Epidemiology of Chronic Hepatitis B Infection in the Cohort of College Students with Vaccination in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Materials
2.2. Statistical Analysis
3. Results
3.1. Characteristics of Subjects
3.2. Increase in ALT, AST, and BMI for HBV Carrier Subjects
3.3. Impacts of Predictive Factors on Abnormal ALT Status with ASSLD Criteria
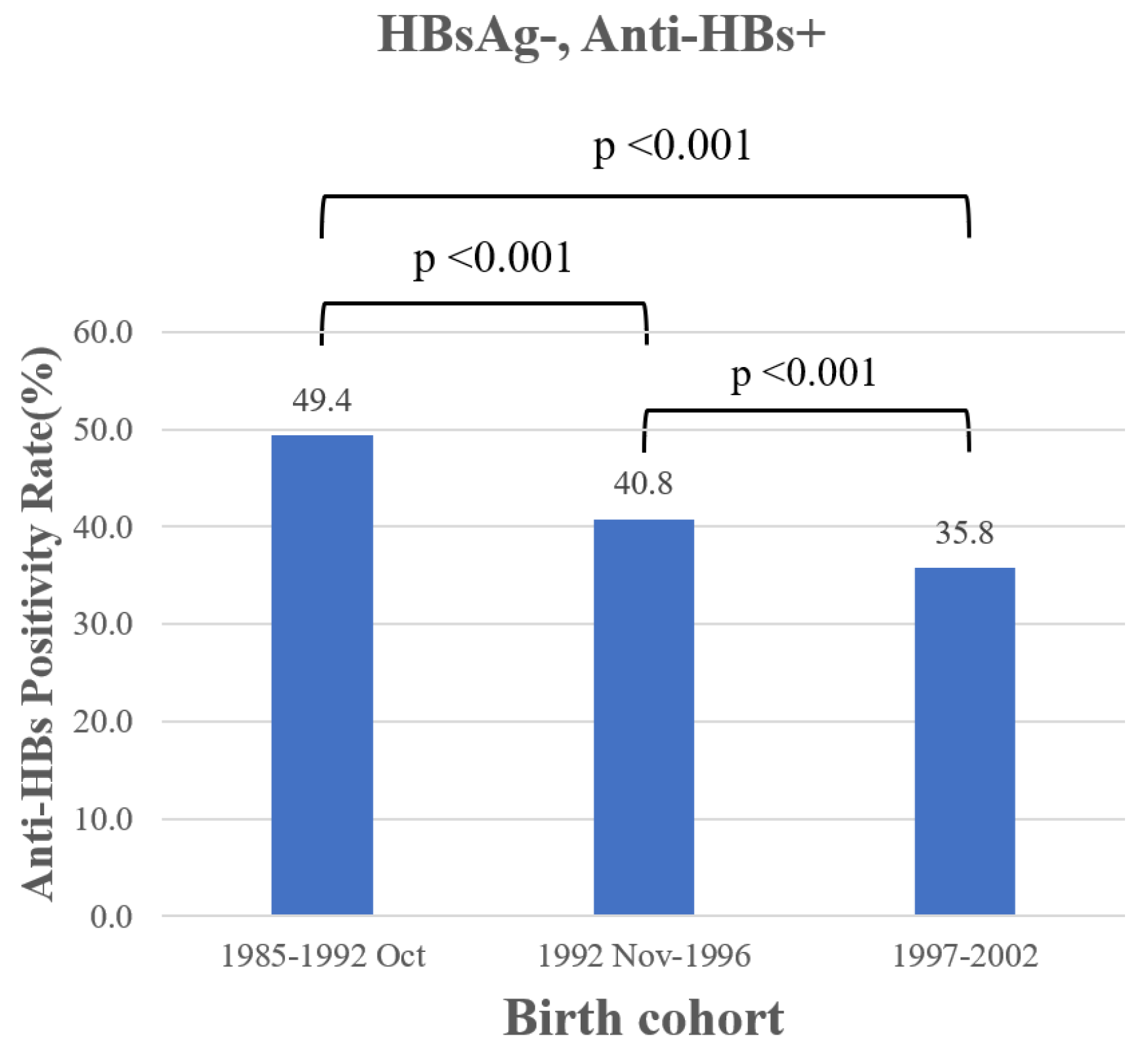
3.4. Cohort Effect in College Students
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veronese, P.; Dodi, I.; Esposito, S.; Indolfi, G. Prevention of vertical transmission of hepatitis B virus infection. World J. Gastroenterol. 2021, 27, 4182. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.-F.; Chu, C.-M. Hepatitis B virus infection. Lancet 2009, 373, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.A.; Wedemeyer, H.; Harrison, P.M. Hepatitis delta virus. Lancet 2011, 378, 73–85. [Google Scholar] [CrossRef]
- Chien, R.-N.; Kao, J.-H.; Peng, C.-Y.; Chen, C.-H.; Liu, C.-J.; Huang, Y.-H.; Hu, T.-H.; Yang, H.-I.; Lu, S.-N.; Ni, Y.-H. Taiwan consensus statement on the management of chronic hepatitis B. J. Formos. Med. Assoc. 2019, 118, 7–38. [Google Scholar] [CrossRef]
- Ni, Y.-H.; Chang, M.-H.; Huang, L.-M.; Chen, H.-L.; Hsu, H.-Y.; Chiu, T.-Y.; Tsai, K.-S.; Chen, D.-S. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann. Intern. Med. 2001, 135, 796–800. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Yeh, C.-C.; Chen, R.-Y.; Su, C.-T.; Wang, W.-C.; Bai, C.-H.; Chan, C.-F.; Su, F.H. Seroprevalence of hepatitis B virus in Taiwan 30 years after the commencement of the national vaccination program. PeerJ 2018, 6, e4297. [Google Scholar] [CrossRef]
- Ni, Y.-H.; Chang, M.-H.; Jan, C.-F.; Hsu, H.-Y.; Chen, H.-L.; Wu, J.-F.; Chen, D.-S. Continuing decrease in hepatitis B virus infection 30 years after initiation of infant vaccination program in Taiwan. Clin. Gastroenterol. Hepatol. 2016, 14, 1324–1330. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chang, M.-H.; Ni, Y.-H.; Hsu, H.-Y.; Chen, D.-S. Long-term immunogenicity and efficacy of universal hepatitis B virus vaccination in Taiwan. J. Infect. Dis. 2003, 187, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Chang, M.-H.; Chen, H.-L.; Wu, J.-F.; Chang, C.-H.; Hsu, H.-Y.; Ni, Y.-H. Universal infant hepatitis B virus (HBV) vaccination for 35 years: Moving toward the eradication of HBV. J. Infect. Dis. 2022, 225, 431–435. [Google Scholar] [CrossRef]
- Lu, F.-T.; Ni, Y.-H. Elimination of Mother-to-Infant Transmission of Hepatitis B Virus: 35 Years of Experience. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 311. [Google Scholar] [CrossRef] [PubMed]
- WHO. Hepatitis B vaccines: WHO position paper, July 2017–Recommendations. Vaccine 2019, 37, 223–225. [Google Scholar] [CrossRef]
- Su, F.-H.; Chu, F.-Y.; Bai, C.-H.; Lin, Y.-S.; Hsueh, Y.-M.; Sung, F.-C.; Yeh, C.-C. Efficacy of hepatitis B vaccine boosters among neonatally vaccinated university freshmen in Taiwan. J. Hepatol. 2013, 58, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Jan, C.-F.; Liu, T.-H.; Ho, C.-H.; Chien, Y.-C.; Chang, C.-J.; Guo, F.-R.; Huang, K.-C. Doses of hepatitis B revaccination needed for the seronegative youths to be seropositive to antibody against hepatitis B surface antigen. Fam. Pract. 2020, 37, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-Y.; Ni, Y.-H.; Chiang, B.-L.; Chen, P.-J.; Chang, M.-H.; Chang, L.-Y.; Su, I.-J.; Kuo, H.-S.; Huang, L.-M.; Chen, D.-S. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J. Infect. Dis. 2008, 197, 1419–1426. [Google Scholar] [CrossRef]
- Bialek, S.R.; Bower, W.A.; Novak, R.; Helgenberger, L.; Auerbach, S.B.; Williams, I.T.; Bell, B.P. Persistence of protection against hepatitis B virus infection among adolescents vaccinated with recombinant hepatitis B vaccine beginning at birth: A 15-year follow-up study. Pediatr. Infect. Dis. J. 2008, 27, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-Y.; Lee, W.-C. Age-period-cohort analysis with a constant-relative-variation constraint for an apportionment of period and cohort slopes. PLoS ONE 2019, 14, e0226678. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Bzowej, N.H.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. AASLD guidelines for treatment of chronic hepatitis B. Hepatol. (Baltim. Md.) 2016, 63, 261. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown Jr, R.S.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Kasarala, G.; Tillmann, H.L. Standard liver tests. Clin. Liver Dis. 2016, 8, 13. [Google Scholar] [CrossRef]
- Lin, C.L.; Kao, J.H. the prevention of hepatitis B-related hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2018, 48, 5–14. [Google Scholar] [CrossRef]
- Di Giampaolo, L.; Costantini, E.; Di Nicola, M.; Porreca, A.; D’Amore, G.; Coppeta, L.; Mangifesta, R. Titer of anti-HBs in health professions trainees: Prevalence of antibody coverage in a University of Central Italy. Hum. Vaccines Immunother. 2022, 18, 1886805. [Google Scholar] [CrossRef] [PubMed]
- Mahallawi, W. Persistence of hepatitis B surface antibody and immune memory to hepatitis B vaccine among medical college students in Madinah. Ann. Saudi Med. 2018, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Hess, L.; Riesenberg, K.; Rolston, K.V.; Nesher, L. Administering an additional hepatitis B vaccination dose after 18 years maintains adequate long-term protection levels in healthcare workers. Infect. Dis. 2020, 52, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Istrate, A.; Azoicăi, D.; Almaş, A.; Rădulescu, A. Variable anti-HBs antibody titers in vaccinated birth cohorts–A cross-sectional population-based study. Vaccine 2020, 38, 7015–7023. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Chih, A.H.; Lee, Y.C.; Huang, K.C.; Jan, C.F. Higher disappearance rate of anti-HB s in Taiwanese freshers neonatally vaccinated with recombinant yeast hepatitis B vaccine. Liver Int. 2017, 37, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Yang, C.-Y.; Shih, C.-T.; Chen, B.-H.; Huang, Y.-L. Waning immunity and booster responses in nursing and medical technology students who had received plasma-derived or recombinant hepatitis B vaccine during infancy. Am. J. Infect. Control 2011, 39, 408–414. [Google Scholar] [CrossRef]
- Kao, J.-T.; Wang, J.-H.; Hung, C.-H.; Yen, Y.-H.; Hung, S.-F.; Hu, T.-H.; Lee, C.-M.; Lu, S.-N. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine 2009, 27, 1858–1862. [Google Scholar] [CrossRef]
- Su, F.H.; Chen, J.D.; Cheng, S.H.; Lin, C.H.; Liu, Y.H.; Chu, F.Y. Seroprevalence of Hepatitis-B infection amongst Taiwanese university students 18 years following the commencement of a national Hepatitis-B vaccination program. J. Med. Virol. 2007, 79, 138–143. [Google Scholar] [CrossRef]
- Kwon, Y.; Jeong, S.J. Association between body mass index and hepatitis B antibody seropositivity in children. Korean J. Pediatr. 2019, 62, 416. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Keshavarz, J.; Bagheri-Jamebozorgi, M.; Nemati, M.; Frootan, R.; Shokri, F. The association of the vitamin D status with the persistence of anti-HBs antibody at 20 years after primary vaccination with recombinant hepatitis B vaccine in infancy. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 66–74. [Google Scholar] [CrossRef]
- Ho, J.K.-T.; Jeevan-Raj, B.; Netter, H.-J. Hepatitis B virus (HBV) subviral particles as protective vaccines and vaccine platforms. Viruses 2020, 12, 126. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Chang, M.; Ni, Y.; Chen, H.-L. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut 2004, 53, 1499–1503. [Google Scholar] [CrossRef]
- Luongo, M.; Critelli, R.; Grottola, A.; Gitto, S.; Bernabucci, V.; Bevini, M.; Vecchi, C.; Montagnani, G.; Villa, E. Acute hepatitis B caused by a vaccine-escape HBV strain in vaccinated subject: Sequence analysis and therapeutic strategy. J. Clin. Virol. 2015, 62, 89–91. [Google Scholar] [CrossRef]
- Hung, W.L.; Wu, J.F.; Ni, Y.H.; Chen, H.L.; Chiang, C.L.; Chang, M.H.; Hsu, H.Y. Occult hepatitis B virus and surface antigen mutant infection in healthy vaccinated cohorts and children with various forms of hepatitis and multiple transfusions. Liver Int. 2019, 39, 1052–1061. [Google Scholar] [CrossRef]
- Fonzo, M.; Bertoncello, C.; Trevisan, A. Factors influencing long-term persistence of anti-HBs after hepatitis B vaccination. NPJ Vaccines 2022, 7, 173. [Google Scholar] [CrossRef]
- Wen, W.-H.; Chang, M.-H.; Zhao, L.-L.; Ni, Y.-H.; Hsu, H.-Y.; Wu, J.-F.; Chen, P.-J.; Chen, D.-S.; Chen, H.-L. Mother-to-infant transmission of hepatitis B virus infection: Significance of maternal viral load and strategies for intervention. J. Hepatol. 2013, 59, 24–30. [Google Scholar] [CrossRef]
- Funk, A.L.; Lu, Y.; Yoshida, K.; Zhao, T.; Boucheron, P.; van Holten, J.; Chou, R.; Bulterys, M.; Shimakawa, Y. Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 70–84. [Google Scholar] [CrossRef]
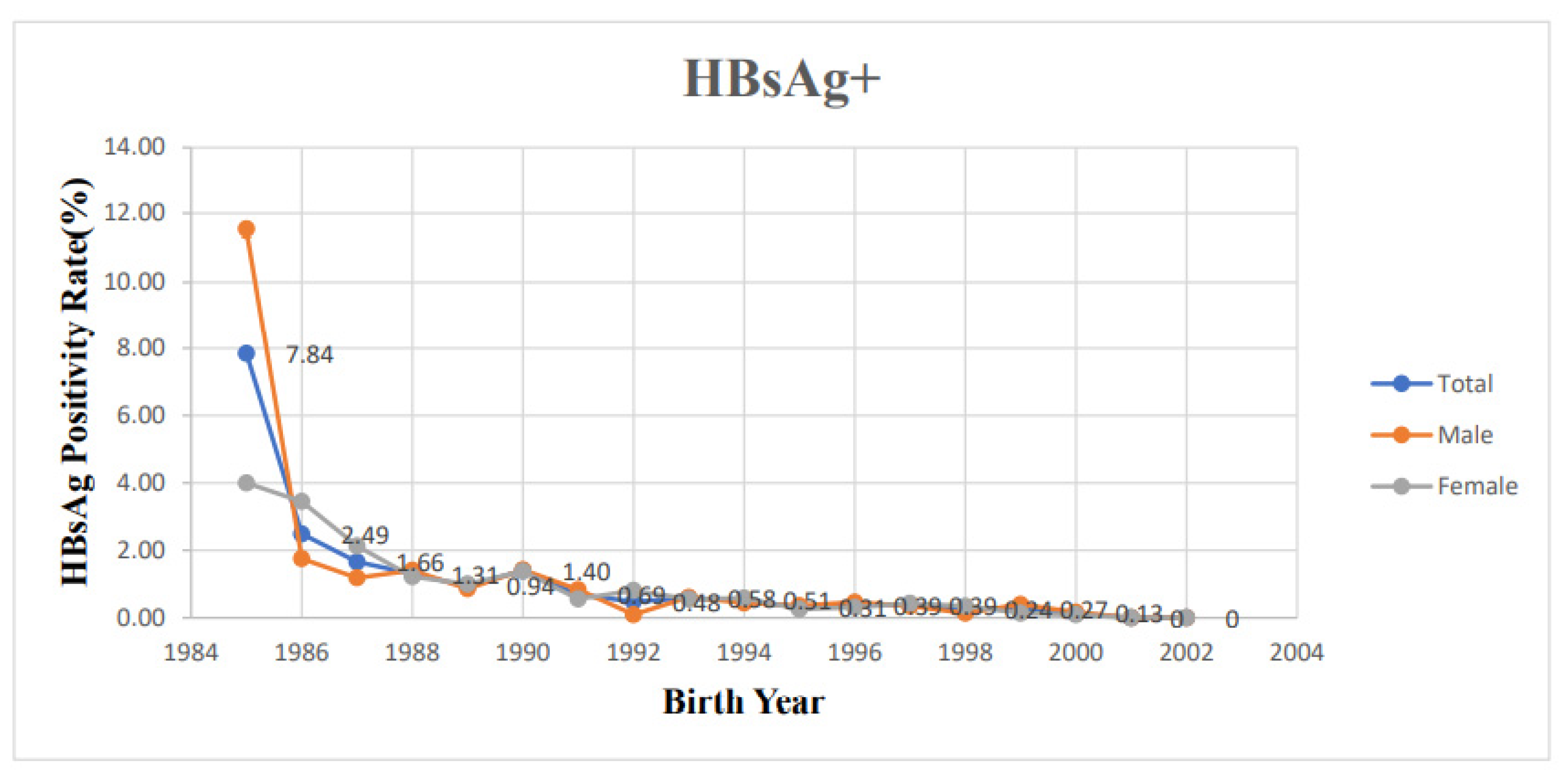
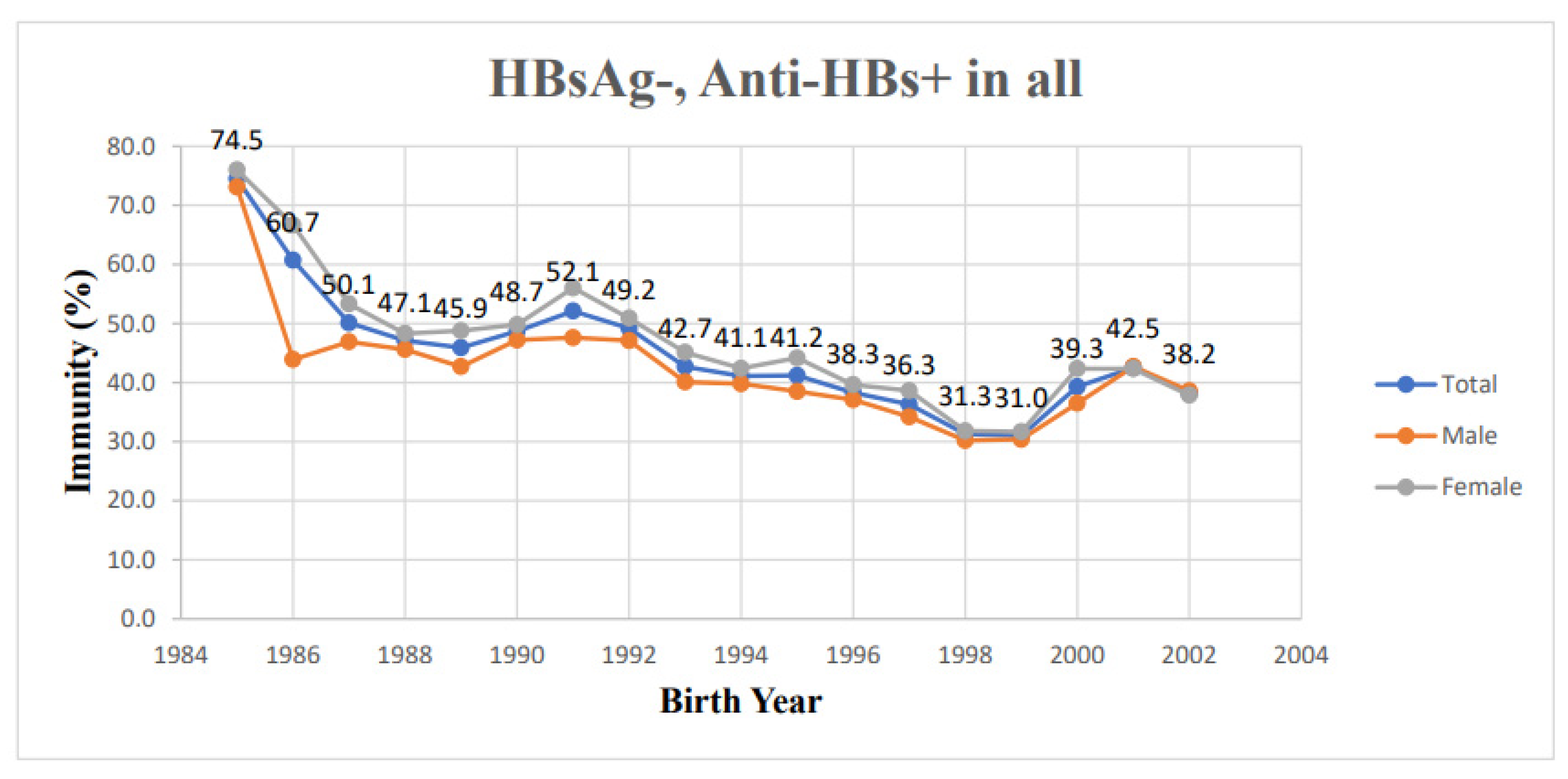
| Birth Year | Sex | HBsAg+ | HBsAg-, Anti-HBs+ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Female | Male | Total | Female | Male | Total | Female | Male | |
| n | n | n | n (%) 1 | n (%) 2 | n (%) 3 | n (%) 1 | n (%) 2 | n (%) 3 | |
| 1985 | 51 | 25 | 26 | 4 (7.84) | 1 (4.00) | 3 (11.54) | 38 (74.5) | 19 (76.0) | 19 (73.1) |
| 1986 | 201 | 87 | 114 | 5 (2.49) | 3 (3.45) | 2 (1.75) | 122 (60.7) | 58 (66.7) | 64 (43.9) |
| 1987 | 844 | 422 | 422 | 14 (1.66) | 9 (2.13) | 5 (1.18) | 423 (50.1) | 225 (53.3) | 198 (46.9) |
| 1988 | 2144 | 1149 | 995 | 28 (1.31) | 14 (1.22) | 14 (1.41) | 1009 (47.1) | 555 (48.3) | 454 (45.6) |
| 1989 | 1708 | 896 | 812 | 16 (0.94) | 9 (1.00) | 7 (0.86) | 784 (45.9) | 437 (48.8) | 347 (42.7) |
| 1990 | 1787 | 1014 | 773 | 25 (1.40) | 14 (1.38) | 11 (1.42) | 870 (48.7) | 505 (49.8) | 365 (47.2) |
| 1991 | 2330 | 1253 | 1077 | 16 (0.69) | 7 (0.56) | 9 (0.84) | 1215 (52.1) | 702 (56.0) | 513 (47.6) |
| 1992 | 2276 | 1230 | 1046 | 11 (0.48) | 10 (0.81) | 1 (0.10) | 1119 (49.2) | 626 (50.9) | 493 (47.1) |
| 1993 | 2408 | 1257 | 1151 | 14 (0.58) | 7 (0.56) | 7 (0.61) | 1028 (42.7) | 567 (45.1) | 461 (40.1) |
| 1994 | 2731 | 1368 | 1363 | 14 (0.51) | 8 (0.58) | 6 (0.44) | 1123 (41.1) | 580 (42.4) | 543 (39.8) |
| 1995 | 3230 | 1558 | 1672 | 10 (0.31) | 4 (0.26) | 6 (0.36) | 1332 (41.2) | 688 (44.2) | 644 (38.5) |
| 1996 | 3370 | 1640 | 1730 | 13 (0.39) | 5 (0.30) | 8 (0.46) | 1292 (38.3) | 650 (39.6) | 642 (37.1) |
| 1997 | 3369 | 1636 | 1733 | 13 (0.39) | 7 (0.43) | 6 (0.35) | 1224 (36.3) | 632 (38.6) | 592 (34.2) |
| 1998 | 2897 | 1436 | 1461 | 7 (0.24) | 5 (0.35) | 2 (0.14) | 908 (31.3) | 467 (31.8) | 441 (30.2) |
| 1999 | 2958 | 1449 | 1509 | 8 (0.27) | 2 (0.14) | 6 (0.40) | 917 (31.0) | 459 (31.7) | 458 (30.4) |
| 2000 | 2290 | 1086 | 1204 | 3 (0.13) | 1 (0.09) | 2 (0.17) | 899 (39.3) | 460 (42.4) | 439 (36.5) |
| 2001 | 1963 | 1013 | 950 | 0 (0) | 0 (0) | 0 (0) | 835 (42.5) | 429 (42.3) | 406 (42.7) |
| 2002 | 1518 | 808 | 710 | 0 (0) | 0 (0) | 0 (0) | 580 (38.2) | 306 (37.9) | 274 (38.6) |
| Variables | HBV Infection | Univariate Analysis | Multivariate Analysis | |
|---|---|---|---|---|
| No (N = 37,874) | Yes (N = 201) | p-Value | p-Value | |
| Age, years, mean ± SD | 18.38 ± 0.76 | 18.48 ± 0.87 | 0.108 | 0.062 |
| Sex | ||||
| Male, N (%) | 19,221 (99.5%) | 106 (0.5%) | 0.623 3 | 0.259 |
| Female, N (%) | 18,653 (99.5%) | 95 (0.5%) | ||
| Height, cm, mean ± SD | 166.1 ± 8.58 | 166.0 ± 8.19 | 0.782 | - |
| Weight, kg, mean ± SD | 59.96 ± 12.87 | 59.16 ± 10.96 | 0.304 | - |
| BMI, kg/m2, mean ± SD | 21.61 ± 3.71 | 21.42 ± 3.37 | 0.414 | 0.005 |
| <24, N (%) | 30,085 (99.5%) | 163 (0.5%) | 0.622 3 | |
| ≥24, N (%) | 7789 (99.5%) | 38 (0.5%) | ||
| ALT 1, U/L, mean ± SD | 18.24 ± 20.00 | 35.17 ± 66.09 | <0.001 | <0.001 |
| ASSLD 2 | ||||
| Normal, N (%) | 34,347 (99.6%) | 142 (0.4%) | <0.001 3 | |
| Abnormal, N (%) | 3527 (98.4%) | 59 (1.6%) | ||
| AST 1, U/L, mean ± SD | 20.83 ± 10.23 | 27.94 ± 24.70 | <0.001 | 0.045 |
| Total cholesterol, mg/dL, mean ± SD | 173.5 ± 30.73 | 175.3 ± 29.61 | 0.398 | 0.979 |
| Model | Abnormal ALT | Abnormal ALT of ASSLD Criteria | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Adjusted Odds Ratio | p-Value | Mean (SD) | Adjusted Odds Ratio | p-Value | |
| Gender (male/female) | 18,748/19,327 | 3.92 (3.44–4.46) | <0.001 | 18,748/19,327 | 1.55 (1.43–1.68) | <0.001 |
| HBsAg | 201/37,874 | 5.56 (3.62–8.54) | <0.001 | 201/37,874 | 5.85 (4.19–8.16) | <0.001 |
| Age, year | 18.38 (0.73) | 1.09 (1.02–1.16) | 0.012 | 18.38 (0.73) | 1.06 (1.01–1.11) | 0.021 |
| BMI, kg/m2 | 21.61 (3.71) | 1.30 (1.29–1.32) | <0.001 | 21.61 (3.71) | 1.30 (1.29–1.31) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, T.-W.; Yang, J.-F.; Chen, Y.-Y.; Wu, K.-T.; Lee, M.-S.; Kuo, H.-J.; Lin, T.-C.; Wang, C.-L.; Hsieh, M.-H.; Lin, C.-Y.; et al. Epidemiology of Chronic Hepatitis B Infection in the Cohort of College Students with Vaccination in Taiwan. Vaccines 2023, 11, 348. https://doi.org/10.3390/vaccines11020348
Cheng T-W, Yang J-F, Chen Y-Y, Wu K-T, Lee M-S, Kuo H-J, Lin T-C, Wang C-L, Hsieh M-H, Lin C-Y, et al. Epidemiology of Chronic Hepatitis B Infection in the Cohort of College Students with Vaccination in Taiwan. Vaccines. 2023; 11(2):348. https://doi.org/10.3390/vaccines11020348
Chicago/Turabian StyleCheng, Te-Wei, Jeng-Fu Yang, Yi-Yu Chen, Kuan-Ta Wu, Meng-Szu Lee, Hsiang-Ju Kuo, Tzu-Chun Lin, Chao-Ling Wang, Meng-Hsuan Hsieh, Chia-Yi Lin, and et al. 2023. "Epidemiology of Chronic Hepatitis B Infection in the Cohort of College Students with Vaccination in Taiwan" Vaccines 11, no. 2: 348. https://doi.org/10.3390/vaccines11020348
APA StyleCheng, T.-W., Yang, J.-F., Chen, Y.-Y., Wu, K.-T., Lee, M.-S., Kuo, H.-J., Lin, T.-C., Wang, C.-L., Hsieh, M.-H., Lin, C.-Y., Batsaikhan, B., Ho, C.-K., & Dai, C.-Y. (2023). Epidemiology of Chronic Hepatitis B Infection in the Cohort of College Students with Vaccination in Taiwan. Vaccines, 11(2), 348. https://doi.org/10.3390/vaccines11020348









