Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting, Design, and Period
2.2. Data Collection and Analysis
3. Results
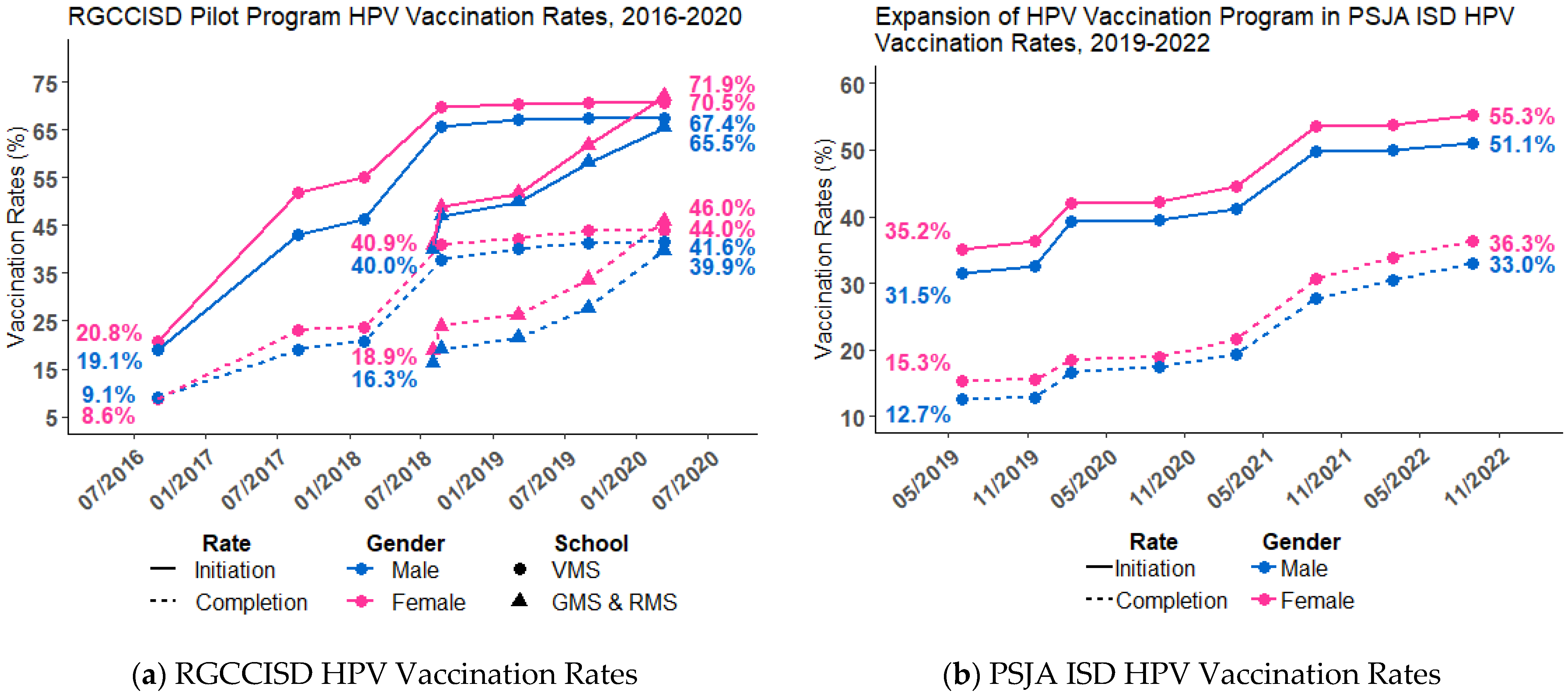
3.1. Descriptive Summary
3.2. HPV Initiation and HPV UTD Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
- Advisory Committee on Immunization Practices (ACIP)
- CDC: Centers for Disease Control and Prevention
- COVID-19: coronavirus Disease 2019
- HPV: human Papillomavirus
- ISD: independent school district
- PSJA ISD: Pharr-San Juan-Alamo Independent School District
- RGV: Rio Grande Valley
- RGCCISD: Rio Grande City Consolidated Independent School District
- SD: standard deviation
- TDAP: tetanus, diphtheria (TD), or tetanus, diphtheria, and pertussis
- US: United States
- UTD: up-to-date
- VFC: vaccines for children
Appendix A
| All | RGCCISD | PSJA ISD | Roma ISD | Zapata ISD | San Isidro ISD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unique Students Reached (as of 31 August 2022) n = 2145 | Unique Students Reached (as of 31 August 2022) n = 968 | Unique Students Reached (as of 31 August 2022) n = 957 | Unique Students Reached (as of 31 August 2022) n = 157 | Unique Students Reached (as of 31 August 2022) n = 58 | Unique Students Reached (as of 31 August 2022) n = 5 | |||||||||||||
| All | Females n = 1074 | Males n = 1071 | All | Females n = 471 | Males n = 497 | All | Females n = 498 | Males n = 459 | All | Females n = 73 | Males n = 84 | All | Females n = 29 | Males n = 29 | All | Females n = 3 | Males n = 2 | |
| Age groups at Initiation | ||||||||||||||||||
| 9–10 | 222 (10.3%) | 115 (10.7%) | 107 (10.0%) | 93 (9.6%) | 45 (9.6%) | 48 (9.7%) | 124 (13.0%) | 68 (13.7%) | 56 (12.2%) | 3 (1.9%) | 0 | 3 (3.6%) | 1 (1.7%) | 1 (3.4%) | 0 | 1 (20.0%) | 1 (33.3%) | 0 |
| 11–12 | 1491 (69.5%) | 750 (69.8%) | 741 (69.2%) | 731 (75.5%) | 366 (77.7%) | 365 (73.4%) | 600 (62.7%) | 306 (61.4%) | 294 (64.1%) | 113 (72.0%) | 56 (76.7%) | 57 (67.9%) | 45 (77.6%) | 20 (69.0%) | 25 (86.2%) | 2 (40.0%) | 2 (66.7%) | 0 |
| 13–14 | 319 (14.9%) | 148 (13.8%) | 171 (16.0%) | 121 (12.5%) | 49 (10.4%) | 72 (14.5%) | 159 (16.6%) | 80 (16.1%) | 79 (17.2%) | 25 (15.9%) | 11 (15.1%) | 14 (16.7%) | 12 (20.7%) | 8 (27.6%) | 4 (13.8%) | 2 (40.0%) | 0 | 2 (100.0%) |
| 15–16 | 64 (3.0%) | 36 (3.4%) | 28 (2.6%) | 15 (1.5%) | 7 (1.5%) | 8 (1.6%) | 41 (4.3%) | 24 (4.8%) | 17 (3.7%) | 8 (5.1%) | 5 (6.8%) | 3 (3.6%) | 0 | 0 | 0 | 0 | 0 | 0 |
| 17+ | 49 (2.3%) | 25 (2.3%) | 24 (2.2%) | 8 (0.8%) | 4 (0.8%) | 4 (0.8%) | 33 (3.4%) | 20 (4.0%) | 13 (2.8%) | 8 (5.1%) | 1 (1.4%) | 7 (8.3%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Age at HPV Initiation | ||||||||||||||||||
| Mean (SD) | 12 (1.5) | 12 (1.6) | 12 (1.5) | 12 (1.2) | 12 (1.2) | 12 (1.3) | 12 (1.8) | 12 (1.9) | 12 (1.6) | 12 (1.8) | 12 (1.5) | 12 (2.0) | 12 (1.0) | 12 (1.2) | 12 (0.8) | 12 (1.6) | 11 (0.6) | 14 (0.7) |
| Median (min, max) | 12 (9, 20) | 11 (9, 20) | 12 (9, 19) | 11 (9, 19) | 11 (9, 18) | 12 (9, 19) | 12 (9, 20) | 11 (9, 20) | 12 (9, 18) | 12 (9, 20) | 12 (11, 20) | 12 (9, 18) | 12 (9, 14) | 12 (9, 14) | 12 (11, 14) | 11 (10, 14) | 11 (10, 11) | 14 (13, 14) |
| School Grade at Initiation | ||||||||||||||||||
| Elementary school | 253 (11.8%) | 128 (11.9%) | 125 (11.7%) | 109 (11.3%) | 54 (11.5%) | 55 (11.1%) | 111 (11.6%) | 61 (12.2%) | 50 (10.9%) | 31 (19.7%) | 11 (15.1%) | 20 (23.8%) | 1 (1.7%) | 1 (3.4%) | 0 | 1 (20.0%) | 1 (33.3%) | 0 |
| Middle school | 1683 (78.5%) | 838 (78.0%) | 845 (78.9%) | 812 (83.9%) | 391 (83.0%) | 421 (84.7%) | 712 (74.4%) | 365 (73.3%) | 347 (75.6%) | 105 (66.9%) | 56 (76.7%) | 49 (58.3%) | 51 (87.9%) | 24 (82.8%) | 27 (93.1%) | 3 (60.0%) | 2 (66.7%) | 1 (50.0%) |
| High school | 209 (9.7%) | 108 (10.1%) | 101 (9.4%) | 47 (4.9%) | 26 (5.5%) | 21 (4.2%) | 134 (14.0%) | 72 (14.5%) | 62 (13.5%) | 21 (13.4%) | 6 (8.2%) | 15 (17.9%) | 6 (10.3%) | 4 (13.8%) | 2 (6.9%) | 1 (20.0%) | 0 | 1 (50.0%) |
| Number of HPV Vaccine Doses | ||||||||||||||||||
| 1 | 1065 (49.7%) | 512 (47.7%) | 553 (51.6%) | 352 (36.4%) | 158 (33.5%) | 194 (39.0%) | 542 (56.6%) | 275 (55.2%) | 267 (58.2%) | 112 (71.3%) | 50 (68.5%) | 62 (73.8%) | 54 (93.1%) | 26 (89.7%) | 28 (96.6%) | 5 (100.0%) | 3 (100.0%) | 2 (100.0%) |
| 2 | 911 (42.5%) | 464 (43.2%) | 447 (41.7%) | 524 (54.1%) | 263 (55.8%) | 261 (52.5%) | 344 (35.9%) | 179 (35.9%) | 165 (35.9%) | 41 (26.1%) | 21 (28.8%) | 20 (23.8%) | 2 (3.4%) | 1 (3.4%) | 1 (3.4%) | 0 | 0 | 0 |
| 3+ | 169 (7.9%) | 98 (9.1%) | 71 (6.6%) | 92 (9.5%) | 50 (10.6%) | 42 (8.5%) | 71 (7.4%) | 44 (8.8%) | 27 (5.9%) | 4 (2.5%) | 2 (2.7%) | 2 (2.4%) | 2 (3.4%) | 2 (6.9%) | 0 | 0 | 0 | 0 |
| Received the initial HPV dose from our program | ||||||||||||||||||
| No | 627 (29.2%) | 336 (31.3%) | 291 (27.2%) | 304 (31.4%) | 160 (34.0%) | 144 (29.0%) | 280 (29.3%) | 153 (30.7%) | 127 (27.7%) | 39 (24.8%) | 20 (27.4%) | 19 (22.6%) | 4 (6.9%) | 3 (10.3%) | 1 (3.4%) | 0 | 0 | 0 |
| Yes | 1518 (70.8%) | 738 (68.7%) | 780 (72.8%) | 664 (68.6%) | 311 (66.0%) | 353 (71.0%) | 677 (70.7%) | 345 (69.3%) | 332 (72.3%) | 118 (75.2%) | 53 (72.6%) | 65 (77.4%) | 54 (93.1%) | 26 (89.7%) | 28 (96.6%) | 5 (100.0%) | 3 (100.0%) | 2 (100.0%) |
| Received Other Vaccinations Bundled with the HPV Vaccine | ||||||||||||||||||
| No | 617 (28.8%) | 307 (28.6%) | 310 (28.9%) | 380 (39.3%) | 191 (40.6%) | 189 (38.0%) | 143 (14.9%) | 67 (13.5%) | 76 (16.6%) | 45 (28.7%) | 23 (31.5%) | 22 (26.2%) | 48 (82.8%) | 25 (86.2%) | 23 (79.3%) | 1 (20.0%) | 1 (33.3%) | 0 |
| Yes | 1528 (71.2%) | 767 (71.4%) | 761 (71.1%) | 588 (60.7%) | 280 (59.4%) | 308 (62.0%) | 814 (85.1%) | 431 (86.5%) | 383 (83.4%) | 112 (71.3%) | 50 (68.5%) | 62 (73.8%) | 10 (17.2%) | 4 (13.8%) | 6 (20.7%) | 4 (80.0%) | 2 (66.7%) | 2 (100.0%) |
| HPV-UTD | ||||||||||||||||||
| No | 1074 (50.1%) | 519 (48.3%) | 555 (51.8%) | 355 (36.7%) | 161 (34.2%) | 194 (39.0%) | 548 (57.3%) | 279 (56.0%) | 269 (58.6%) | 112 (71.3%) | 50 (68.5%) | 62 (73.8%) | 54 (93.1%) | 26 (89.7%) | 28 (96.6%) | 5 (100.0%) | 3 (100.0%) | 2 (100.0%) |
| Yes | 1071 (49.9%) | 555 (51.7%) | 516 (48.2%) | 613 (63.3%) | 310 (65.8%) | 303 (61.0%) | 409 (42.7%) | 219 (44.0%) | 190 (41.4%) | 45 (28.7%) | 23 (31.5%) | 22 (26.2%) | 4 (6.9%) | 3 (10.3%) | 1 (3.4%) | 0 | 0 | 0 |
| Age at HPV-UTD | ||||||||||||||||||
| Mean (SD) | 13 (1.4) | 13 (1.5) | 13 (1.4) | 12 (1.2) | 12 (1.2) | 12 (1.2) | 13 (1.7) | 13 (1.7) | 13 (1.6) | 13 (1.2) | 13 (0.7) | 14 (1.5) | 13 (1.7) | 12 (2.1) | 13 (.) | n/A | n/A | n/A |
| Median (min, max) | 12 (9, 19) | 12 (9, 19) | 13 (9, 18) | 12 (9, 18) | 12 (9, 18) | 12 (9, 18) | 12 (9, 19) | 12 (9, 19) | 13 (9, 18) | 13 (11, 18) | 13 (12, 15) | 13 (11, 18) | 13 (10, 14) | 13 (10, 14) | 13 (13, 13) | |||
| Days between Initiation and UTD | ||||||||||||||||||
| Mean (SD) | 480 (405.1) | 478 (410.1) | 482 (400.2) | 393 (297.0) | 395 (314.1) | 392 (279.1) | 564 (487.3) | 563 (495.9) | 567 (478.4) | 889 (460.5) | 809 (345.7) | 972 (552.3) | 525 (371.0) | 390 (310.1) | 932 (.) | n/A | n/A | n/A |
| Median (min, max) | 324 (146, 2855) | 310 (146, 2855) | 339 (150, 2843) | 268 (149, 2341) | 260 (149, 2016) | 272 (150, 2341) | 374 (146, 2855) | 364 (146, 2855) | 403 (167, 2843) | 874 (160, 2523) | 829 (160, 1469) | 875 (188, 2523) | 489 (191, 932) | 231 (191, 747) | 932 (932, 932) | |||
References
- Oyo-Ita, A.; Wiysonge, C.; Oringanje, C.; Nwachukwu, C.E.; Oduwole, O.; Meremikwu, M.M. Interventions for improving coverage of childhood immunisation in low- and middle-income countries. Cochrane Database Syst. Rev. 2016, 7, CD008145. [Google Scholar] [CrossRef]
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef]
- Bishop, J.M.; Real, F.J.; McDonald, S.L.; Klein, M.; DeBlasio, D.; Kahn, J.A.; Kreps, G.L.; Rosen, B.L. Evaluation of HPV Vaccine: Same Way, Same DayTM: A Pilot Study. J. Health Commun. 2021, 26, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Brandt, H.M.; Vanderpool, R.C.; Pilar, M.; Zubizarreta, M.; Stradtman, L.R. A narrative review of HPV vaccination interventions in rural U.S. communities. Prev. Med. 2021, 145, 106407. [Google Scholar] [CrossRef]
- Kornides, M.L.; McRee, A.L.; Gilkey, M.B. Parents Who Decline HPV Vaccination: Who Later Accepts and Why? Acad Pediatr. 2018, 18, S37–S43. [Google Scholar] [CrossRef]
- Newman, P.A.; Logie, C.H.; Lacombe-Duncan, A.; Baiden, P.; Tepjan, S.; Rubincam, C.; Doukas, N.; Asey, F. Parents’ uptake of human papillomavirus vaccines for their children: A systematic review and meta-analysis of observational studies. BMJ Open 2018, 8, e019206. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Taylor, Z.; Georges, R.; Carlson-Cosentino, M.; Nguyen, L.; Salas, M.; Vice, A.; Bernal, N.; Bhaloo, T. Primary Care Physicians’ Role in Parental Decision to Vaccinate with HPV Vaccine: Learnings from a South Texas Hispanic Patient Population. J. Immigr. Minor. Health 2017, 20, 1236–1242. [Google Scholar] [CrossRef]
- Donahue, K.L.; Hendrix, K.S.; Sturm, L.A.; Zimet, G.D. Human papillomavirus vaccine initiation among 9–13-year-olds in the United States. Prev. Med. Rep. 2015, 2, 892–898. [Google Scholar] [CrossRef]
- Brown, B.; Gabra, M.I.; Pellman, H. Reasons for acceptance or refusal of Human Papillomavirus Vaccine in a California pediatric practice. Papillomavirus Res. 2017, 3, 42–45. [Google Scholar] [CrossRef]
- Henry, K.A.; Swiecki-Sikora, A.L.; Stroup, A.M.; Warner, E.L.; Kepka, D. Area-based socioeconomic factors and Human Papillomavirus (HPV) vaccination among teen boys in the United States. BMC Public Health 2017, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Victory, M.; Do, T.Q.N.; Kuo, Y.-F.; Rodriguez, A.M. Parental knowledge gaps and barriers for children receiving human papillomavirus vaccine in the Rio Grande Valley of Texas. Hum. Vaccines Immunother. 2019, 15, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.Y.; Elam-Evans, L.D.; Singleton, J.A.; Yankey, D.; Markowitz, L.E.; Fredua, B.; Williams, C.L.; Meyer, S.A.; Stokley, S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2016. Morb. Mortal. Wkly. Rep. 2017, 66, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Texas Department of State Health Services. 2016 National Immunization Survey (NIS)—Vaccination Coverage Levels. Available online: https://www.dshs.texas.gov/immunization-unit/immunization-coverage-levels/national-immunization-survey/2016-national-immunization-survey#NIS (accessed on 24 January 2023).
- Shah, P.D.; Gilkey, M.B.; Pepper, J.K.; Gottlieb, S.L.; Brewer, N.T. Promising alternative settings for HPV vaccination of US adolescents. Expert Rev. Vaccines 2013, 13, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Do, T.Q.N.; Hsu, E.; Schmeler, K.M.; Montealegre, J.R.; Rodriguez, A.M. School-based human papillomavirus vaccination program for increasing vaccine uptake in an underserved area in Texas. Papillomavirus Res. 2019, 8, 100189. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.; Coker, A.L.; Eggleston, K.S.; Fernandez, M.E.; Arrastia, C.D.; Fadden, M.K. HPV Vaccine Acceptance among Latina Mothers by HPV Status. J. Women’s Health 2009, 18, 1793–1799. [Google Scholar] [CrossRef]
- Center for Reproductive Rights. Nuestro Voz, Nuestro Salud, Nuestro Texas: The Fight for Women’s Reproductive Health in the Rio Grande Valley (November 2013). New York, NY 10005, USA. Available online: http://nuestrotexas.org/pdf/NT-spread.pdf (accessed on 3 August 2021).
- Texas Cancer Registry. Age-Adjusted Invasive Cancer Incidence Rates in Texas (2015-2019). Available online: http://www.cancer-rates.info/tx/ (accessed on 1 September 2021).
- Mission Regional Medical Center. A Community Health Needs Assessment & Implementation Plan (May 2013). Available online: http://missionrmc.org/uploads/file/2013_mrmc_community-needs-assessment.pdf (accessed on 2 September 2021).
- US Census Bureau. Historical County Level Poverty Estimates Tool. Available online: https://www.census.gov/library/visualizations/time-series/demo/census-poverty-tool.html (accessed on 3 August 2021).
- Paul, P.; Fabio, A. Literature review of HPV vaccine delivery strategies: Considerations for school- and non-school based immunization program. Vaccine 2014, 32, 320–326. [Google Scholar] [CrossRef]
- Abdullahi, L.H.; Kagina, B.M.; Wiysonge, C.S.; Hussey, G.D. Improving vaccination uptake among adolescents. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Perman, S.; Turner, S.; Ramsay, A.I.G.; Baim-Lance, A.; Utley, M.; Fulop, N.J. School-based vaccination programmes: A systematic review of the evidence on organisation and delivery in high income countries. BMC Public Health 2017, 17, 252. [Google Scholar] [CrossRef]
- Vermandere, H.; Naanyu, V.; Mabeya, H.; Broeck, D.V.; Michielsen, K.; Degomme, O. Determinants of Acceptance and Subsequent Uptake of the HPV Vaccine in a Cohort in Eldoret, Kenya. PLoS ONE 2014, 9, e109353. [Google Scholar] [CrossRef]
- Lorini, C.; Santomauro, F.; Donzellini, M.; Capecchi, L.; Bechini, A.; Boccalini, S.; Bonanni, P.; Bonaccorsi, G. Health literacy and vaccination: A systematic review. Hum. Vaccines Immunother. 2018, 14, 478–488. [Google Scholar] [CrossRef]
- Davies, C.; Marshall, H.S.; Zimet, G.; McCaffery, K.; Brotherton, J.M.L.; Kang, M.; Garland, S.; Kaldor, J.; McGeechan, K.; Skinner, S.R.; et al. Effect of a School-Based Educational Intervention About the Human Papillomavirus Vaccine on Psychosocial Outcomes Among Adolescents: Analysis of Secondary Outcomes of a Cluster Randomized Trial. JAMA Netw. Open 2021, 4, e2129057. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.R.; Davies, C.; Cooper, S.; Stoney, T.; Marshall, H.; Jones, J.; Collins, J.; Hutton, H.; Parrella, A.; Zimet, G.; et al. HPV.edu study protocol: A cluster randomised controlled evaluation of education, decisional support and logistical strategies in school-based human papillomavirus (HPV) vaccination of adolescents. BMC Public Health 2015, 15, 896. [Google Scholar] [CrossRef] [PubMed]
- Do, T.Q.N.; Jibaja-Weiss, M.L.; Chen, L.; Schmeler, K.M.; Montealegre, J.R.; Kuo, Y.-F. Human Papillomavirus Vaccinations During the COVID-19 Pandemic in Middle Schools in the Rio Grande Valley of Texas. Am. J. Public Health 2022, 112, 1269–1272. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Do, T.Q.N.; Chen, L.; Schmeler, K.M.; Montealegre, J.R.; Kuo, Y.-F. Human papillomavirus vaccinations at recommended ages: How a middle school-based educational and vaccination program increased uptake in the Rio Grande Valley. Hum. Vaccines Immunother. 2022, 18, 2133315. [Google Scholar] [CrossRef]
- Meites, E.S.P.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. Am. J. Transplant. 2019, 19, 3202–3206. [Google Scholar] [CrossRef]
- CDC. HPV Vaccine Schedule and Dosing. August 2019. Available online: https://www.cdc.gov/hpv/hcp/schedules-recommendations.html (accessed on 3 August 2021).
- Donahue, K.L.; Stupiansky, N.W.; Alexander, A.B.; Zimet, G.D. Acceptability of the human papillomavirus vaccine and reasons for non-vaccination among parents of adolescent sons. Vaccine 2014, 32, 3883–3885. [Google Scholar] [CrossRef]
- Bednarczyk, R.A.; Birkhead, G.S.; Morse, D.L.; Doleyres, H.; McNutt, L.-A. Human papillomavirus vaccine uptake and barriers: Association with perceived risk, actual risk and race/ethnicity among female students at a New York State university, 2010. Vaccine 2011, 29, 3138–3143. [Google Scholar] [CrossRef]
- Allen, J.D.; Coronado, G.D.; Williams, R.S.; Glenn, B.; Escoffery, C.; Fernandez, M.; Tuff, R.A.; Wilson, K.M.; Mullen, P.D. A systematic review of measures used in studies of human papillomavirus (HPV) vaccine acceptability. Vaccine 2010, 28, 4027–4037. [Google Scholar] [CrossRef]
- Bloem, P.; Ogbuanu, I. Vaccination to prevent human papillomavirus infections: From promise to practice. PLOS Med. 2017, 14, e1002325. [Google Scholar] [CrossRef]
- Margolis, M.A.; Brewer, N.T.; Shah, P.D.; Calo, W.A.; Alton Dailey, S.; Gilkey, M.B. Talking about recommended age or fewer doses: What motivates HPV vaccination timeliness? Hum. Vaccin. Immunother. 2021, 17, 3077–3080. [Google Scholar] [CrossRef]
- Ejezie, C.L.; Osaghae, I.; Ayieko, S.; Cuccaro, P. Adherence to the Recommended HPV Vaccine Dosing Schedule among Adolescents Aged 13 to 17 Years: Findings from the National Immunization Survey-Teen, 2019–2020. Vaccines 2022, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Flood, T.; Wilson, I.M.; Prue, G.; McLaughlin, M.; Hughes, C.M. Impact of school-based educational interventions in middle adolescent populations (15-17yrs) on human papillomavirus (HPV) vaccination uptake and perceptions/knowledge of HPV and its associated cancers: A systematic review. Prev. Med. 2020, 139, 106168. [Google Scholar] [CrossRef] [PubMed]
- Sitaresmi, M.N.; Rozanti, N.M.; Simangunsong, L.B.; Wahab, A. Improvement of Parent’s awareness, knowledge, perception, and acceptability of human papillomavirus vaccination after a structured-educational intervention. BMC Public Health 2020, 20, 1836. [Google Scholar] [CrossRef] [PubMed]


| Variable 1 | Total | RGCCISD Pilot Program | Expansion into PSJA ISD | Expansion into Roma ISD, Zapata ISD, San Isidro ISD |
|---|---|---|---|---|
| Number of middle schools | 16 | 3 | 8 | 5 |
| Number of HPV vaccine initiations delivered at school campus interventions | 1549 | 497 | 677 | 375 |
| Number of HPV vaccine completions delivered at school campus interventions | 1042 | 578 | 378 | 86 |
| Number of HPV vaccine initiations delivered by collaborating healthcare practices | 18,172 | 4231 | 10,713 | 3228 |
| Number of HPV vaccine completions delivered by collaborating healthcare practices | 17,075 | 3936 | 10,829 | 2310 |
| Number of school campus interventions | 178 | 41 | 110 | 27 |
| Variable | All Unique Students Vaccinated Directly or Indirectly 1 (n = 19,951) | All Unique Students Vaccinated Directly (n = 2145) | ||||
|---|---|---|---|---|---|---|
| All (n = 19,951) | Females (n = 10,289) | Males (n = 9662) | All (n = 2145) | Females (n = 1074) | Males (n = 1071) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age Groups at Initiation | ||||||
| 9–10 | 4680 (23.5%) | 2618 (25.4%) | 2062 (21.3%) | 222 (10.3%) | 115 (10.7%) | 107 (10.0%) |
| 11–12 | 13,135 (65.8%) | 6687 (65.0%) | 6448 (66.7%) | 1491 (69.5%) | 750 (69.8%) | 741 (69.2%) |
| 13–14 | 1460 (7.3%) | 673 (6.5%) | 787 (8.1%) | 319 (14.9%) | 148 (13.8%) | 171 (16.0%) |
| 15–16 | 507 (2.5%) | 239 (2.3%) | 268 (2.8%) | 64 (3.0%) | 36 (3.4%) | 28 (2.6%) |
| 17+ | 169 (0.8%) | 72 (0.7%) | 97 (1.0%) | 49 (2.3%) | 25 (2.3%) | 24 (2.2%) |
| Age at HPV Initiation | ||||||
| Mean (SD) | 11 (1.4) | 11 (1.4) | 11 (1.5) | 12 (1.5) | 12 (1.6) | 12 (1.5) |
| Median (min, max) | 11 (9, 21) | 11 (9, 20) | 11 (9, 21) | 12 (9, 20) | 11 (9, 20) | 12 (9, 19) |
| School District | ||||||
| RGCCISD | 5583 (28.0%) | 2916 (28.3%) | 2667 (27.6%) | 968 (45.1%) | 471 (43.9%) | 497 (46.4%) |
| PSJA ISD | 11,390 (57.1%) | 5905 (57.4%) | 5485 (56.8%) | 957 (44.6%) | 498 (46.4%) | 459 (42.9%) |
| Roma ISD | 1523 (7.6%) | 782 (7.6%) | 741 (7.7%) | 157 (7.3%) | 73 (6.8%) | 84 (7.8%) |
| Zapata ISD | 1011 (5.1%) | 481 (4.7%) | 530 (5.5%) | 58 (2.7%) | 29 (2.7%) | 29 (2.7%) |
| San Isidro ISD | 103 (0.5%) | 51 (0.5%) | 52 (0.5%) | 5 (0.2%) | 3 (0.3%) | 2 (0.2%) |
| Jim Hogg ISD | 341 (1.7%) | 154 (1.5%) | 187 (1.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| School District | ||||||
| Elementary | 6816 (342%) | 3644 (35.4%) | 3172 (32.8%) | 253 (1.8%) | 128 (11.9%) | 125 (11.7%) |
| Middle school | 12,079 (60.5%) | 6147(59.7%) | 5932 (61.4%) | 1683 (78.5%) | 838 (78.0%) | 845 (78.9%) |
| High school | 1056 (5.3%) | 498 (4.8%) | 558 (5.8%) | 209 (9.7%) | 108 (10.1%) | 101 (9.4%) |
| Number of Doses | ||||||
| 1 | 8154 (40.9%) | 3998 (38.9%) | 4156 (43.0%) | 1065 (49.7%) | 512 (47.7%) | 553 (51.6%) |
| 2 | 9595 (48.1%) | 5082 (49.4%) | 4513 (46.7%) | 911 (42.5%) | 464 (43.2%) | 447 (41.7%) |
| 3+ | 2202 (11.0%) | 1209 (11.8%) | 993 (10.3%) | 169 (7.9%) | 98 (9.1%) | 71 (6.6%) |
| Received the Initial HPV Dose from Our Program | ||||||
| No | 18,433 (92.4%) | 9551 (92.8%) | 8882 (91.9%) | 627 (29.2%) | 336 (31.3%) | 291 (27.2%) |
| Yes | 1518 (7.6%) | 738 (7.2%) | 780 (8.1%) | 1518 (70.8%) | 738 (68.7%) | 780 (72.8%) |
| Received Other Vaccinations Bundled with HPV Vaccine 2 | ||||||
| No | Not available | Not available | Not available | 617 (29.2%) | 336 (31.3%) | 291 (27.2%) |
| Yes | Not available | Not available | Not available | 1528 (70.8%) | 738 (68.7%) | 780 (72.8%) |
| HPV-UTD 3 | ||||||
| No | 8220 (41.2%) | 4031 (39.2%) | 4189 (43.4%) | 1074 (50.1%) | 519 (48.3%) | 555 (51.8%) |
| Yes | 11,731 (58.8%) | 6258 (60.8%) | 5473 (56.6%) | 1071 (49.9%) | 555 (51.7%) | 516 (48.2%) |
| Age at HPV-UTD 2 | ||||||
| Mean (SD) | 12 (1.6) | 12 (1.6) | 12 (1.6) | 13 (1.4) | 13 (1.5) | 13 (1.4) |
| Median (min, max) | 12 (9, 20) | 11 (9, 19) | 12 (9, 20) | 12 (9, 19) | 12 (9, 19) | 13 (9, 18) |
| Days Between HPV Initiation and UTD | ||||||
| Mean (SD) | 403 (325.6) | 404 (330.0) | 401 (320.4) | 480 (405.1) | 478 (410.1) | 482 (400.2) |
| Median (min, max) | 291 (146, 2968) | 286 (146, 2967) | 300 (146, 2968) | 324 (146, 2855) | 310 (146, 2855) | 339 (150, 2843) |
| Variable | All Students Vaccinated Indirectly and Directly 1 (n = 19,951) | Students Vaccinated Directly (n = 2145) | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Age at initiation (1-year increase) | 0.676 (0.641–0.712) | <0.0001 | 0.626 (0.549–0.714) | <0.0001 | |
| Gender | Female | 1.000 | 1.000 | ||
| Male | 0.902 (0.833–0.976) | 0.0103 | 0.792 (0.642–0.976) | 0.0288 | |
| School grade at initiation | Elementary | 1.385 (1.219–1.573) | <0.0001 | 1.008 (0.676–1.505) | 0.9671 |
| Middle school | 1.000 | 1.000 | |||
| High school | 1.96 (1.097–1.776) | 0.0068 | 0.517 (0.285–0.937) | 0..0296 | |
| School district | RGCCISD | 1.000 | 1.000 | ||
| PSJA ISD | 1.205 (1.091–1.331) | 0.0002 | 1.141 (0.861–1.512) | 0.3589 | |
| Roma ISD | 0.623 (0.521–0.743) | <0.0001 | 1.679 (1.030–2.735) | 0.0375 | |
| San Isidro ISD | 1.445 (0.833–2.507) | 0.1904 | 0.929 (0.033–26.408) | 0.9658 | |
| Zapata ISD | 1.701 (1.422–2.034) | <0.0001 | 1.094 (0.373–3.212) | 0.8702 | |
| Jim Hogg ISD | 0.976 (0.741–1.285) | 0.8621 | |||
| Received the initial HPV dose through our program | Yes | 1.000 | |||
| No | 0.636 (0.544–0.743) | <0.0001 | |||
| Intervention year 2 | 2016 | 1.000 | |||
| 2017 | 0.694 (0.595–0.810) | <0.0001 | 1.000 | ||
| 2018 | 0.515 (0.442–0.601) | <0.0001 | 0.418 (0.199–0.882) | 0.022 | |
| 2019 | 0.226 (0.194–0.264) | <0.0001 | 0.226 (0.110–0.465) | <0.0001 | |
| 2020 | 0.142 (0.118–0.171) | <0.0001 | 0.089 (0.041–0.193) | <0.0001 | |
| 2021 | 0.046 (0.035–0.060) | <0.0001 | 0.050 (0.024–0.106) | <0.0001 | |
| 2022 | 0.007 (0.003–0.013) | <0.0001 | 0.008 (0.003–0.017) | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, A.M.; Do, T.Q.N.; Eyada, M.F.; Chen, L.; Schmeler, K.M.; Montealegre, J.R. Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion. Vaccines 2023, 11, 329. https://doi.org/10.3390/vaccines11020329
Rodriguez AM, Do TQN, Eyada MF, Chen L, Schmeler KM, Montealegre JR. Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion. Vaccines. 2023; 11(2):329. https://doi.org/10.3390/vaccines11020329
Chicago/Turabian StyleRodriguez, Ana M., Thuy Quynh N. Do, Mostafa F. Eyada, Lu Chen, Kathleen M. Schmeler, and Jane R. Montealegre. 2023. "Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion" Vaccines 11, no. 2: 329. https://doi.org/10.3390/vaccines11020329
APA StyleRodriguez, A. M., Do, T. Q. N., Eyada, M. F., Chen, L., Schmeler, K. M., & Montealegre, J. R. (2023). Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion. Vaccines, 11(2), 329. https://doi.org/10.3390/vaccines11020329






