Impact of the COVID-19 Pandemic on HPV Vaccinations in Switzerland and Greece: Road to Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Population
2.1.1. Switzerland
2.1.2. Greece
2.2. Data Sources
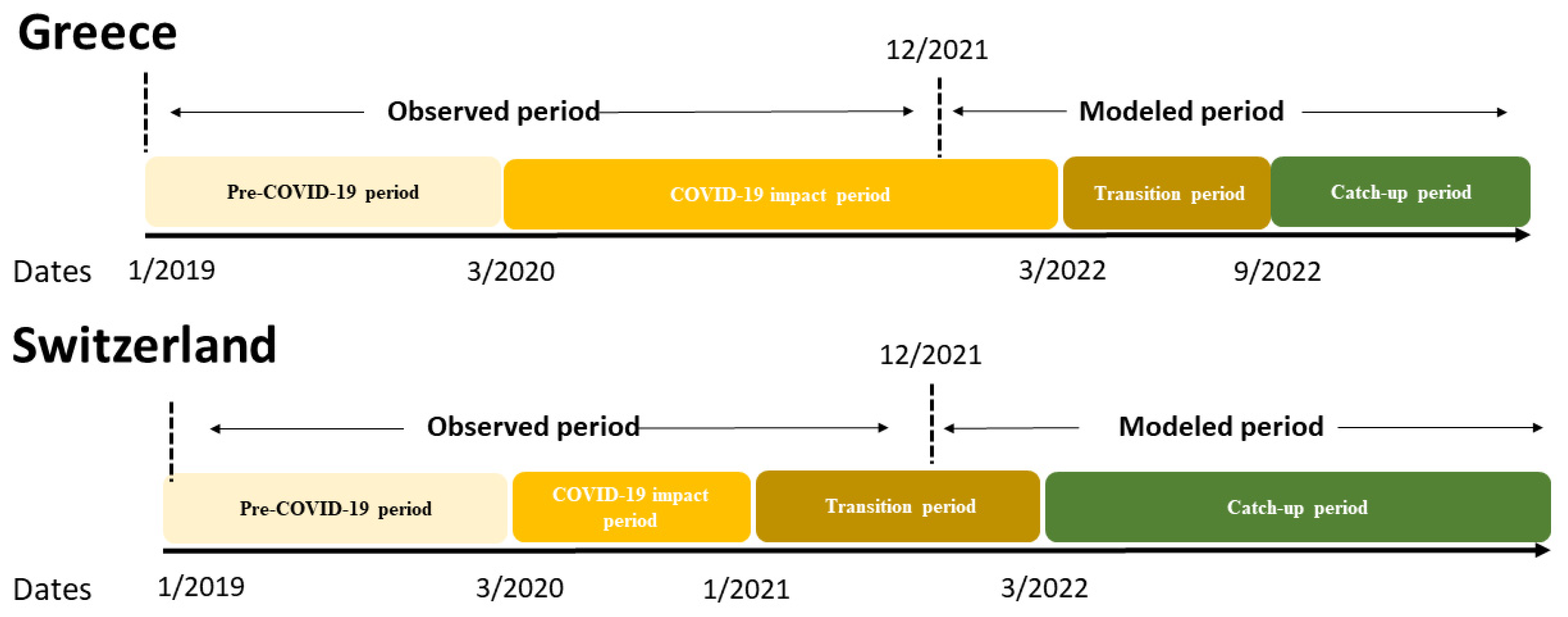
2.3. The COVID-19 Recovery Tool
2.4. Estimating the HPV Dose Deficit
2.5. Examined Scenarios
3. Results
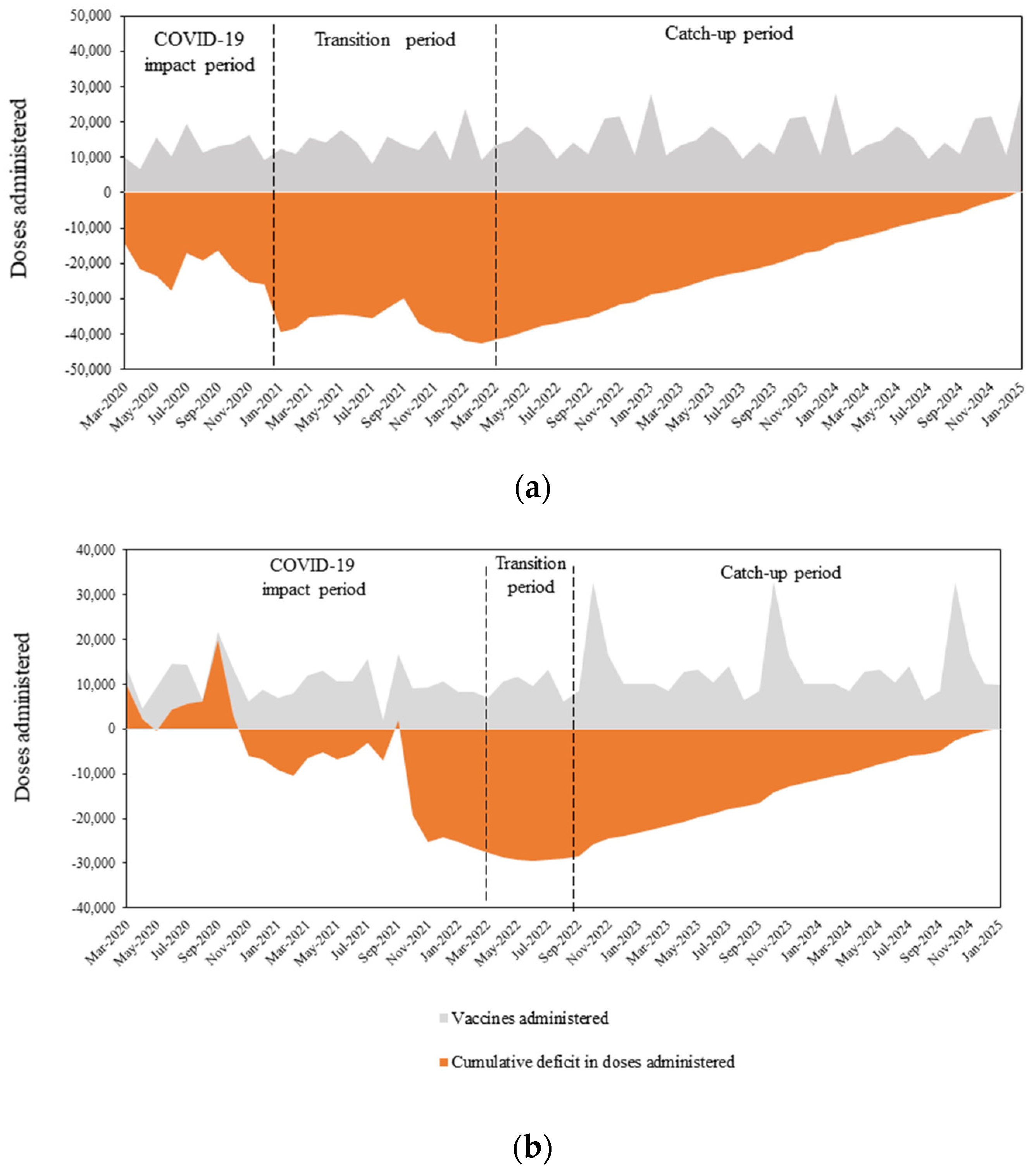
3.1. HPV Vaccination Deficit Accumulation
3.1.1. Switzerland
3.1.2. Greece
3.2. Cumulative Deficit of HPV Vaccine Doses
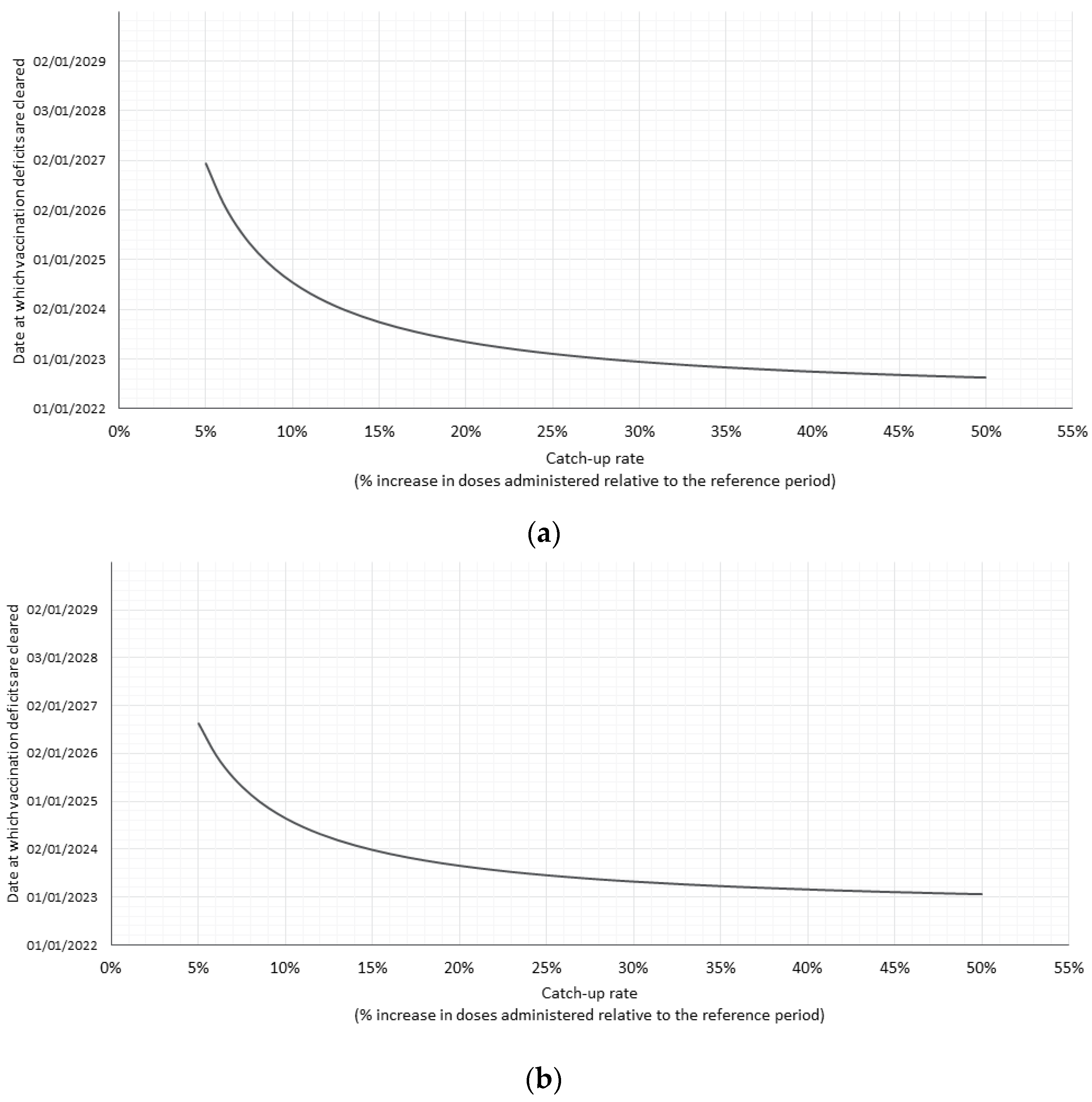
3.3. Catch-Up Rate Scenarios
4. Discussion
4.1. Study Implications
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.; Giuliano, A.; Iversen, O.-E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.M., Jr.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.-N.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R.; et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.; Hernandez-Avila, M.; Wheeler, C.; Perez, G.; Harper, D.; Leodolter, S.; Tang, G.; Ferris, D.; Steben, M.; Bryan, J.; et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007, 356, 1928–1943. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.; Faivre, P.; Lopalco, P.L.; Joura, E.; Bergroth, T.; Varga, S.; Gemayel, N.; Drury, R. The status of human papillomavirus vaccination recommendation, funding, and coverage in WHO Europe countries (2018–2019). Expert Rev. Vaccines 2020, 19, 1073–1083. [Google Scholar] [CrossRef]
- Colzani, E.; Johansen, K.; Johnson, H.; Pastore Celentano, L. Human papillomavirus vaccination in the European Union/European Economic Area and globally: A moral dilemma. Eurosurveillance 2021, 26, 2001659. [Google Scholar] [CrossRef]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. 2020. Available online: who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-may-2020 (accessed on 2 September 2022).
- Shet, A.; Carr, K.; Danovaro-Holliday, M.C.; Sodha, S.; Prosperi, C.; Wunderlich, J.; Wonodi, C.; Reynolds, H.; Mirza, I.; Gacic-Dobo, M.; et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: Evidence of disruption and recovery from 170 countries and territories. Lancet Glob. Health 2022, 10, e186–e194. [Google Scholar] [CrossRef]
- WHO. WHO and UNICEF Warn of a Decline in Vaccinations during COVID-19. 2020. Available online: https://www.who.int/news/item/15-07-2020-who-and-unicef-warn-of-a-decline-in-vaccinations-during-covid-19 (accessed on 2 September 2022).
- Sato, A.P.S. Pandemic and vaccine coverage: Challenges of returning to schools. Rev. De Saúde Pública 2020, 54, 115. [Google Scholar] [CrossRef]
- SeyedAlinaghi, S.; Karimi, A.; Mojdeganlou, H.; Alilou, S.; Mirghaderi, S.P.; Noori, T.; Shamsabadi, A.; Dadras, O.; Vahedi, F.; Mohammadi, P.; et al. Impact of COVID-19 pandemic on routine vaccination coverage of children and adolescents: A systematic review. Health Sci. Rep. 2022, 5, e00516. [Google Scholar] [CrossRef] [PubMed]
- Langdon-Embry, M.; Papadouka, V.; Cheng, I.; Almashhadani, M.; Ternier, A.; Zucker, J.R. Notes from the field: Rebound in routine childhood vaccine administration following decline during the COVID-19 pandemic—New York City, 1 March–27 June 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 999. [Google Scholar] [CrossRef]
- Santoli, J.M.L.M.; DeSilva, M.B. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration —United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 591–593. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. COVID-19 Pandemic Leads to Major Backsliding on Childhood Vaccinations; New WHO, UNICEF Data Shows; UNICEF: New York, NY, USA, 2021. [Google Scholar]
- Saxena, S.; Skirrow, H.; Bedford, H. Routine vaccination during COVID-19 pandemic response. BMJ 2020, 369, m2392. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein Hagander, K.; Aronsson, B.; Danielsson, M.; Lepp, T.; Kulane, A.; Schollin Ask, L. National Swedish survey showed that child health services and routine immunisation programmes were resilient during the early COVID-19 pandemic. Acta Paediatr. 2021, 110, 2559–2566. [Google Scholar] [CrossRef]
- Daniels, V.; Saxena, K.; Roberts, C.; Kothari, S.; Corman, S.; Yao, L.; Niccolai, L. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: A model based analysis. Vaccine 2021, 39, 2731–2735. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guiding Principles for Immunization Activities during the COVID-19 Pandemic. 2020. Available online: https://apps.who.int/iris/handle/10665/331590 (accessed on 2 September 2022).
- Saxena, K.; Marden, J.R.; Carias, C.; Bhatti, A.; Patterson-Lomba, O.; Gomez-Lievano, A.; Yao, L. Impact of the COVID-19 pandemic on adolescent vaccinations: Projected time to reverse deficits in routine adolescent vaccination in the United States. Curr. Med. Res. Opin. 2021, 37, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- MoH. G Σύσταση της Εθνικής Επιτροπής Εμβολιασμών για τον εμβολιασμό αγοριών και κοριτσιών έναντι του ιού των ανθρωπίνων θηλωμάτων (in Greek). 2022. Available online: https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko-programma-emboliasmwn-epe-paidiwn-kai-efhbwn/10314-systash-ths-ethnikhs-epitrophs-emboliasmwn-gia-ton-emboliasmo-agoriwn-kai-koritsiwn-enanti-toy-ioy-twn-anthrwpinwn-thhlwmatwn (accessed on 23 August 2022).
- Bundesamt für Gesundheit BAG - Abteilung Übertragbare Krankheiten. Humane Papillomaviren (HPV). 2022. Available online: https://www.bag.admin.ch/bag/en/home/krankheiten/krankheiten-im-ueberblick/hpv.html (accessed on 23 August 2022).
- Riesen, M.; Konstantinoudis, G.; Lang, P.; Low, N.; Hatz, C.; Maeusezahl, M.; Spaar, A.; Bühlmann, M.; Spycher, B.; Althaus, C. Exploring variation in human papillomavirus vaccination uptake in Switzerland: A multilevel spatial analysis of a national vaccination coverage survey. BMJ Open 2018, 8, e021006. [Google Scholar] [CrossRef]
- MoH. Greek Vaccination Program for Children and Adolescents. 2020. Available online: https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko-programma-emboliasmwn-epe-paidiwn-kai-efhbwn/7246-programma-emboliasmwn-paidiwn-efhbwn-2020 (accessed on 16 January 2022).
- IQVIA. IQVIA Sell-In data for Channels: Pharmacies, SD-Physicians, Hospitals. Data Release: April 2022. 2022. Available online: https://www.iqvia.com/insights/the-iquivia-institute/available-iqvia-data (accessed on 17 May 2022).
- Zeitouny, S.; Suda, K.J.; Mitsantisuk, K.; Law, M.R.; Tadrous, M. Mapping global trends in vaccine sales before and during the first wave of the COVID-19 pandemic: A cross-sectional time-series analysis. BMJ Glob. Health 2021, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Gountas, I.; Hillas, G.; Souliotis, K. Act early, save lives: Managing COVID-19 in Greece. Public Health 2020, 187, 136–139. [Google Scholar] [CrossRef] [PubMed]
- WHO. Varied Impact of COVID-19 on Routine Immunization in the European Region. 2021. Available online: https://www.who.int/europe/news/item/16-07-2021-varied-impact-of-covid-19-on-routine-immunization-in-the-european-region (accessed on 25 August 2022).
- Rao, S.R.; Kampan, N.; Chew, K.T.; Shafiee, M.N. The impact of the COVID-19 pandemic on the national HPV immunization program in Malaysia. Front. Public Health 2022, 10, 907720. [Google Scholar] [CrossRef]
- ANSM. Use of Community Drugs in France during the COVID-19 Epidemic: Update until April 25, 2021. 2021. Available online: https://ansm.sante.fr/actualites/usage-des-medicaments-de-ville-en-france-durant-lepidemie-de-covid-19-point-de-situation-jusquau-25-avril-2021 (accessed on 25 August 2022).
- Mennini, F.S.; Silenzi, A.; Marcellusi, A.; Conversano, M.; Siddu, A.; Rezza, G. HPV Vaccination during the COVID-19 Pandemic in Italy: Opportunity Loss or Incremental Cost. Vaccines 2022, 10, 1133. [Google Scholar] [CrossRef]
- Bundesamt für Gesundheit BAG. Informationsmagazin für Medizinische Fachpersonen und Medienschaffende. 2021. Available online: https://www.bag.admin.ch/dam/bag/de/dokumente/cc/Kampagnen/Bulletin/2021/bu-16-21.pdf.download.pdf/BU_16_21_DE.pdf (accessed on 25 August 2022).
- Mancarella, M.; Natarelli, F.; Bertolini, C.; Zagari, A.; Enrica Bettinelli, M.; Castaldi, S. Catch-up vaccination campaign in children between 6 and 8 years old during COVID-19 pandemic: The experience in a COVID hub in Milan, Italy. Vaccine 2022, 40, 3664–3669. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Kennedy, A.M.; Wooten, K.; Gust, D.A.; Pickering, L.K. Association between health care providers’ influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics 2006, 118, e1287–e1292. [Google Scholar] [CrossRef] [PubMed]
- Kempe, A.; Saville, A.W.; Dickinson, L.M.; Beaty, B.; Eisert, S.; Gurfinkel, D.; Brewer, S.; Shull, H.; Herrero, D.; Herlihy, R. Collaborative centralized reminder/recall notification to increase immunization rates among young children: A comparative effectiveness trial. JAMA Pediatr. 2015, 169, 365–373. [Google Scholar] [CrossRef]
- Hofstetter, A.M.; DuRivage, N.; Vargas, C.Y.; Camargo, S.; Vawdrey, D.; Fisher, A.; Stockwell, M. Text message reminders for timely routine MMR vaccination: A randomized controlled trial. Vaccine 2015, 33, 5741–5746. [Google Scholar] [CrossRef]
- Bigaard, J.; Franceschi, S. Vaccination against HPV: Boosting coverage and tackling misinformation. Mol. Oncol. 2021, 15, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Greek MoH. Greek National Immunization Program 2022. Available online: https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko-programma-emboliasmwn-epe-paidiwn-kai-efhbwn/10289-ethniko-programma-emboliasmwn-paidiwn-kai-efhbwn-2022 (accessed on 23 August 2022).
- Hutton, D.W.; Brandeau, M.L. Too much of a good thing? When to stop catch-up vaccination. Med. Decis. Mak. 2013, 33, 920–936. [Google Scholar] [CrossRef] [PubMed]
- Lindley, M.C.; Boyer-Chu, L.; Fishbein, D.B.; Kolasa, M.; Middleman, A.; Wilson, T.; Wolicki, J.; Wooley, S.; Working Group on the Role of Schools in Delivery of Adolescent Vaccinations. The role of schools in strengthening delivery of new adolescent vaccinations. Pediatrics 2008, 121 (Suppl. S1), S46–S54. [Google Scholar] [CrossRef]
- EFPIA. EFPIA and Vaccines Europe Position on the European Health Emergency Preparedness and Response Authority (HERA). 2022. Available online: https://www.efpia.eu/news-events/the-efpia-view/statements-press-releases/efpia-and-vaccines-europe-position-on-the-european-health-emergency-preparedness-and-response-authority-hera/ (accessed on 31 August 2022).
- NHS. Primary Care Network: ‘Drive Thru’ Services Offering a Safe Alternative to Immunisation Clinics. 2021. Available online: https://www.england.nhs.uk/gp/case-studies/drive-thru-services-offering-a-safe-alternative-to-immunisation-clinics/ (accessed on 31 August 2022).
- WHO. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. 2020. Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 11 July 2022).
- European Commission. Europe’s Beating Cancer Plan. 2021. Available online: https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf (accessed on 11 July 2022).
| Switzerland | Greece | |
|---|---|---|
| Accumulated deficit of doses at start of the transition period | NA 1 | 27,527 |
| Accumulated deficit of doses at start of the catch-up period | 42,680 | 31,000 |
| Accumulated deficit at start of the catch-up period as % of total annual doses administered during 2019 | 24.4% | 21.7% |
| Accumulated deficit as a function of the number of months of 2019 2 | 2.9 | 2.6 |
| Switzerland | Greece | |
|---|---|---|
| Base case: End of 2024 | ||
| Additional number of HPV doses per month that must be administered compared to 2019 | 1226 | 975 |
| Catch-up as % of total annual doses administered during 2019 | 8.4% | 8.2% |
| Optimistic scenario: End of 2023 | ||
| Additional number of HPV doses per month that must be administered compared to 2019 | 1840 | 1545 |
| Catch-up as % of total annual doses administered during 2019 | 12.6% | 13% |
| Pessimistic scenario: End of 2025 | ||
| Additional number of HPV doses per month that must be administered compared to 2019 | 930 | 715 |
| Catch-up as % of total annual doses administered during 2019 | 6.3% | 6.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gountas, I.; Favre-Bulle, A.; Saxena, K.; Wilcock, J.; Collings, H.; Salomonsson, S.; Skroumpelos, A.; Sabale, U. Impact of the COVID-19 Pandemic on HPV Vaccinations in Switzerland and Greece: Road to Recovery. Vaccines 2023, 11, 258. https://doi.org/10.3390/vaccines11020258
Gountas I, Favre-Bulle A, Saxena K, Wilcock J, Collings H, Salomonsson S, Skroumpelos A, Sabale U. Impact of the COVID-19 Pandemic on HPV Vaccinations in Switzerland and Greece: Road to Recovery. Vaccines. 2023; 11(2):258. https://doi.org/10.3390/vaccines11020258
Chicago/Turabian StyleGountas, Ilias, Andrea Favre-Bulle, Kunal Saxena, Jessica Wilcock, Hannah Collings, Stina Salomonsson, Anastasios Skroumpelos, and Ugne Sabale. 2023. "Impact of the COVID-19 Pandemic on HPV Vaccinations in Switzerland and Greece: Road to Recovery" Vaccines 11, no. 2: 258. https://doi.org/10.3390/vaccines11020258
APA StyleGountas, I., Favre-Bulle, A., Saxena, K., Wilcock, J., Collings, H., Salomonsson, S., Skroumpelos, A., & Sabale, U. (2023). Impact of the COVID-19 Pandemic on HPV Vaccinations in Switzerland and Greece: Road to Recovery. Vaccines, 11(2), 258. https://doi.org/10.3390/vaccines11020258






