1. Introduction
Dysentery is an acute infectious colitis caused by an enteric bacterial pathogen known as
Shigella. Dysentery is ubiquitous. About 188 million global cases of shigellosis are reported annually [
1]. The main risk groups for shigellosis are composed of children under 5 years of age, underprivileged elderly people, and travelers in endemic areas [
2,
3]. Shigellosis caused by
Shigella flexneri is predominantly found in low- and middle-income countries [
1]. Despite the high epidemiological significance of Flexner’s dysentery and progressive antibiotic resistance, a licensed vaccine against
S. flexneri has not yet been developed.
Shigella vaccine development is being pursued in several directions: a live attenuated vaccine [
4], a whole-cell inactivated vaccine [
5], and a conjugated vaccine, including chemically conjugated or the most modern bioconjugate preparations (O-polysaccharide (O-PS) conjugated to the carrier protein, exotoxin A of
P. aeruginosa, using gene engineering techniques) [
6].
Live and inactivated whole-cell vaccines against
S. flexneri have been immunogenic in clinical and preclinical studies [
7,
8,
9]. However, a large number of observed adverse events and short-lived immune responses in clinical study subjects limits their regulatory approval and use in the clinic [
10]. To reduce the adverse events of whole-cell vaccines, the oral route of administration [
7] and/or attenuated strains have been used [
4]. The main challenge for whole-cell vaccine developers is to strike a balance between reactogenicity and immunogenicity [
11].
According to published clinical studies, conjugated dysentery vaccines, including bioconjugates, are safe. An increase in serum-specific antibodies of IgG and IgA classes by 2–10 times was recorded one month after the first vaccination, and levels remained unchanged for 56 days after the repeated vaccination [
12].
S. flexneri can be divided into 13 serotypes: 1a, 1b, 2a, 2b, 3a, 3b, 4a, 4b, 5a, 5b, 6, X, and Y. Type antigens are determined by saccharide residues linked to the main chain. Additional sugars or conformation of the chemical structure form group antigens (serotypes X and Y lack type antigens and have only group antigens) [
13]. Serotypes/serogroups isolated from approximately 90% of patients with dysentery include
S. flexneri 1b, 2a, 2b, 3a, and 6 [
14].
Thus, the morbidity of shigellosis caused by S. flexneri, in contrast to shigellosis caused by S. sonnei, is due to the presence of multiple serotypes of the pathogen. When developing a vaccine against shigellosis caused by S. flexneri, in addition to solving the problem of creating an effective vaccine immunogen, the choice and technological development of a set of antigenic vaccine components that provide protection against infection in a given geographic area are equally important.
Protective immunity to
Shigella is induced by O-polysaccharide part of lipopolysaccharide (LPS), but LPS is highly endotoxic [
15]. Therefore, the use of LPS in its native form (nLPS) as an active component of a vaccine is impossible. In an earlier study, we applied the LPS detoxification method to create a modified tri-acylated highly homogenous LPS form (Ac
3-S-LPS) from
S. flexneri 2a [
16]. This paper describes a novel
Shigella vaccine based on original pentavalent LPS preparation that, in addition to Ac
3-S-LPS from
S. flexneri 2a, includes four other Ac
3-S-LPS compounds from
S. flexneri 1b, 3a, 6, and Y (PLVF). The preclinical safety profile of PLVF was studied in rabbits. Antibody levels after immunization of mice with PLVF were assessed. Functional evaluation of anti-PLVF antibodies’ potency for lysis of virulent
Shigella of selected serotypes was also performed. The protective potency of PLVF was assessed in a Sereni test.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
The biomass of strains of S. flexneri 1b 1818, 2a 1605, 3a 2167, 6 281-55, and Y 2643, which represented the smooth (S) form of bacteria each serotyped with a homologous Shigella serum, was obtained through fermentation using Hottinger’s broth (Nutrient Media, State Research Center for Applied Biotechnology and Microbiology, Obolensk Russia) in a 250 L fermenter (BioR 250, Prointech-bio, Pushchino, Russia) with stirring and forced aeration. Bacterial cells were separated from the liquid phase by flow centrifugation (Z-41, CEPA, Lahr, Germany). Wet cells were subsequently washed with sterile saline and water, and then lyophilized.
For serum bactericidal antibody (SBA) assay, S. flexneri 1b, 2a, 3a, 6, and Y were streaked on tryptone soy agar plates and incubated overnight at 37 °C. Then, bacterial cells were washed off plate surfaces using sterile 0.9% NaCl and further diluted in phosphate-buffered saline.
2.2. Isolation and Degradation of S-LPS
Freeze-dried bacterial cells were extracted with 45% aqueous phenol (Sigma-Aldrich, St. Louis, MO, USA) at 68–70 °C. The aqueous phase was separated, dialyzed, and lyophilized to give a crude LPS preparation. The preparation was dissolved in TRIS buffer containing 0.01% (w/w) CaCl2 and MgCl2 solution (pH 7.2). RNAse (Sigma-Aldrich, St. Louis, MO, USA) (100 μg mL−1) and DNAse (Sigma-Aldrich, St. Louis, MO, USA) (10 μg mL−1) were added, and the solution was stirred at 37 °C for 16 h. The reaction mixture was treated with proteinase K (Sigma-Aldrich, St. Louis, MO, USA) (20 μg mL−1) for 2 h at 55 °C and then dialyzed using ultrafiltration with a 50 kDa cut-off membrane (Vladisart, Vladimir, Russia), concentrated, and lyophilized to give a purified LPS preparation. S-LPS was obtained using gel-permeation chromatography on Sephadex G-150 (Sigma-Aldrich, St. Louis, MO, USA) in the presence of Na-deoxycholate (Sigma-Aldrich, St. Louis, MO, USA) as detergent. Fractions that contained S-LPS were combined and freeze-dried.
After the removal of lipid A (2% AcOH, 100 °C, 1 h), the carbohydrate portions of nLPS and S-LPS were profiled by an Agilent 1260 HPLC system with UV- and RI-detection on a TSK gel G3000PW (Toyopearl, Mainz, Germany) (7.8 mm I.D. 30 cm) column using 0.2 M NaCl elution solution and a flow rate of 0.5 mL min−1. The content of low molecular mass compounds in S-LPS did not exceed 5–10%.
Partial de-acetylation of S-LPS was performed when S-LPS (300 mg) solution was heated with stirring in 8.3% aqueous ammonia and water (100 mL) containing 100 mg of Na-deoxycholate at 30 °C for 8 h, then cooled to 5–10 °C, diluted with 200 mL of water, neutralized with AcOH, and freeze-dried. The product was treated with 100% ethanol (200 mL), the precipitate was separated by centrifugation, washed with 100% ethanol (2 × 200 mL), vacuum-dried, dissolved in water, and freeze-dried to give Ac
3-S-LPS (213 mg) [
17].
2.3. Production of PLVF
The candidate vaccine product was manufactured based on Ac
3-S-LPS compounds from
S. flexneri 1b, 2a, 3a, 6, and Y (PLVF) as the active substances at a dose of 125 μg (25 μg of each antigen compound) and contained the following formulation excipients: phenol (preservative) 0.75 mg, NaCl 4.15 mg, Na
2HPO
4 0.052 mg, and NaH
2PO
4 0.017 mg (all Sigma-Aldrich, St. Louis, MO, USA), and 0.5 mL sterile pyrogen-free water for injection [
16]. The final form of PLVF product was formulated and dispensed aseptically in ampoules at a GMP-compliant manufacturing suite of vaccine enterprise of the Chumakov Institute of Poliomyelitis and Viral Encephalitides, Russian Academy of Sciences.
2.4. Chemical and Physical Analyses
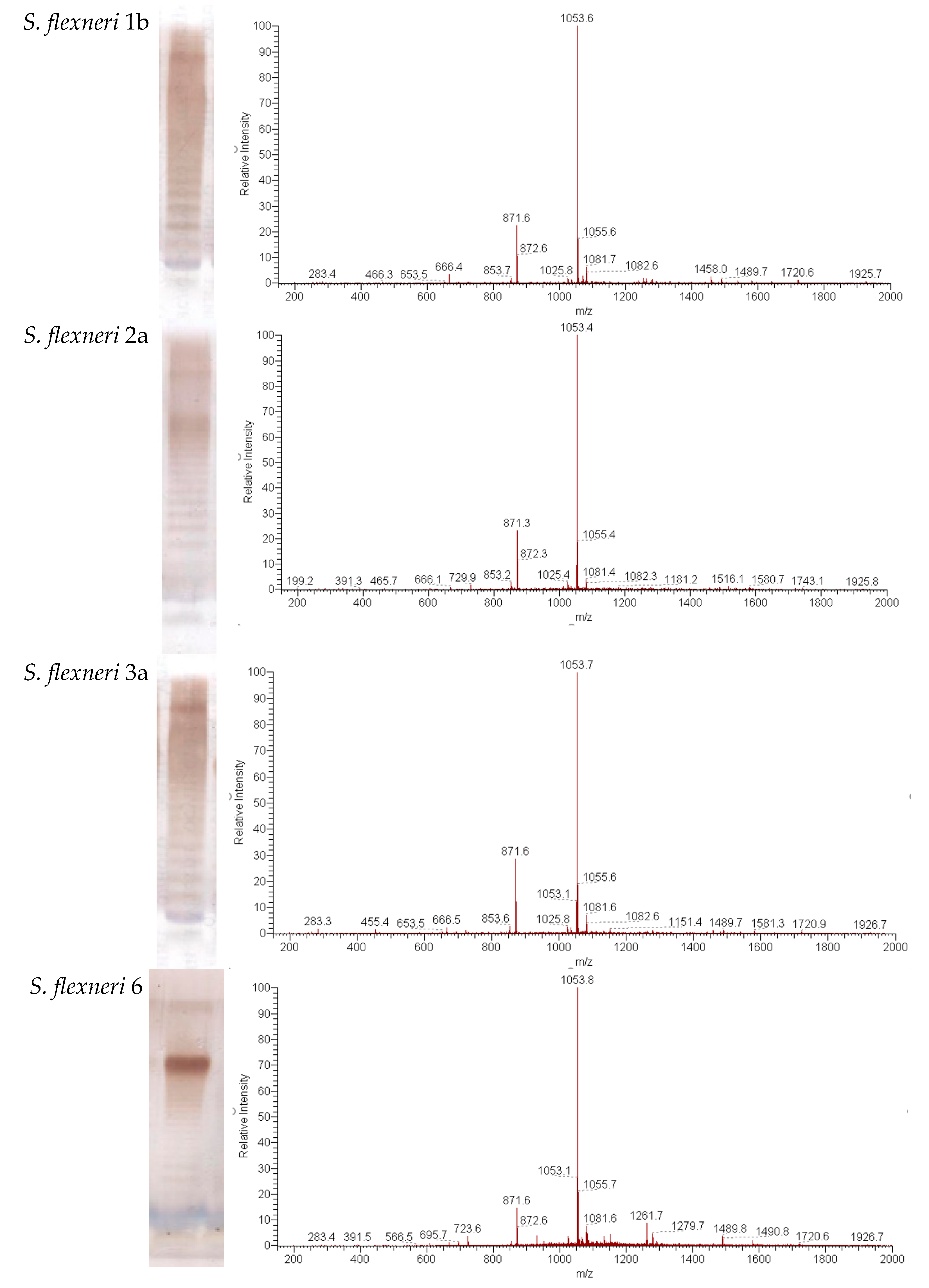
SDS-PAGE was performed on a 12% acrylamide gel according to the Laemmli method using a Bio-Rad Mini-Protean electrophoresis system. Gels were stained with silver nitrate reagent (Sigma-Aldrich, St. Louis, MO, USA).
Electrospray ionization high-resolution mass spectra were recorded in the negative ion mode using a micrOTOF II instrument (Bruker Daltonics, Billerica, MA, USA). A sample (~50 ng μL−1) was dissolved in a 1:1 (v/v) water–acetonitrile mixture and sprayed at a flow rate of 3 μLmin−1. End plate offset voltage was set to 0.5 kV and capillary voltage to 4.5 kV. Drying gas temperature was 180 °C. Mass range was from m/z 50 to 3000 Da.
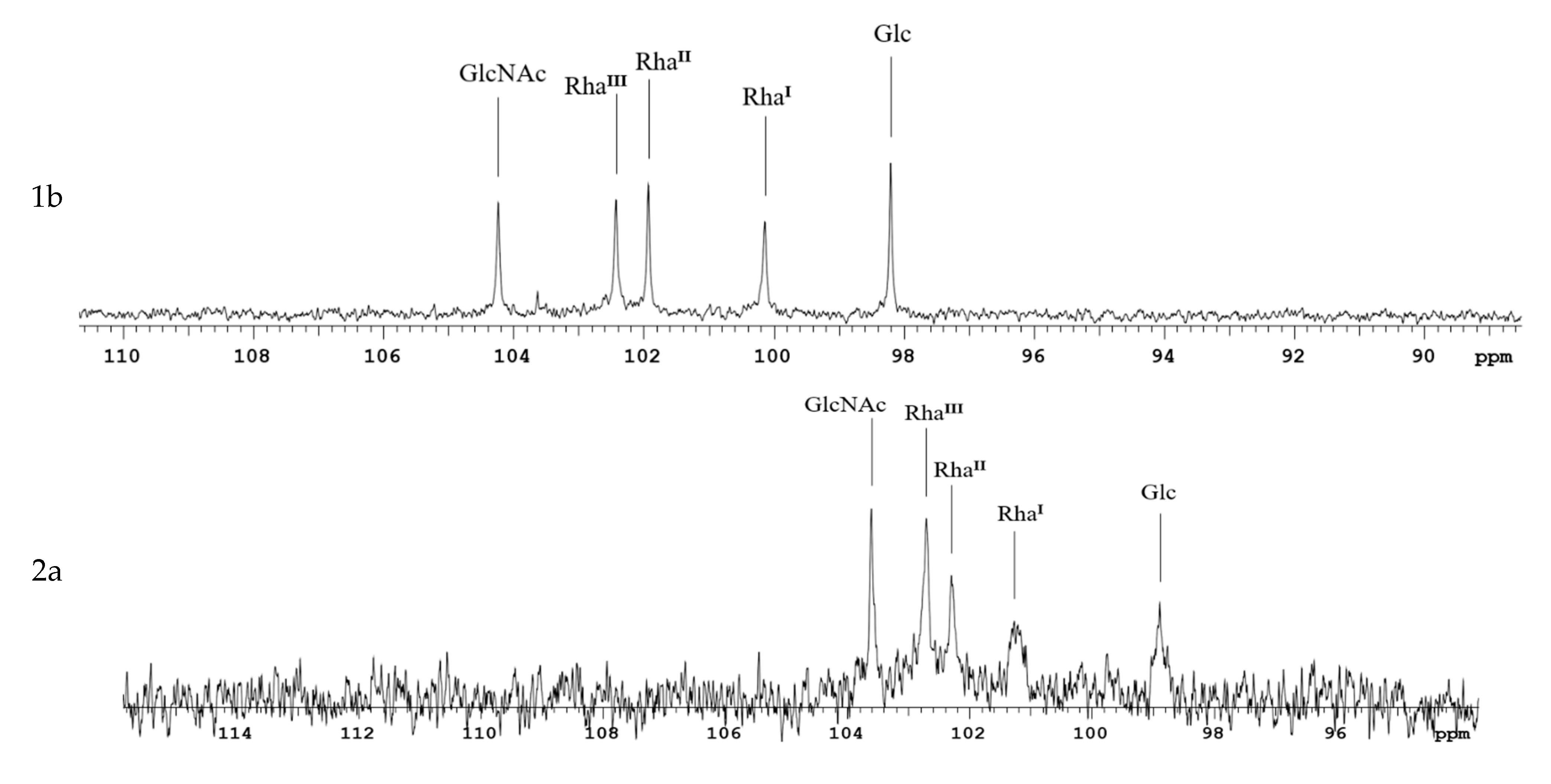
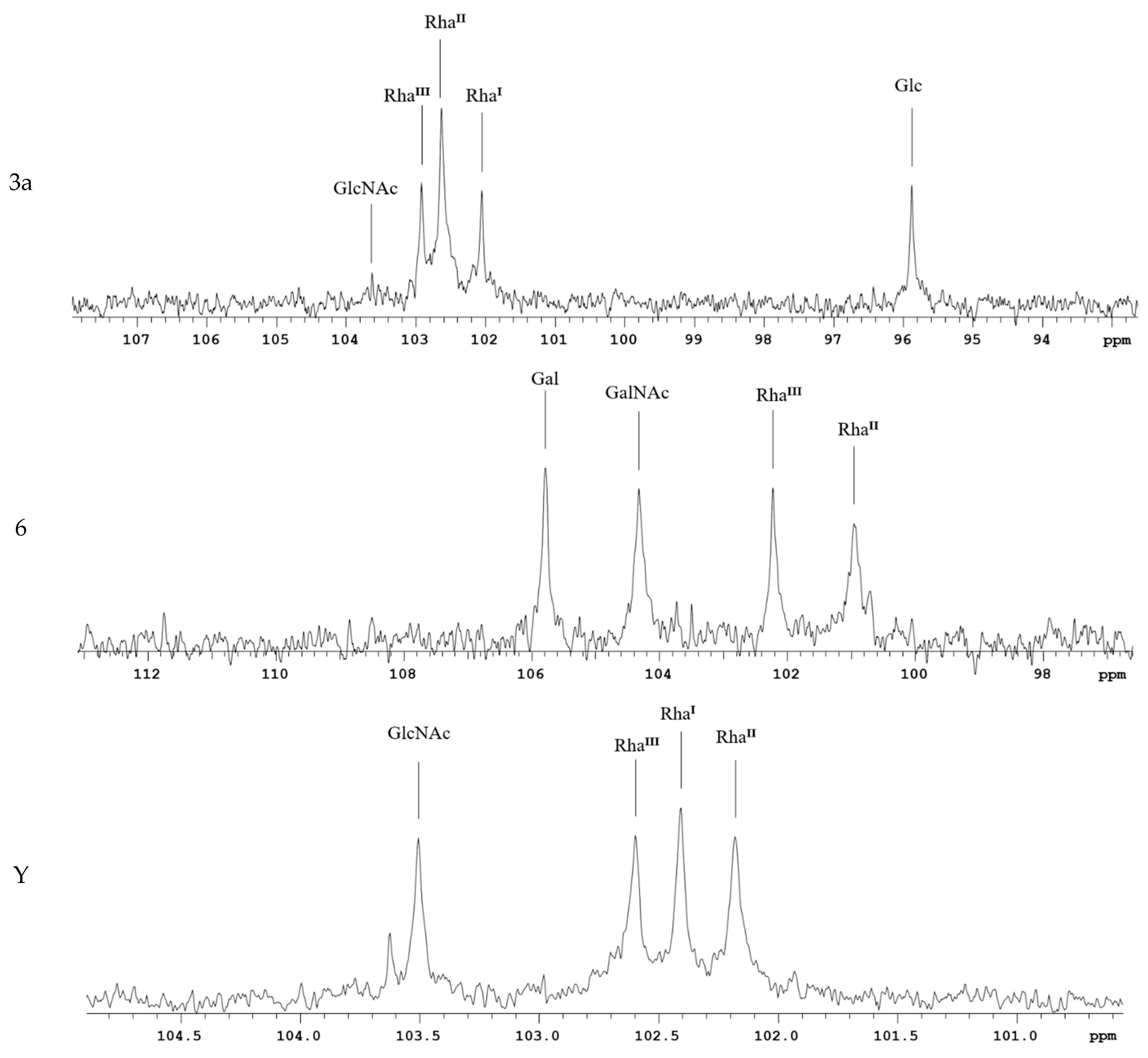
1H- and 13C-NMR spectroscopy were performed for solutions in 99.95% D2O at 323 K on a Bruker DRX-500 spectrometer (Bruker Daltonics, Billerica, MA, USA) using sodium3-trimethylsilylpropanoate-2,2,3,3-d4 (δH0) and acetone (δC 31.45) as references for calibration. Prior to analysis, samples were freeze-dried from 99.5% D2O. The Bruker Topspin 2.1 program was employed to acquire and process the NMR data.
2.5. Animal Studies
The study on animal models was carried out in accordance with the ethical principles approved by the order of the Ministry of Health of the Russian Federation No. 199n from 4 January 2016. The study protocols were approved by the local ethics committees of the research organizations. Animals were housed in accredited animal facilities with free access to food and water. Before the start of a study, animals were placed in a separate room for a period of quarantine (14–21 days, depending on the animal species) and health-monitored.
2.6. Pyrogenicity
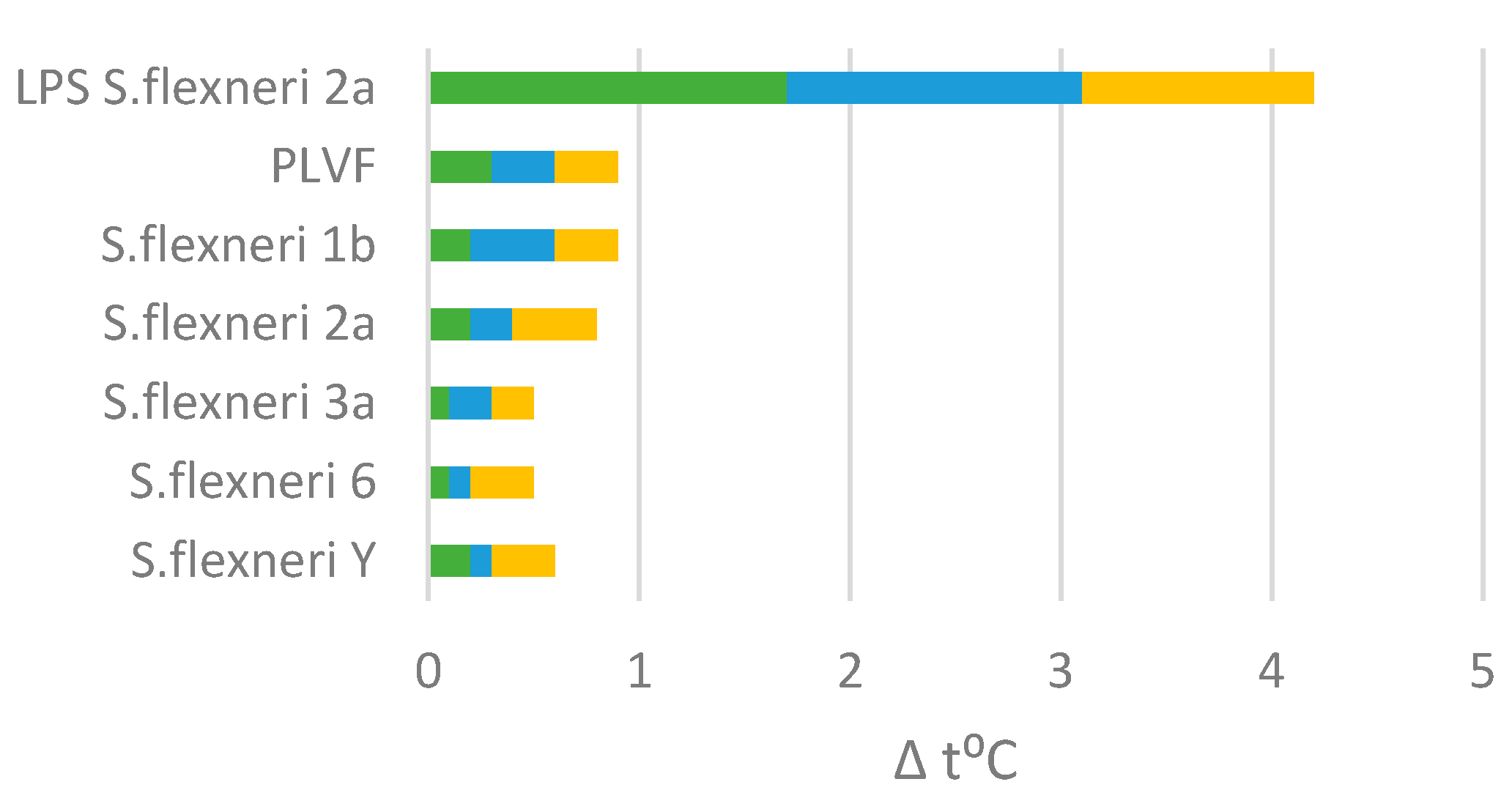
The standard method for determining the pyrogenicity is the rabbit pyrogen test (RPT). RPT was conducted on 21 adult Chinchilla rabbits (aged 3 months at the start of the experiment and weighing 2.8–3.05 g). Rabbits were randomized by weight into 7 groups. Three rabbits per group were intravenously injected with PLVF, each Ac3-S-LPS vaccine component or unmodified nLPS of S. flexneri 2a (as pyrogenicity control) at a dose of 0.025 µg kg−1.
A substance was considered apyrogenic if the cumulative temperature rise of three rabbits did not exceed 1.15 °C in accordance with the European Pharmacopoeia requirements [
18]. The PLVF doze used for RPT was chosen by analogy to the WHO-approved Vi-vaccine pyrogenicity test dose of 0.025 µg kg
−1.
2.7. Toxicology Study
A standard PLVF toxicity study was performed in rabbits. Chinchilla male and female rabbits were purchased from the Federal State Budgetary Institution of Science “Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency”, Russia. Acute toxicity studies were performed with a single subcutaneous injection of 125 μg/0.5 mL of PLVF (5 male and 5 female rabbits per group, aged 3 months at the start of experiment and weighing 2708 ± 71 g), followed by observation for 14 days. Animals in the control group were injected with 0.5 mL of PBS. Chronic toxicity studies were performed with daily subcutaneous immunization of rabbits with 125 μg/0.5 mL PLVF for a week (5 males and 5 females per group), followed by observation for 7 days. Animals in the control group were injected with 0.5 mL of PBS. Blood samples for hematology and biochemistry were collected before vaccination, and 7 and 14 days after vaccination. On the 14th day after vaccination, necropsy, histological examination of 16 internal organs and tissues of each rabbit, and the blood differential test were performed. The local irritation was studied by histopathological evaluation of the site of repeated subcutaneous injection of the vaccine preparation (macro- and microscopic description of the skin at the injection site).
Tissue samples for histological examination were fixed in 10% neutral buffered formalin, dehydrated in ascending concentrations of alcohol, and embedded in paraffin. Paraffin sections 5 μm thick were cut on a SM 2000R microtome (Leica, Wetzlar, Germany), stained with hematoxylin and eosin, and examined using a DM1000 microscope (Leica, Wetzlar, Germany).
The study of acute and chronic toxicity, as well as the study of the local tolerance of PLVF, was carried out in accordance with the recommendations and the requirements of local legislation (Federal Law of 12.04.10 N 61 “On the Circulation of Medicines”).
2.8. Immunochemical Identification of PLVF
We utilized standard sandwich ELISA with monovalent rabbit antisera against type antigens I, II, III, and VI, and group antigen 3,4 (Agnolla®, SPbSRIVS, Saint Petersburg, Russia) and PLVF-coated microwells for identification of PLVF components 1b, 2a, 3a, 6, and Y, respectively, and intact S. flexneri S-LPS 1b-, 2a-, 3a-, 6-, and Y-coated microwells for control antigenic activity.
2.9. Immunogenicity in Mice
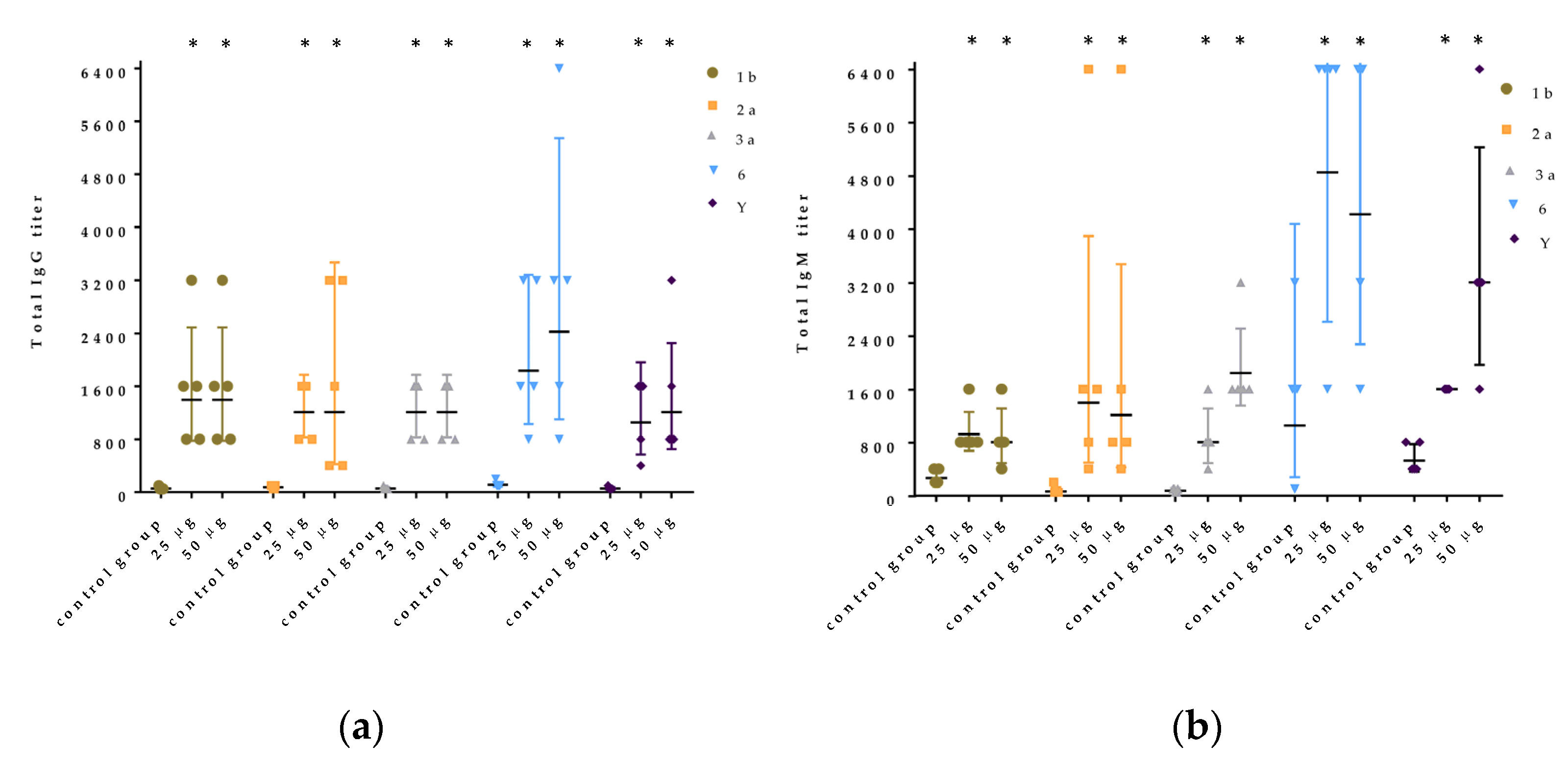
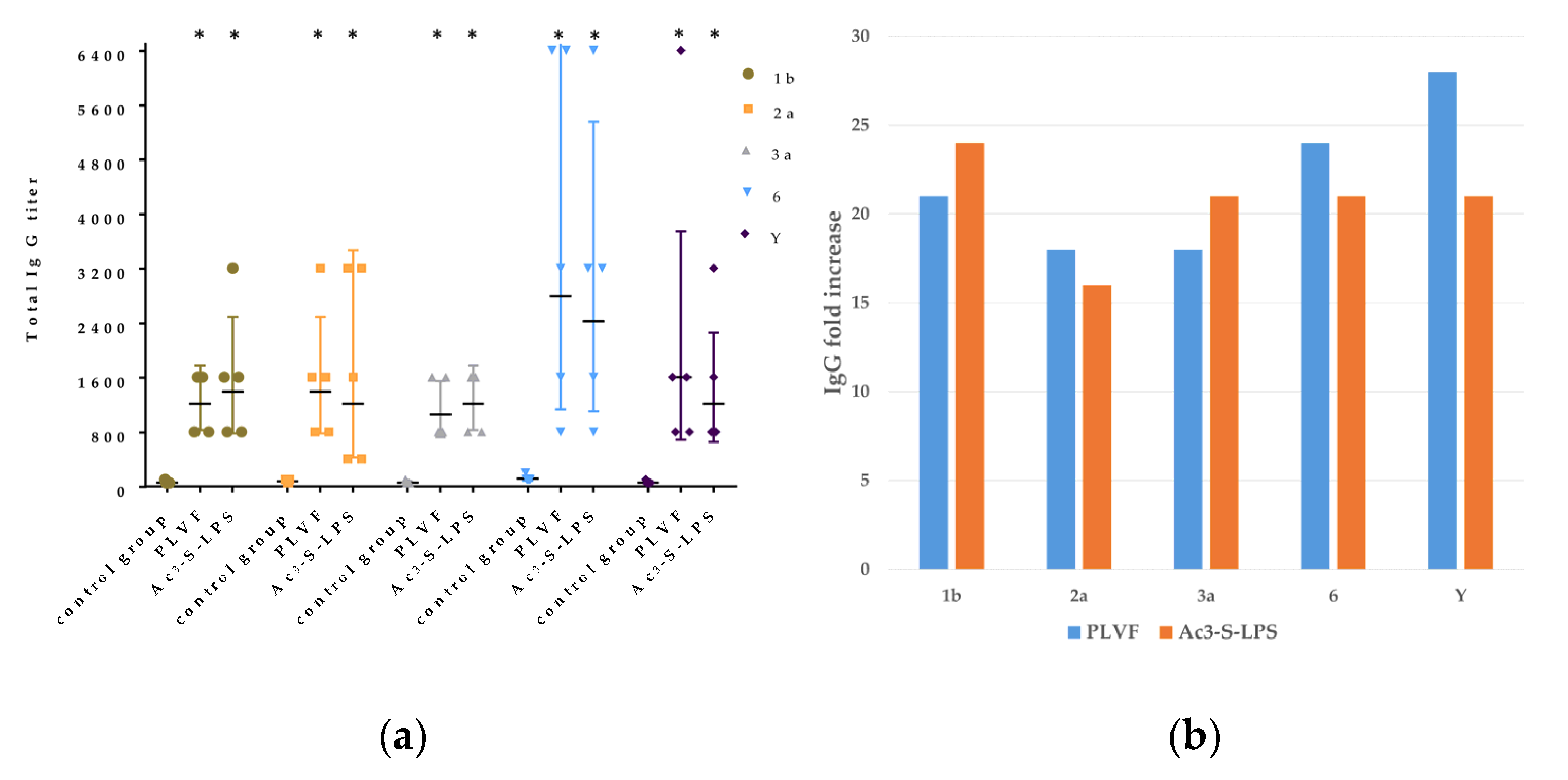
Sixty (CBA × C57BL/6) F1 female mice (5 mice per group, 8-weeks-old at the start of the experiment and weighing 18 ± 0.3 g) were purchased from the Federal State Budgetary Institution of Science “Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency”, Russia and immunized intraperitoneally with 125 μg per mouse of PLVF or with two doses of 25 μg and 50 μg of Ac3-S-LPS 1b, 2a, 3a, 6, and Y. Two weeks after the primary injection, the mice were boosted with the same dose. At day 15 after the secondary immunization, serum samples were collected and levels of LPS-specific total IgG and IgM were evaluated by ELISA (using a standard protocol) with native S. flexneri LPS (nLPS) 1b, 2a, 3a, 6, and Y adsorbed on microplates (Greiner, Kremsmünster, Austria). Control animals were given saline.
2.10. SBA Assay
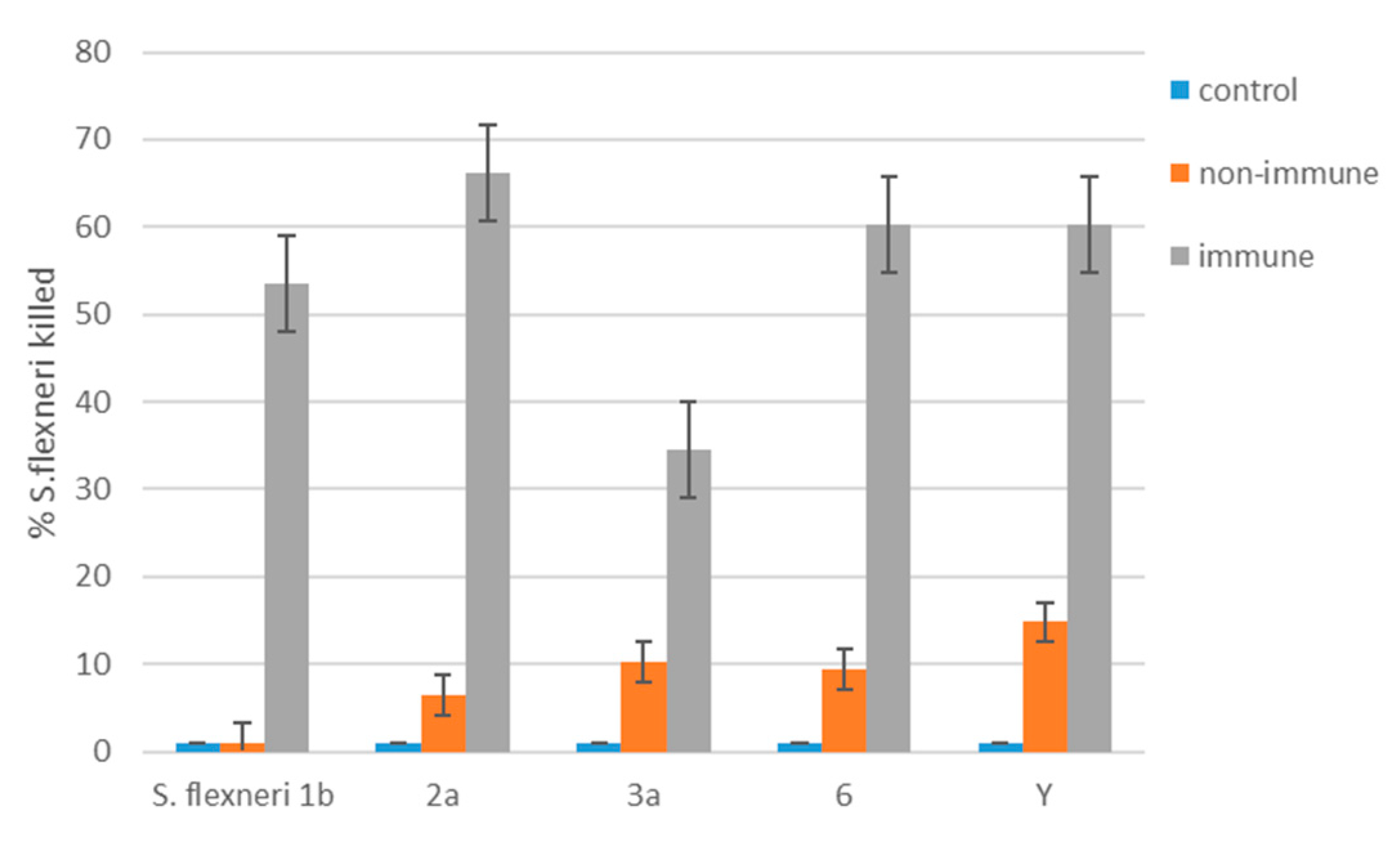
Heat-inactivated mouse serum samples were diluted 1:3000 in PBS and 100 µL were added into 96-well U-bottom plates (Medpolymer, Saint Petersburg, Russia). A 100 µL aliquot containing 104 CFU of each S. flexneri serotype 1b, 2a, 3a, 6, and Y separately and 25 µL of guinea pig complement (made in-house) were added into the wells. The final volume was 300 µL and serum dilution 1:9000. Plates were incubated for 1.5 h at 37 °C in a shaker at 200 rpm. Serum bactericidal activity was assessed individually for each mouse. The assay included complement control wells containing S. flexneri bacteria with guinea pig complement with no serum. This complement control was used to define 0% killing in the SBA killing rate calculation.
The percentage of bacteria killing (SBA rate) was determined by the equation [1- (colony forming units (CFU) of surviving bacteria/total CFU)] × 100.
2.11. Efficacy Evaluation of PLVF
Male (Agouti strain) guinea pigs were purchased from the Federal State Budgetary Institution of Science “Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency”, Russia. To examine the PLVF for eye protection against virulent
S. flexneri stains, groups of 10 guinea pigs (aged 3 months at the start of the experiment and weighing 275 ± 3 g) were twice immunized subcutaneously dorsally with a dose of 125 µg of vaccine at an interval of 10 days. Control animals were given saline. Ten days after the last immunization,
S. flexneri keratoconjunctivitis was induced in experimental and control groups of animals by inoculation into the mucosal surface of the conjunctiva of both eyes with a suspension of a virulent strain at a dose of 2 × 10
9 cells in 30 µL of sterile saline. Each group was inoculated with one of
S. flexneri 1b, 2a, 3a, 6, and Y serotypes. Keratoconjunctivitis was assessed 7 days after challenge by visual inspection. The efficacy of PLVF was calculated by the formula: PLVF Efficacy = 100 × (Control attack rate – PLVF attack rate)/Control attack rate, where attack rate = number of infected eyes/total eyes [
4].
2.12. Statistical Analysis
Statistical analyses of immunogenicity data were performed using GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, CA, USA). For statistical significance while comparing groups of mice, the Student’s t test was performed. Statistical significance was defined as p < 0.05.
4. Discussion
Flexner’s shigellosis represents a longstanding, difficult public health problem in many countries around the world. The achievement of field protection after vaccination against S. flexneri is an extremely difficult task.
Shigella-specific antibodies play an important role in promoting host defense against shigellosis. Children and adults living in areas endemic for shigellosis develop circulating antibody secreting cells and serum antibodies specific for
Shigella LPS and invasion plasmid antigen. Sera of volunteers infected with
S. flexneri 2a have a pronounced bactericidal efficacy [
19].
Recent studies of functional antibodies after immunization with protein-lipopolysaccharide outer membrane vesicles (OMV)
S. sonnei vaccine based on generalized modules for membrane antigen (GMMA) have shown that anti-LPS antibodies are the main drivers of bactericidal activity. On the contrary, anti-protein antibodies had limited ability to either bind to
Shigella cells or kill them in the presence of complement [
20].
In modern LPS-enriched
Shigella vaccines (Invaplex
AR-Detox, GMMA) genetically modified Shigella strains (Δ
msbB orΔ
htrB) are being used to prevent production of the most endotoxic form of LPS with hexa-acylated lipid A domain [
21,
22]. However, in these vaccines, LPS remains heterogeneous like classical endotoxin and contains penta- and tetra-acylated lipid A and, therefore, is still relatively endotoxic and pyrogenic. As a result, the doses of preparations containing genetically modified LPS proposed for the safe parenteral administration by the authors of the abovementioned studies are relatively low (in the range of 0.1–15 μg). Additional fractions of protein antigens, Ipa molecules (Invaplex
AR-Detox) or OMV proteins (GMMA), were also present in these vaccine preparations. Further studies aiming to increase the content of LPS, and thus change the design of GMMA-based vaccines to enhance their immunogenicity and efficacy, have been announced [
20].
Our previous studies have shown that Ac
3-S-LPS is apyrogenic and is the most immunogenic form of
S. flexneri 2a S-LPS [
16]. As we have already demonstrated the possibility of safe clinical use of
S. flexneri 2a Ac
3-S-LPS, it was reasonable to use this approach to obtain Ac
3-S-LPS components of other serotypes/serogroups for the development of PLVF. Therefore, PLVF was designed to include in the vaccine dose the maximum number of
S. flexneri antigens. Thus, PLVF contains five Ac
3-S-LPS of
S. flexneri, namely 1b, 2a, 3a, 6, and Y.
Each Ac3-S-LPS is a macromolecule, and at the same time is a full-fledged bioconjugate vaccine. In this respect, the O-PS conjugate is naturally produced by the bacterial cell and contains a built-in adjuvant-lipid A domain, the structure of which is deacylated to an apyrogenic form. The low content of pyrogens in PLVF preparation was confirmed using the rabbit pyrogenicity test. Preclinical toxicology studies in rabbits demonstrated the complete absence of signs of acute and chronic toxicity, local irritant action, damage to internal organs, and changes in biochemical and hematological parameters when PLVF was administered at a dose of 125 μg in 0.5 mL intended for human administration.
Thus, we achieved a significant extension of the dose range for a single parenteral administration to animals of Ac3-S-LPS as an active substance of PLVF to over 100 μg. Under the chronic toxicity regimen, multiple administration up to 875 μg of Ac3-S-LPS did not cause any endotoxic effects. These data once again demonstrate the critical difference between Ac3-S-LPS and natural endotoxin, the safe parenteral dose of which is sharply lower—just a few nanograms.
We used SDS-PAGE and mass spectrometry to determine the structure of Ac3-S-LPS of S. flexneri 1b, 2a, 3a, 6, and Y. Ac3-S-LPS from PLVF contains the long-chain S-LPS (O-PS chain length is about 20 repeating units) and has a triacylated lipid A. It should be emphasized that such vaccine grade Ac3-S-LPS is completely free from the most endotoxic highly acylated molecules of LPS—its hexa- and penta-acylated derivatives. The structure of the polysaccharide component of Ac3-S-LPS was determined using 13C NMR and was distinct for each serotype.
Each Ac3-S-LPS of PLVF was identified using a homologous monovalent antiserum. The serological properties of PLVF components and nLPS of the same S. flexneri serotypes were similar. Thus, the methods used to obtain Ac3-S-LPS did not cause the alteration of antigenic determinants.
In double immunized mice, we have registered high IgG levels (16-28-fold rise) to all components of PLVF without an aluminum hydroxide adjuvant. High immune response in mice correlated with protection against dysentery eye infection in guinea pigs. We have registered 50–75% efficacy against dysenteric keratoconjunctivitis after double immunization of guinea pigs. In a recent paper [
21], a 75% protection rate in the
S. flexneri 2a Sereny test after triple subcutaneous immunization with Invaplex
AR-Detox vaccine was reported. Thus, in addition to the 2a component, the simultaneous induction of a systemic immune response in mice and mucosal protection in guinea pigs has now been confirmed for four more
S. flexneri O-antigens.
The correctness of the choice of Ac3-S-LPS with long-chain OPS as a protective antigen of PLVF was confirmed by the presence in the sera of mice immunized with the vaccine of significant amounts of bactericidal antibodies to each of the homologous virulent strains of S. flexneri, serotypes 1b, 2a, 3a, 6, and Y.
The results of this preclinical study substantiated a wide dose interval for the safe administration of the candidate vaccine product and, thus, successfully validated the design of a pentavalent vaccine combination. If the goal of protection in the field against Flexner’s shigellosis using Ac3-S-LPS vaccine is achieved, the problem of comprehensive protection against shigellosis may be solved by the combined use of PLVF and the first available vaccine against Sonne’s shigellosis. This vaccine is based on S. sonnei exopolysaccharide, which elicits protective antibodies, and has been successfully used for prophylactic immunization for 15 years. In addition, the comprehensive data on the safety of PLVF offers the possibility of extending our vaccine construction strategy to various serotypes of Enterobacteriaceae.