Antibody and T-Cell Responses against SARS-CoV-2 after Booster Vaccination in Patients on Dialysis: A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Outcome Measures
2.3. Serological Assay
2.4. T Cell Response Measurement
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
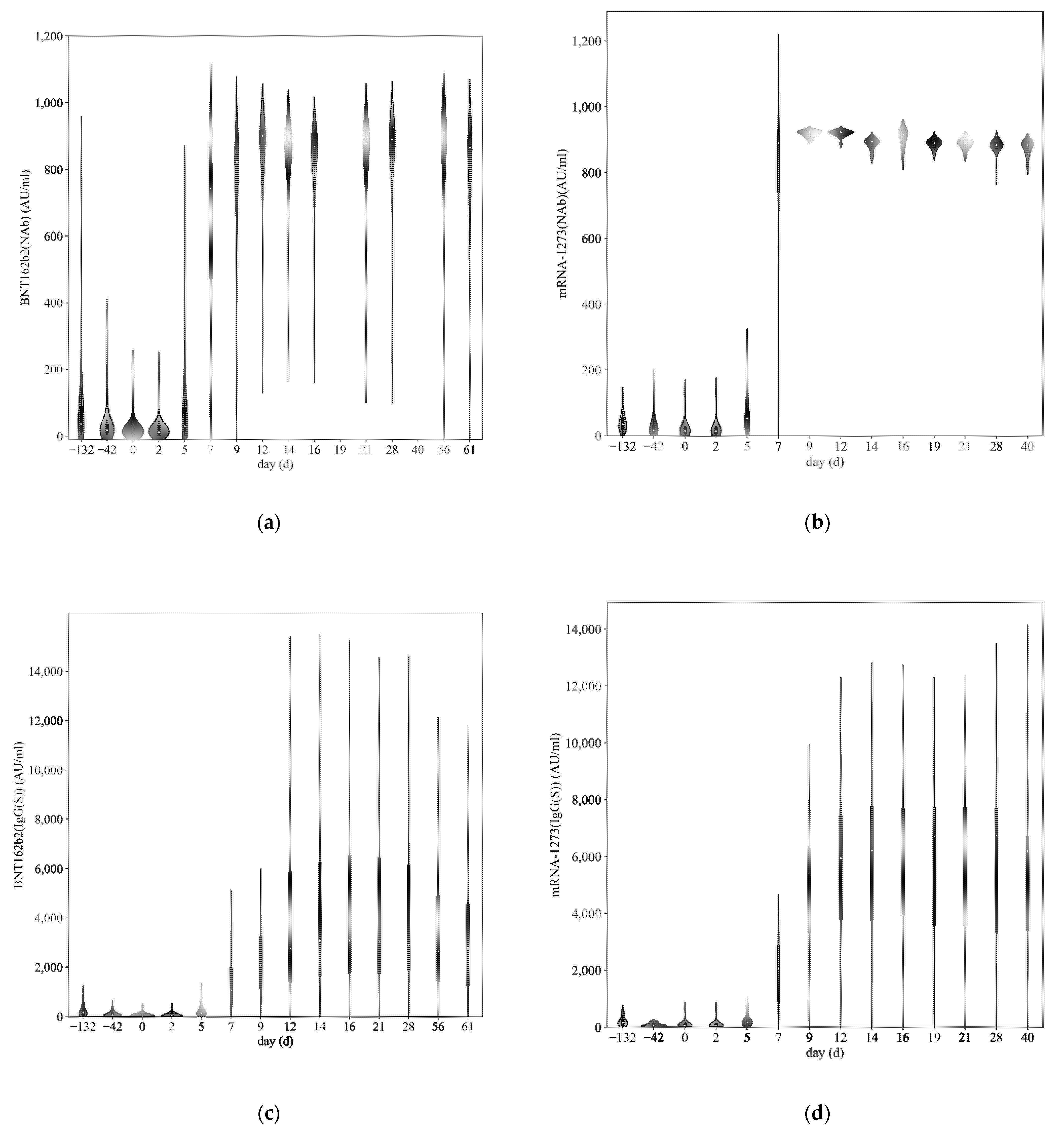
3.2. SARS-CoV-2 Antibody Titer
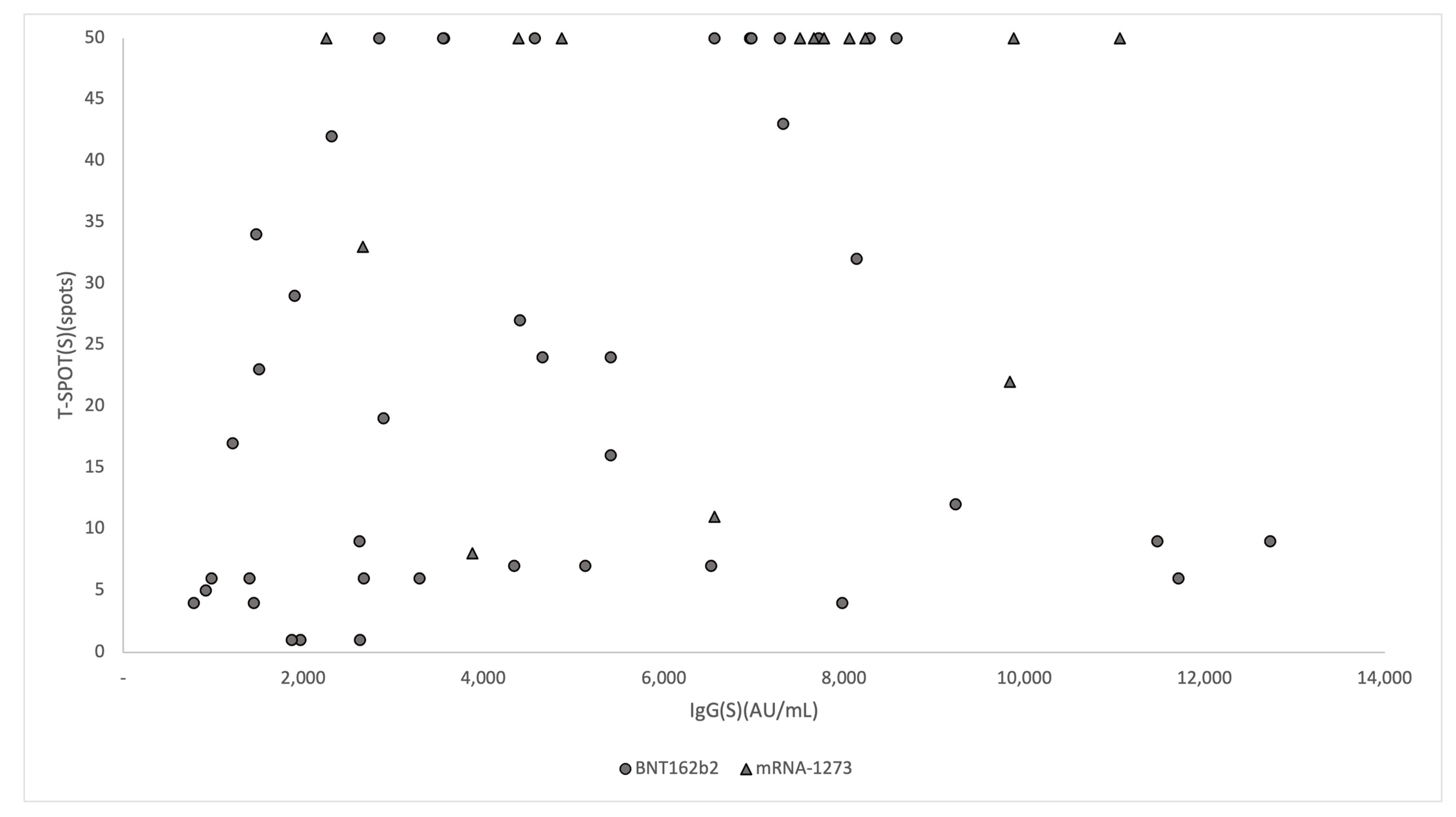
3.3. Association of IgG(S) Antibody Titers with T-SPOT
3.4. Factors Associated with Maximum IgG(S) Antibody Titer
4. Discussion
- (1)
- To collect a larger number of dialysis cases;
- (2)
- To measure immunity over time; and
- (3)
- To observe the dynamics of immunity after further additional vaccination among dialysis patients.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razzaghi, H.; Wang, Y.; Lu, H.; Marshall, K.E.; Dowling, N.F.; Paz-Bailey, G.; Twentyman, E.R.; Peacock, G.; Greenlund, K.J. Estimated County-Level Prevalence of Selected Underlying Medical Conditions Associated with Increased Risk for Severe COVID-19 Illness-United States, 2018. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 945–950. [Google Scholar] [CrossRef]
- Hoxha, E.; Suling, A.; Turner, J.E.; Haubitz, M.; Floege, J.; Huber, T.B.; Galle, J.C. COVID-19 Prevalence and Mortality in Chronic Dialysis Patients. Dtsch. Arztebl. Int. 2021, 118, 195–196. [Google Scholar] [CrossRef]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef]
- Corbett, R.W.; Blakey, S.; Nitsch, D.; Loucaidou, M.; McLean, A.; Duncan, N.; Ashby, D.R.; West London, R.; Transplant, C. Epidemiology of COVID-19 in an Urban Dialysis Center. J. Am. Soc. Nephrol. 2020, 31, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Klein, K.; Morath, C.; Bartenschlager, M.; Kim, H.; Buylaert, M.; Reineke, M.; Tollner, M.; Nusshag, C.; Kalble, F.; et al. Neutralizing Antibody Activity Against the B.1.617.2 (delta) Variant Before and After a Third BNT162b2 Vaccine Dose in Hemodialysis Patients. Front. Immunol. 2022, 13, 840136. [Google Scholar] [CrossRef] [PubMed]
- Shashar, M.; Nacasch, N.; Grupper, A.; Benchetrit, S.; Halperin, T.; Erez, D.; Rozenberg, I.; Shitrit, P.; Sela, Y.; Wand, O.; et al. Humoral Response to Pfizer BNT162b2 Vaccine Booster in Maintenance Hemodialysis Patients. Am. J. Nephrol. 2022, 53, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Stockinger, R.; Sprenger-Mahr, H.; Lhotta, K.; Zitt, E. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: Time for a boost. Kidney Int. 2021, 100, 1334–1335. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.S.F.; Lianne Messchendorp, A.; de Vries, R.D.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Geers, D.; Schmitz, K.S.; van Kessel, C.H.G.; et al. Antibody and T-cell responses 6 months after COVID-19 mRNA-1273 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Clin. Infect. Dis. 2022, ciac557. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Bao, W.; Fu, S.; Jin, H. Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 2070. [Google Scholar] [CrossRef]
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 13 July 2022).
- Digital Agency. Vaccination Status against COVID-19. Available online: https://info.vrs.digital.go.jp/dashboard (accessed on 30 August 2022).
- Kobashi, Y.; Nishikawa, Y.; Kawamura, T.; Kodama, T.; Shimazu, Y.; Obara, D.; Zhao, T.; Tsubokura, M. Seroprevalence of SARS-CoV-2 antibodies among hospital staff in rural Central Fukushima, Japan: A historical cohort study. Int. Immunopharmacol. 2021, 98, 107884. [Google Scholar] [CrossRef]
- Kobashi, Y.; Shimazu, Y.; Nishikawa, Y.; Kawamura, T.; Kodama, T.; Obara, D.; Tsubokura, M. The difference between IgM and IgG antibody prevalence in different serological assays for COVID-19; lessons from the examination of healthcare workers. Int. Immunopharmacol. 2021, 92, 107360. [Google Scholar] [CrossRef]
- Kobashi, Y.; Shimazu, Y.; Kawamura, T.; Nishikawa, Y.; Omata, F.; Kaneko, Y.; Kodama, T.; Tsubokura, M. Factors associated with anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein antibody titer and neutralizing activity among healthcare workers following vaccination with the BNT162b2 vaccine. PLoS ONE 2022, 17, e0269917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Nishi-Uchi, T.; Omata, F.; Takita, M.; Kawashima, M.; Nishikawa, Y.; Yamamoto, C.; Kobashi, Y.; Kawamura, T.; Shibuya, K.; et al. Humoral response to SARS-CoV-2 vaccination in haemodialysis patients and a matched cohort. BMJ Open 2022, 12, e065741. [Google Scholar] [CrossRef] [PubMed]
- Fucci, A.; Giacobbe, S.; Guerriero, I.; Suzumoto, Y.; D’Andrea, E.L.; Scrima, M.; Nolli, M.L.; Iervolino, A.; Chiuchiolo, L.A.; Salvatore, E.; et al. The DiaCoVAb Study in South Italy: Immune Response to SARS-CoV-2 Vaccination in Dialysis Patients. Kidney Blood Press. Res. 2022, 47, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Bruminhent, J.; Setthaudom, C.; Kitpermkiat, R.; Kiertiburanakul, S.; Malathum, K.; Assanatham, M.; Nongnuch, A.; Phuphuakrat, A.; Chaumdee, P.; Janphram, C.; et al. Immunogenicity of ChAdOx1 nCoV-19 vaccine after a two-dose inactivated SARS-CoV-2 vaccination of dialysis patients and kidney transplant recipients. Sci. Rep. 2022, 12, 3587. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.J.; Wu, M.; Harvey, R.; Billany, R.E.; Wall, E.C.; Kelly, G.; Haemodialysis Covid-19 Consortium, C.C.I.P.; Howell, M.; Kassiotis, G.; Swanton, C.; et al. Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients. Lancet 2022, 399, 800–802. [Google Scholar] [CrossRef]
- Lau, C.S.; Phua, S.K.; Liang, Y.L.; Oh, M.L.H.; Aw, T.C. SARS-CoV-2 Spike and Neutralizing Antibody Kinetics 90 Days after Three Doses of BNT162b2 mRNA COVID-19 Vaccine in Singapore. Vaccines 2022, 10, 331. [Google Scholar] [CrossRef]
- Bachelet, T.; Bourdenx, J.P.; Martinez, C.; Mucha, S.; Martin-Dupont, P.; Perier, V.; Pommereau, A. Humoral response after SARS-CoV-2 mRNA vaccines in dialysis patients: Integrating anti-SARS-CoV-2 Spike-Protein-RBD antibody monitoring to manage dialysis centers in pandemic times. PLoS ONE 2021, 16, e0257646. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Housset, P.; Kubab, S.; Hanafi, L.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Caudwell, V.; Faucon, A.L. Humoral response after a fourth “booster” dose of a Coronavirus disease 2019 vaccine following a 3-dose regimen of mRNA-based vaccination in dialysis patients. Kidney Int. 2022, 101, 1289–1290. [Google Scholar] [CrossRef]
- Falsey, A.R.; Frenck, R.W.J.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Bailey, R.; Swanson, K.A.; Xu, X.; et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Pross, V.; Bergmann, Y.; et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021, 131, 150175. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemee, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef]
- Perez-Alos, L.; Armenteros, J.J.A.; Madsen, J.R.; Hansen, C.B.; Jarlhelt, I.; Hamm, S.R.; Heftdal, L.D.; Pries-Heje, M.M.; Moller, D.L.; Fogh, K.; et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat. Commun. 2022, 13, 1614. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.T.; Harding, A.C.; Gilbert-Jaramillo, J.; Knight, M.L.; Longet, S.; Brown, A.; Adele, S.; Adland, E.; Brown, H.; Medawar Laboratory, T.; et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat. Commun. 2021, 12, 5061. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of COVID-19 mRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.J.; Thomas, D.; Balamchi, S.; Ip, J.; Naylor, K.; Dixon, S.N.; McArthur, E.; Kwong, J.; Perl, J.; Atiquzzaman, M.; et al. Vaccine Effectiveness Against SARS-CoV-2 Infection and Severe Outcomes in the Maintenance Dialysis Population in Ontario, Canada. J. Am. Soc. Nephrol. 2022, 33, 839–849. [Google Scholar] [CrossRef] [PubMed]
| Total Dialysis Patients (n = 58) | 3rd Vaccine Type | |||
|---|---|---|---|---|
| BNT162b2 (n = 43) | mRNA-1273 (n = 15) | p-Value | ||
| Age (years, median [IQR]) | 71 (48–89) | 72.7 (51–89) | 66 (50–84) | |
| Females; N (%) * | 18 (31.0) | 0.03 | ||
| Interval between 2nd and 3rd vaccinations | 232 (198–252) | 231 (198–238) | 236 (219–252) | |
| Medical history; N (%) | ||||
| Hypertension | 53 (91.4) | 40 (93.0) | 13 (86.7) | 0.90 |
| Hyperlipidemia ** | 7 (12.0) | 3 (7.0) | 4 (26.7) | <0.01 |
| Bronchial asthma | 1 (1.7) | 1 (2.3) | 0 (0.0) | 0.23 |
| Diabetes | 27 (48.5) | 20 (46.5) | 7 (46.7) | 0.06 |
| Cardiovascular disease | 8 (19.1) | 8 (18.6) | 0 (0.0) | 0.89 |
| Gout ** | 5 (8.8) | 3 (7.0) | 2 (13.3) | <0.01 |
| Anaphylaxis | 1 (1.7) | 1 (2.3) | 0 (0.0) | 0.23 |
| Respiratory disease | 3 (5.2) | 2 (4.7) | 1 (6.7) | 0.58 |
| Rheumatoid arthritis | 1 (1.7) | 1 (2.3) | 0 (0.0) | 0.23 |
| Medications; N (%) | ||||
| Antihistamine | 8 (13.8) | 6 (14.0) | 2 (13.3) | 0.25 |
| NSAIDs | 2 (3.4) | 2 (4.7) | 0 (0.0) | 0.23 |
| Steroids | 2 (3.4) | 2 (4.7) | 0 (0.0) | 0.23 |
| Acetaminophen ** | 4 (6.9) | 4 (9.3) | 0 (0.0) | <0.01 |
| Antitumor agents ** | 1 (1.7) | 0 (0.0) | 1 (6.7) | <0.01 |
| Adverse reaction (3rd vaccination); N (%) | ||||
| Pain * | 9 (15.5) | 5 (11.6) | 4 (26.7) | 0.02 |
| Malaise | 5 (8.6) | 3 (7.0) | 2 (13.3) | 0.17 |
| Joint pain ** | 41 (70.7) | 29 (67.4) | 12 (80.0) | <0.01 |
| Fever (≥37.5 °C) ** | 12 (20.7) | 6 (14.0) | 6 (40.0) | <0.01 |
| Headache | 5 (8.6) | 4 (9.3) | 1 (6.7) | 0.50 |
| Fever (<37.5 °C) | 6 (10.3) | 4 (9.3) | 2 (13.3) | 0.43 |
| Nausea | 1 (1.7) | 1 (2.3) | 0 (0.0) | 0.23 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Coef (95% CI) | p-Value | Coef (95% CI) | p-Value | |
| Age (years) * | −81.8 (−159–4.99) | 0.04 | −32.02 (−123–58.87) | 0.48 |
| Females | 939 (−918–2796) | 0.32 | 962 (−905–2829) | 0.31 |
| 3rd Vaccine type(BNT162b2) | 1.48 (−484–3234) | 0.14 | 1000 (−987–2986) | 0.32 |
| Medications | ||||
| Antihistamine | 14.69 (−6.77–36.15) | 0.18 | ||
| Steroids | −3111 (−7678–1456) | 0.18 | ||
| Acetaminophen | −125 (−3472–3223) | 1.00 | ||
| Antitumor agents | 1265 (−5236–7765) | 0.70 | ||
| Medical history | ||||
| Hypertension | −1537 (−4858–1784) | 0.36 | ||
| Hyperlipidemia | 395 (−2210–2999) | 0.76 | ||
| Bronchial asthma | 2040 (−4446–8525) | 0.53 | ||
| Diabetes | 862 (−850–2575) | 0.32 | ||
| Cardiovascular disease | −672 (−3128–1785) | 0.59 | ||
| Gout | 212 (−2810–3235) | 0.89 | ||
| Anaphylaxis | −1791 (−8283–4699) | 0.58 | ||
| Respiratory disease | 2335 (−1440–6110) | 0.22 | ||
| Rheumatoid arthritis | −4620 (−11,000–1766) | 0.15 | ||
| Adverse reaction (3rd vaccination) | ||||
| Inoculation site reaction ** | 3025 (828–5223) | <0.01 | ||
| Malaise | 2453 (−495–5401) | 0.10 | ||
| Joint pain, myalgia | 1147 (−735–3029) | 0.23 | ||
| Fever (≥37.5 °C) * | 2505 (519–4492) | 0.01 | ||
| Headache | 786 (−2229–3801) | 0.60 | ||
| Fever (<37.5 °C) | −251 (−3037–2536) | 0.86 | ||
| Nausea | 3990 (−2427–10,400) | 0.22 | ||
| Presence of systemic adverse effects | 1373 (−537–3283) | 0.16 | 886 (−1117–2890) | 0.38 |
| Presence of local adverse effects | 1657 (−75.57–3389) | 0.06 | 1291 (−577–3159) | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawashima, M.; Saito, H.; Nishiuchi, T.; Yoshimura, H.; Wakui, M.; Tani, Y.; Nishikawa, Y.; Omata, F.; Takita, M.; Zhao, T.; et al. Antibody and T-Cell Responses against SARS-CoV-2 after Booster Vaccination in Patients on Dialysis: A Prospective Observational Study. Vaccines 2023, 11, 260. https://doi.org/10.3390/vaccines11020260
Kawashima M, Saito H, Nishiuchi T, Yoshimura H, Wakui M, Tani Y, Nishikawa Y, Omata F, Takita M, Zhao T, et al. Antibody and T-Cell Responses against SARS-CoV-2 after Booster Vaccination in Patients on Dialysis: A Prospective Observational Study. Vaccines. 2023; 11(2):260. https://doi.org/10.3390/vaccines11020260
Chicago/Turabian StyleKawashima, Moe, Hiroaki Saito, Takamitsu Nishiuchi, Hiroki Yoshimura, Masatoshi Wakui, Yuta Tani, Yoshitaka Nishikawa, Fumiya Omata, Morihito Takita, Tianchen Zhao, and et al. 2023. "Antibody and T-Cell Responses against SARS-CoV-2 after Booster Vaccination in Patients on Dialysis: A Prospective Observational Study" Vaccines 11, no. 2: 260. https://doi.org/10.3390/vaccines11020260
APA StyleKawashima, M., Saito, H., Nishiuchi, T., Yoshimura, H., Wakui, M., Tani, Y., Nishikawa, Y., Omata, F., Takita, M., Zhao, T., Yamamoto, C., Kobashi, Y., Kawamura, T., Sugiyama, A., Nakayama, A., Kaneko, Y., Sawano, T., Shibuya, K., Kazama, J., ... Tsubokura, M. (2023). Antibody and T-Cell Responses against SARS-CoV-2 after Booster Vaccination in Patients on Dialysis: A Prospective Observational Study. Vaccines, 11(2), 260. https://doi.org/10.3390/vaccines11020260





