Patients With Non-Infectious Complications Have Reduced Naive B, Treg, Th17, and Tfh17 Cells. Front. Immunol. 2019, 10, 2593. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Stratton, C.W.; Tang, Y.-W. Outbreak of Pneumonia of Unknown Etiology in Wuhan, China: The Mystery and the Miracle. J. Med. Virol. 2020, 92, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Liu, M.; Shi, S.; Tian, J. Impacts of Immunosuppression and Immunodeficiency on COVID-19: A Systematic Review and Meta-Analysis. J. Infect. 2020, 81, e93. [Google Scholar] [CrossRef]
- Van der Made, C.I.; Netea, M.G.; van der Veerdonk, F.L.; Hoischen, A. Clinical Implications of Host Genetic Variation and Susceptibility to Severe or Critical COVID-19. Genome Med. 2022, 14, 96. [Google Scholar] [CrossRef]
- Liu, B.M.; Hill, H.R. Role of Host Immune and Inflammatory Responses in COVID-19 Cases with Underlying Primary Immunodeficiency: A Review. J. Interferon Cytokine Res. 2020, 40, 549–554. Available online: https://home.liebertpub.com/jir (accessed on 26 October 2022). [CrossRef]
- Milito, C.; Lougaris, V.; Giardino, G.; Punziano, A.; Vultaggio, A.; Carrabba, M.; Cinetto, F.; Scarpa, R.; Delle Piane, R.M.; Baselli, L.; et al. Clinical Outcome, Incidence, and SARS-CoV-2 Infection-Fatality Rates in Italian Patients with Inborn Errors of Immunity. J. Allergy Clin. Immunol. Pract. 2021, 9, 2904–2906.e2. [Google Scholar] [CrossRef]
- Bucciol, G.; Tangye, S.G.; Meyts, I. Coronavirus Disease 2019 in Patients with Inborn Errors of Immunity: Lessons Learned. Curr. Opin. Pediatr. 2021, 33, 648–656. [Google Scholar] [CrossRef]
- Katzenstein, T.L.; Rasmussen, L.D.; Drabe, C.H.; Larsen, C.S.; Hansen, A.-B.E.; Stærkind, M.; Knudsen, L.S.; Hansen, C.H.; Obel, N. Outcome of SARS-CoV-2 Infection among Patients with Common Variable Immunodeficiency and a Matched Control Group: A Danish Nationwide Cohort Study. Front. Immunol. 2022, 13, 994253. [Google Scholar] [CrossRef]
- Tangye, S.G.; Bucciol, G.; Meyts, I. Mechanisms Underlying Host Defense and Disease Pathology in Response to Severe Acute Respiratory Syndrome (SARS)-CoV2 Infection: Insights from Inborn Errors of Immunity. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 515–524. [Google Scholar] [CrossRef]
- Shields, A.M.; Burns, S.O.; Savic, S.; Richter, A.G.; Anantharachagan, A.; Arumugakani, G.; Baker, K.; Bahal, S.; Bermingham, W.; Bhole, M.; et al. COVID-19 in Patients with Primary and Secondary Immunodeficiency: The United Kingdom Experience. J. Allergy Clin. Immunol. 2021, 147, 870–875.e1. [Google Scholar] [CrossRef]
- Goudouris, E.S.; Pinto-Mariz, F.; Mendonça, L.O.; Aranda, C.S.; Guimarães, R.R.; Kokron, C.; Barros, M.T.; Anísio, F.; Alonso, M.L.O.; Marcelino, F.; et al. Outcome of SARS-CoV-2 Infection in 121 Patients with Inborn Errors of Immunity: A Cross-Sectional Study. J. Clin. Immunol. 2021, 41, 1479–1489. [Google Scholar] [CrossRef]
- Kołtan, S.; Ziętkiewicz, M.; Grześk, E.; Becht, R.; Berdej-Szczot, E.; Cienkusz, M.; Ewertowska, M.; Heropolitańska-Pliszka, E.; Krysiak, N.; Lewandowicz-Uszyńska, A.; et al. COVID-19 in Unvaccinated Patients with Inborn Errors of Immunity—Polish Experience. Front. Immunol. 2022, 13, 5656. [Google Scholar] [CrossRef]
- Çölkesen, F.; Kandemir, B.; Arslan, Ş.; Çölkesen, F.; Yıldız, E.; Korkmaz, C.; Vatansev, H.; Evcen, R.; Aykan, F.S.; Kılınç, M.; et al. Relationship between Selective IgA Deficiency and COVID-19 Prognosis. Jpn. J. Infect. Dis. 2022, 75, 228–233. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Yamamoto, T.; Watanabe, S. Association between Selective IgA Deficiency and COVID-19. J. Clin. Biochem. Nutr. 2020, 67, 122–125. [Google Scholar] [CrossRef]
- ESID-European Society for Immunodeficiencies. Available online: https://esid.org/COVID-19/ESID-COVID-19-Statement-March-2022 (accessed on 25 October 2022).
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of MRNA-1273 Vaccine–Induced Antibodies against SARS-CoV-2 Variants. Science 2021, 373, 1372. [Google Scholar] [CrossRef]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody Response to SARS-CoV-2 Infection in Humans: A Systematic Review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 MRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639. [Google Scholar] [CrossRef]
- Shields, A.M.; Faustini, S.E.; Hill, H.J.; Al-Taei, S.; Tanner, C.; Ashford, F.; Workman, S.; Moreira, F.; Verma, N.; Wagg, H.; et al. Increased Seroprevalence and Improved Antibody Responses Following Third Primary SARS-CoV-2 Immunisation: An Update From the COV-AD Study. Front. Immunol. 2022, 13, 2704. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.N.; Murugesan, K.; Banaei, N.; Pinsky, B.A.; Tang, M.; Hoyte, E.; Lewis, D.B.; Gernez, Y. Immunogenicity and Tolerability of COVID-19 Messenger RNA Vaccines in Primary Immunodeficiency Patients with Functional B-Cell Defects. J. Allergy Clin. Immunol. 2022, 149, 907–911.e3. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.M.; Faustini, S.E.; Hill, H.J.; Al-Taei, S.; Tanner, C.; Ashford, F.; Workman, S.; Wagg, H.; Heritage, G.; Campton, N.; et al. SARS-CoV-2 Vaccine Responses in Individuals with Antibody Deciency: Findings From The COV-AD Study upon Tyne Hospitals NHS Foundation Trust Teaching Hospitals NHS Trust. J. Clin. Immunol. 2022, 42, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Del Pino-Molina, L.; López-Granados, E.; Lecrevisse, Q.; Torres Canizales, J.; Pérez-Andrés, M.; Blanco, E.; Wentink, M.; Bonroy, C.; Nechvatalova, J.; Milota, T.; et al. Dissection of the Pre-Germinal Center B-Cell Maturation Pathway in Common Variable Immunodeficiency Based on Standardized Flow Cytometric EuroFlow Tools. Front. Immunol. 2021, 11, 603972. [Google Scholar] [CrossRef]
- Amodio, D.; Ruggiero, A.; Sgrulletti, M.; Pighi, C.; Cotugno, N.; Medri, C.; Morrocchi, E.; Colagrossi, L.; Russo, C.; Zaffina, S.; et al. Humoral and Cellular Response Following Vaccination With the BNT162b2 MRNA COVID-19 Vaccine in Patients Affected by Primary Immunodeficiencies. Front. Immunol. 2021, 12, 727850. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 Vaccine Induces Neutralizing Antibodies and Poly-Specific T Cells in Humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early Induction of Functional SARS-CoV-2-Specific T Cells Associates with Rapid Viral Clearance and Mild Disease in COVID-19 Patients. Cell Rep. 2021, 34, 108728. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive Immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Hagin, D.; Freund, T.; Navon, M.; Halperin, T.; Adir, D.; Marom, R.; Levi, I.; Benor, S.; Alcalay, Y.; Freund, N.T. Immunogenicity of Pfizer-BioNTech COVID-19 Vaccine in Patients with Inborn Errors of Immunity. J. Allergy Clin. Immunol. 2021, 148, 739. [Google Scholar] [CrossRef]
- Salinas, A.F.; Mortari, E.P.; Terreri, S.; Quintarelli, C.; Pulvirenti, F.; Di Cecca, S.; Guercio, M.; Milito, C.; Bonanni, L.; Auria, S.; et al. SARS-CoV-2 Vaccine Induced Atypical Immune Responses in Antibody Defects: Everybody Does Their Best. J. Clin. Immunol. 2021, 41, 1709–1722. [Google Scholar] [CrossRef]
- Delmonte, O.M.; Bergerson, J.R.E.; Burbelo, P.D.; Durkee-Shock, J.R.; Dobbs, K.; Bosticardo, M.; Keller, M.D.; McDermott, D.H.; Rao, V.K.; Dimitrova, D.; et al. Antibody Responses to the SARS-CoV-2 Vaccine in Individuals with Various Inborn Errors of Immunity. J. Allergy Clin. Immunol. 2021, 148, 1192. [Google Scholar] [CrossRef]
- Shin, J.J.; Par-Young, J.; Unlu, S.; McNamara, A.; Park, H.J.; Shin, M.S.; Gee, R.J.; Doyle, H.; Afinogenova, Y.; Zidan, E.; et al. Defining Clinical and Immunological Predictors of Poor Immune Responses to COVID-19 MRNA Vaccines in Patients with Primary Antibody Deficiency. J. Clin. Immunol. 2022, 42, 1137–1150. [Google Scholar] [CrossRef]
- Ameratunga, R.; Longhurst, H.; Steele, R.; Lehnert, K.; Leung, E.; Brooks, A.E.S.; Woon, S.T. Common Variable Immunodeficiency Disorders, T-Cell Responses to SARS-CoV-2 Vaccines, and the Risk of Chronic COVID-19. J. Allergy Clin. Immunol. Pract. 2021, 9, 3575. [Google Scholar] [CrossRef]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. Personal View A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Tormo, N.; Navalpotro, D.; Martínez-Serrano, M.; Moreno, M.; Grosson, F.; Tur, I.; Guna, M.R.; Soriano, P.; Tornero, A.; Gimeno, C. Commercial Interferon-Gamma Release Assay to Assess the Immune Response to First and Second Doses of MRNA Vaccine in Previously COVID-19 Infected versus Uninfected Individuals. Diagn. Microbiol. Infect. Dis. 2022, 102, 115573. [Google Scholar] [CrossRef]
- Chapel, H.; Lucas, M.; Lee, M.; Bjorkander, J.; Webster, D.; Grimbacher, B.; Fieschi, C.; Thon, V.; Abedi, M.R.; Hammarstrom, L. Common Variable Immunodeficiency Disorders: Division into Distinct Clinical Phenotypes. Blood 2008, 112, 277–286. [Google Scholar] [CrossRef]
- Ameratunga, R. Assessing Disease Severity in Common Variable Immunodeficiency Disorders (CVID) and CVID-Like Disorders. Front. Immunol. 2018, 9, 2130. [Google Scholar] [CrossRef]
- Piqueras, B.; Lavenu-Bombled, C.; Galicier, L.; Bergeron-Van Der Cruyssen, F.; Mouthon, L.; Chevret, S.; Debré, P.; Schmitt, C.; Oksenhendler, E. Common Variable Immunodeficiency Patient Classification Based on Impaired B Cell Memory Differentiation Correlates with Clinical Aspects. J. Clin. Immunol. 2003, 23, 385–400. [Google Scholar] [CrossRef]
- Warnatz, K.; Denz, A.; Dräger, R.; Braun, M.; Groth, C.; Wolff-Vorbeck, G.; Eibel, H.; Schlesier, M.; Peter, H.H. Severe Deficiency of Switched Memory B Cells (CD27(+)IgM(-)IgD(-)) in Subgroups of Patients with Common Variable Immunodeficiency: A New Approach to Classify a Heterogeneous Disease. Blood 2002, 99, 1544–1551. [Google Scholar] [CrossRef]
- Wehr, C.; Kivioja, T.; Schmitt, C.; Ferry, B.; Witte, T.; Eren, E.; Vlkova, M.; Hernandez, M.; Detkova, D.; Bos, P.R.; et al. The EUROclass Trial: Defining Subgroups in Common Variable Immunodeficiency. Blood 2008, 111, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Driessen, G.J.; Van Zelm, M.C.; Van Hagen, P.M.; Hartwig, N.G.; Trip, M.; Warris, A.; De Vries, E.; Barendregt, B.H.; Pico, I.; Hop, W.; et al. B-Cell Replication History and Somatic Hypermutation Status Identify Distinct Pathophysiologic Backgrounds in Common Variable Immunodeficiency. Blood 2011, 118, 6814–6823. [Google Scholar] [CrossRef] [PubMed]
- Prokofjeva, T.; Lucane, Z.; Kovalova, Z.; Kurjane, N. Inborn Errors of Immunity in Latvia: Analysis of Data from 1994 to 2020. J. Clin. Immunol. 2022, 42, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; Chen, P.; Söderdahl, G.; Österborg, A.; Smith, C.I.E.; Wullimann, D.; et al. Safety and Efficacy of the MRNA BNT162b2 Vaccine against SARS-CoV-2 in Five Groups of Immunocompromised Patients and Healthy Controls in a Prospective Open-Label Clinical Trial. EBioMedicine 2021, 74, 103705. [Google Scholar] [CrossRef]
- Squire, J.; Joshi, A. Seroconversion after Coronavirus Disease 2019 Vaccination in Patients with Immune Deficiency. Ann. Allergy Asthma Immunol. 2021, 127, 383. [Google Scholar] [CrossRef]
- Babaha, F.; Rezaei, N. Primary Immunodeficiency Diseases in COVID-19 Pandemic: A Predisposing or Protective Factor? Am. J. Med. Sci. 2020, 360, 740–741. [Google Scholar] [CrossRef]
- Göschl, L.; Mrak, D.; Grabmeier-Pfistershammer, K.; Stiasny, K.; Haslacher, H.; Schneider, L.; Deimel, T.; Kartnig, F.; Tobudic, S.; Aletaha, D.; et al. Reactogenicity and Immunogenicity of the Second COVID-19 Vaccination in Patients with Inborn Errors of Immunity or Mannan-Binding Lectin Deficiency. Front. Immunol. 2022, 13, 5063. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Friedmann, D.; Goldacker, S.; Peter, H.H.; Warnatz, K. Preserved Cellular Immunity Upon Influenza Vaccination in Most Patients with Common Variable Immunodeficiency. J. Allergy Clin. Immunol. Pract. 2020, 8, 2332–2340.e5. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the Elderly: The Challenge of Immune Changes with Aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef]
- Gardulf, A.; Abolhassani, H.; Gustafson, R.; Eriksson, L.E.; Hammarström, L. Predictive Markers for Humoral Influenza Vaccine Response in Patients with Common Variable Immunodeficiency. J. Allergy Clin. Immunol. 2018, 142, 1922–1931.e2. [Google Scholar] [CrossRef]
- Beck, C.R.; McKenzie, B.C.; Hashim, A.B.; Harris, R.C.; Nguyen-Van-Tam, J.S. Influenza Vaccination for Immunocompromised Patients: Systematic Review and Meta-Analysis by Etiology. J. Infect. Dis. 2012, 206, 1250–1259. [Google Scholar] [CrossRef]
| Parameters | CVID | sIgAD | Healthy Controls | p |
|---|---|---|---|---|
| Number | 17 | 15 | 15 | - |
| Sex | ||||
| Female, n (%) | 10 (58.8%) | 12 (80%) | 10 (66.6%) | 0.460 |
| Male, n (%) | 7 (41.2%) | 3 (20%) | 5 (33.4%) | |
| Age, median (IQR) | 40 (24) | 37 (21) | 37 (19) | 0.467 |
| Ethnicity, Caucasian, n | 17 (100%) | 15 (100%) | 15 (100%) | - |
| Age at diagnosis, median (IQR) | 34 (28) | 33 (45) | 0.664 | |
| Positive family history, n (%) | 1 (5.8%) | 1 (6.6%) | 0.755 | |
| Clinical characteristics | ||||
| Recurrent infections, n (%) | 17 (100%) | 13 (86.6%) | ||
| Recurrent pneumonia, n (%) | 15 (88.2%) | 1 (6.6%) | ||
| Recurrent otitis media, n (%) | 8 (47%) | 2 (13.3%) | ||
| Recurrent sinusitis, n (%) | 12 (70.5%) | 3 (20%) | ||
| Recurrent urinary tract infections, n (%) | 2 (11.7%) | 3 (20%) | ||
| Sepsis in personal medical history, n (%) | 0 (0%) | 1 (6.6%) | ||
| Bronchiectasis, n (%) | 6 (35.3%) | 0 (0%) | ||
| Conductive hearing impairment, n (%) | 3 (17.6%) | 0 (0%) | ||
| Autoimmunity, n (%) | 6 (35.3%) | 8 (53.3%) | 0.784 | |
| Splenomegaly, n (%) | 5 (29.4%) | 1 (6.7%) | 0.178 | |
| Hepatomegaly, n (%) | 3 (17.6%) | 1 (6.7%) | 0.603 | |
| Enteropathy, n (%) | 4 (23.5%) | 3 (20.0%) | 0.576 | |
| Malignancy, n (%) | 2 (11.8%) | 1 (6.7%) | 0.548 | |
| Allergy or atopy, n (%) | 10 (58.8%) | 8 (53.3%) | 0.517 | |
| CVID severity score, median | 15 points (IQR = 19) | |||
| Positive SARS-CoV-2 PCR in personal medical history, n (%) | 10 (58.8%) | 9 (60.0%) | 8 (53.8%) | 0.918 |
| Severity of COVID-19 according to WHO clinical progression scale * | ||||
| Asymptomatic (score 1), n | 1 | 0 | 0 | |
| Mild (not hospitalized 2–3), n | 6 | 8 | 8 | |
| Moderate (hospitalized 4–5), n | 2 | 1 | 0 | |
| Severe (hospitalized 6 + ), n | 1 | 0 | 0 |
| Parameter | CVID | sIgAD | Healthy Controls | p |
|---|---|---|---|---|
| Number of vaccinated individuals | 15 | 13 | 15 | |
| Vaccination | 0.711 | |||
| Pfizer BioNTechBNT162b2, n | 9 | 9 | 12 | |
| Spikevax mRNA-1273, n | 5 | 4 | 3 | |
| Jcovden Ad26.COV2, n | 1 | 0 | 0 | |
| Booster dose (3rd dose) received, n | 10 | 4 | 8 | 0.118 |
| Days after vaccination, median (IQR) | 167 (77) | 148 (300) | 215 (201) | 0.156 |
| Positive humoral response, n (%) | 14 (93.3%) | 13 (100%) | 15 (100%) | 1.000 |
| Positive T-cell response n/yes | 14 (93.3%) | 12 (92.3%) | 12 (80%) | 0.596 |
| Level of anti-S IgG, ratio, median (IQR) | 5.4720 (4.08) | 8.6260 (5.12) | 9.4350 (2.13) | 0.035 |
| CD4+ cell response (INF-y) to S1 pool-specific protein, IU/mL, median (IQR) | 0.4662 (0.86) | 0.5845 (0.62) | 0.2949 (0.70) | 0.765 |
| CD4+ and CD8+ cell response (INF-y) to S1 and S2 pool-specific proteins, IU/mL, median (IQR) | 0.6451 (1.28) | 0.5052 (0.92) | 0.5503 (0.68) | 0.966 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucane, Z.; Slisere, B.; Ozola, L.; Rots, D.; Papirte, S.; Vilne, B.; Gailite, L.; Kurjane, N. Long-Term Immunological Memory of SARS-CoV-2 Is Present in Patients with Primary Antibody Deficiencies for up to a Year after Vaccination. Vaccines 2023, 11, 354. https://doi.org/10.3390/vaccines11020354
Lucane Z, Slisere B, Ozola L, Rots D, Papirte S, Vilne B, Gailite L, Kurjane N. Long-Term Immunological Memory of SARS-CoV-2 Is Present in Patients with Primary Antibody Deficiencies for up to a Year after Vaccination. Vaccines. 2023; 11(2):354. https://doi.org/10.3390/vaccines11020354
Chicago/Turabian StyleLucane, Zane, Baiba Slisere, Lota Ozola, Dmitrijs Rots, Sindija Papirte, Baiba Vilne, Linda Gailite, and Natalja Kurjane. 2023. "Long-Term Immunological Memory of SARS-CoV-2 Is Present in Patients with Primary Antibody Deficiencies for up to a Year after Vaccination" Vaccines 11, no. 2: 354. https://doi.org/10.3390/vaccines11020354
APA StyleLucane, Z., Slisere, B., Ozola, L., Rots, D., Papirte, S., Vilne, B., Gailite, L., & Kurjane, N. (2023). Long-Term Immunological Memory of SARS-CoV-2 Is Present in Patients with Primary Antibody Deficiencies for up to a Year after Vaccination. Vaccines, 11(2), 354. https://doi.org/10.3390/vaccines11020354






