Vaccine Formulation Strategies and Challenges Involved in RNA Delivery for Modulating Biomarkers of Cardiovascular Diseases: A Race from Laboratory to Market
Abstract
1. Introduction
2. Therapeutics
2.1. Addressing Pressing Issues of CVD Using miRNA Inhibitors
2.2. Antisense Oligonucleotides (ASO)
2.3. siRNAs and other Inhibitory Techniques
2.4. Antagomirs as Therapeutic Agents
2.5. CVD Treatment with lncRNA Inhibition
3. Pharmaceutics of RNA-Based Vaccine Delivery
3.1. Hurdles in the Systemic Delivery of siRNA
3.2. Stability in the Circulatory System
3.3. Vascular Endothelium as the Semiselective Barrier
3.4. Extracellular Matrix Diffusion
3.5. Cytoplasmic Delivery
4. Delivery Strategies to Improve the Targeting and Therapeutic Efficacy of Non-Coding RNA-Based Vaccines
4.1. Lipid and Lipid-Based Nanoparticle Vaccines
4.2. Polymer-Based Nanoparticle Vaccines
4.3. Device-Based Methods for RNA Vaccines
4.4. RNA Viral Vector-Mediated Vaccine Delivery
4.5. Vaccine Based on Tissue Enrichment via RNA Therapeutics Modification
4.6. miRNA Encapsulation
5. Challenges Associated with RNA Vaccine Therapy
6. Six Qualities of Vaccine Delivery Systems to Be Clinically Relevant
7. Clinical Angle
8. Challenges Associated with Long Non-Coding RNA Therapeutics
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Small, E.M.; Olson, E.N. Pervasive Roles of MicroRNAs in Cardiovascular Biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. Correction: MicroRNAs: Small RNAs with a Big Role in Gene Regulation. Nat. Rev. Genet. 2004, 5, 631. [Google Scholar] [CrossRef]
- Thum, T. MicroRNA Therapeutics in Cardiovascular Medicine. EMBO Mol. Med. 2012, 4, 3–14. [Google Scholar] [CrossRef]
- Boon, R.A.; Dimmeler, S. MicroRNAs in Myocardial Infarction. Nat. Rev. Cardiol. 2015, 12, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The Landscape of Long Noncoding RNAs in the Human Transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef]
- Boon, R.A.; Jaé, N.; Holdt, L.; Dimmeler, S. Long Noncoding RNAs: From Clinical Genetics to Therapeutic Targets? J. Am. Coll. Cardiol. 2016, 67, 1214–1226. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, D.P. LincRNAs: Genomics, Evolution, and Mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef]
- Perry, R.B.-T.; Ulitsky, I. The Functions of Long Noncoding RNAs in Development and Stem Cells. Development 2016, 143, 3882–3894. [Google Scholar] [CrossRef]
- Van Rooij, E.; Olson, E.N. MicroRNA Therapeutics for Cardiovascular Disease: Opportunities and Obstacles. Nat. Rev. Drug Discov. 2012, 11, 860–872. [Google Scholar] [CrossRef]
- Hullinger, T.G.; Montgomery, R.L.; Seto, A.G.; Dickinson, B.A.; Semus, H.M.; Lynch, J.M.; Dalby, C.M.; Robinson, K.; Stack, C.; Latimer, P.A.; et al. Inhibition of MiR-15 Protects against Cardiac Ischemic Injury. Circ. Res. 2012, 110, 71–81. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of Neonatal and Adult Mammalian Heart Regeneration by the MiR-15 Family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef]
- Fiedler, J.; Jazbutyte, V.; Kirchmaier, B.C.; Gupta, S.K.; Lorenzen, J.; Hartmann, D.; Galuppo, P.; Kneitz, S.; Pena, J.T.G.; Sohn-Lee, C.; et al. MicroRNA-24 Regulates Vascularity after Myocardial Infarction. Circulation 2011, 124, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Boon, R.A.; Iekushi, K.; Lechner, S.; Seeger, T.; Fischer, A.; Heydt, S.; Kaluza, D.; Tréguer, K.; Carmona, G.; Bonauer, A.; et al. MicroRNA-34a Regulates Cardiac Ageing and Function. Nature 2013, 495, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Gao, X.-M.; Winbanks, C.E.; Boey, E.J.H.; Tham, Y.K.; Kiriazis, H.; Gregorevic, P.; Obad, S.; Kauppinen, S.; Du, X.-J.; et al. Therapeutic Inhibition of the MiR-34 Family Attenuates Pathological Cardiac Remodeling and Improves Heart Function. Proc. Natl. Acad. Sci. USA 2012, 109, 17615–17620. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, H.-W.; Qiu, Y.; Dupee, D.; Noonan, M.; Lin, Y.-D.; Fisch, S.; Unno, K.; Sereti, K.-I.; Liao, R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ. Res. 2015, 117, 450–459. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef]
- Hinkel, R.; Penzkofer, D.; Zühlke, S.; Fischer, A.; Husada, W.; Xu, Q.F.; Baloch, E.; Van Rooij, E.; Zeiher, A.M.; Kupatt, C.; et al. Inhibition of MicroRNA-92a Protects against Ischemia/Reperfusion Injury in a Large-Animal Model. Circulation 2013, 128, 1066–1075. [Google Scholar] [CrossRef]
- Bellera, N.; Barba, I.; Rodriguez-Sinovas, A.; Ferret, E.; Asín, M.A.; Gonzalez-Alujas, M.T.; Pérez-Rodon, J.; Esteves, M.; Fonseca, C.; Toran, N.; et al. Single Intracoronary Injection of Encapsulated Antagomir-92a Promotes Angiogenesis and Prevents Adverse Infarct Remodeling. J. Am. Heart Assoc. 2014, 3, e000946. [Google Scholar] [CrossRef]
- Ma, S.; Tian, X.Y.; Zhang, Y.; Mu, C.; Shen, H.; Bismuth, J.; Pownall, H.J.; Huang, Y.; Wong, W.T. E-Selectin-Targeting Delivery of MicroRNAs by Microparticles Ameliorates Endothelial Inflammation and Atherosclerosis. Sci. Rep. 2016, 6, 22910. [Google Scholar] [CrossRef]
- Cao, L.; Chai, S. Is Involved in Morphine Pre Conditioning to Protect Rat Cardiomyocytes from Ischemia/Reperfusion Injury through Targeting Akt3. Mol. Med. Rep. 2020, 320, 1480–1488. [Google Scholar] [CrossRef]
- Tan, J.; Pan, W.; Chen, H.; Du, Y.; Jiang, P.; Zeng, D.; Wu, J.; Peng, K. Circ_0124644 Serves as a CeRNA for MiR-590-3p to Promote Hypoxia-Induced Cardiomyocytes Injury via Regulating SOX4. Front. Genet. 2021, 12, 667724. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; Zhang, X.; Lughmani, H.Y.; Kennedy, D.J.; Haller, S.T.; Pierre, S.V.; Shapiro, J.I.; Tian, J. A Strategic Expression Method of MiR-29b and Its Anti-Fibrotic Effect Based on RNA-Sequencing Analysis. PLoS ONE 2020, 15, e0244065. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 Contributes to Myocardial Disease by Stimulating MAP Kinase Signalling in Fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Karakikes, I.; Chaanine, A.H.; Kang, S.; Mukete, B.N.; Jeong, D.; Zhang, S.; Hajjar, R.J.; Lebeche, D. Therapeutic Cardiac-Targeted Delivery of MiR-1 Reverses Pressure Overload-Induced Cardiac Hypertrophy and Attenuates Pathological Remodeling. J. Am. Heart Assoc. 2013, 2, e000078. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.; Tuazon, J.P.; Borlongan, C.V.; Yu, G. MicroRNA-133a and Myocardial Infarction. Cell Transplant. 2019, 28, 831–838. [Google Scholar] [CrossRef]
- Zhang, X.-T.; Xu, M.-G. Potential Link between MicroRNA-208 and Cardiovascular Diseases. J. Xiangya Med. 2021, 6, 12. [Google Scholar] [CrossRef]
- Wahlquist, C.; Jeong, D.; Rojas-Muñoz, A.; Kho, C.; Lee, A.; Mitsuyama, S.; van Mil, A.; Park, W.J.; Sluijter, J.P.G.; Doevendans, P.A.F.; et al. Inhibition of MiR-25 Improves Cardiac Contractility in the Failing Heart. Nature 2014, 508, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wahlquist, C.; El Azzouzi, H.; Deddens, J.C.; Kuster, D.; van Mil, A.; Rojas-Munoz, A.; Huibers, M.M.; Mercola, M.; de Weger, R.; et al. MiR-132/212 Impairs Cardiomyocytes Contractility in the Failing Heart by Suppressing SERCA2a. Front. Cardiovasc. Med. 2021, 8, 592362. [Google Scholar] [CrossRef] [PubMed]
- Loyer, X.; Potteaux, S.; Vion, A.-C.; Guérin, C.L.; Boulkroun, S.; Rautou, P.-E.; Ramkhelawon, B.; Esposito, B.; Dalloz, M.; Paul, J.-L.; et al. Inhibition of MicroRNA-92a Prevents Endothelial Dysfunction and Atherosclerosis in Mice. Circ. Res. 2014, 114, 434–443. [Google Scholar] [CrossRef]
- Mondadori dos Santos, A.; Metzinger, L.; Haddad, O.; M’baya-Moutoula, E.; Taïbi, F.; Charnaux, N.; Massy, Z.A.; Hlawaty, H.; Metzinger-Le Meuth, V. MiR-126 Is Involved in Vascular Remodeling under Laminar Shear Stress. Biomed. Res. Int. 2015, 2015, 497280. [Google Scholar] [CrossRef]
- Petrkova, J.; Borucka, J.; Kalab, M.; Klevcova, P.; Michalek, J.; Taborsky, M.; Petrek, M. Increased Expression of MiR-146a in Valvular Tissue from Patients with Aortic Valve Stenosis. Front. Cardiovasc. Med. 2019, 6, 86. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, J.; Zhang, F.; Lei, Q.; Zhang, T.; Li, K.; Guo, J.; Hong, Y.; Bu, G.; Lv, X.; et al. MicroRNA-181a-5p and MicroRNA-181a-3p Cooperatively Restrict Vascular Inflammation and Atherosclerosis. Cell Death Dis. 2019, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Thum, T. Noncoding RNAs and Myocardial Fibrosis. Nat. Rev. Cardiol. 2014, 11, 655–663. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of Stress-Dependent Cardiac Growth and Gene Expression by a MicroRNA. Science 2007, 316, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Schütz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjärn, M.; Hansen, H.F.; Berger, U.; et al. LNA-Mediated MicroRNA Silencing in Nonhuman Primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Silahtaroglu, A.; Bak, M.; Christensen, M.; Lind-Thomsen, A.; Hedtjärn, M.; Hansen, J.B.; Hansen, H.F.; Straarup, E.M.; et al. Antagonism of MicroRNA-122 in Mice by Systemically Administered LNA-AntimiR Leads to up-Regulation of a Large Set of Predicted Target MRNAs in the Liver. Nucleic Acids Res. 2008, 36, 1153–1162. [Google Scholar] [CrossRef]
- Obad, S.; dos Santos, C.O.; Petri, A.; Heidenblad, M.; Broom, O.; Ruse, C.; Fu, C.; Lindow, M.; Stenvang, J.; Straarup, E.M.; et al. Silencing of MicroRNA Families by Seed-Targeting Tiny LNAs. Nat. Genet. 2011, 43, 371–378. [Google Scholar] [CrossRef]
- Nahar, S.; Singh, A.; Morihiro, K.; Moai, Y.; Kodama, T.; Obika, S.; Maiti, S. Systematic Evaluation of Biophysical and Functional Characteristics of Selenomethylene-Locked Nucleic Acid-Mediated Inhibition of MiR-21. Biochemistry 2016, 55, 7023–7032. [Google Scholar] [CrossRef]
- Meng, L.; Liu, C.; Lü, J.; Zhao, Q.; Deng, S.; Wang, G.; Qiao, J.; Zhang, C.; Zhen, L.; Lu, Y.; et al. Small RNA Zippers Lock MiRNA Molecules and Block MiRNA Function in Mammalian Cells. Nat. Commun. 2017, 8, 13964. [Google Scholar] [CrossRef]
- Fabani, M.M.; Abreu-Goodger, C.; Williams, D.; Lyons, P.A.; Torres, A.G.; Smith, K.G.C.; Enright, A.J.; Gait, M.J.; Vigorito, E. Efficient Inhibition of MiR-155 Function in Vivo by Peptide Nucleic Acids. Nucleic Acids Res. 2010, 38, 4466–4475. [Google Scholar] [CrossRef]
- Paulasova, P.; Pellestor, F. The Peptide Nucleic Acids (PNAs): A New Generation of Probes for Genetic and Cytogenetic Analyses. Ann. Genet. 2004, 47, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Behlke, M.A. Chemical Modification of SiRNAs for in Vivo Use. Oligonucleotides 2008, 18, 305–319. [Google Scholar] [CrossRef]
- Li, Y.-G.; Zhang, P.-P.; Jiao, K.-L.; Zou, Y.-Z. Knockdown of MicroRNA-181 by Lentivirus Mediated SiRNA Expression Vector Decreases the Arrhythmogenic Effect of Skeletal Myoblast Transplantation in Rat with Myocardial Infarction. Microvasc. Res. 2009, 78, 393–404. [Google Scholar] [CrossRef]
- Choi, W.-Y.; Giraldez, A.J.; Schier, A.F. Target Protectors Reveal Dampening and Balancing of Nodal Agonist and Antagonist by MiR-430. Science 2007, 318, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Langlet, F.; Chachlaki, K.; Roa, J.; Rasika, S.; Jouy, N.; Gallet, S.; Gaytan, F.; Parkash, J.; Tena-Sempere, M.; et al. Corrigendum: A MicroRNA Switch Regulates the Rise in Hypothalamic GnRH Production before Puberty. Nat. Neurosci. 2016, 19, 1115. [Google Scholar] [CrossRef]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of MicroRNAs in Vivo with “Antagomirs”. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Iaconetti, C.; Polimeni, A.; Sorrentino, S.; Sabatino, J.; Pironti, G.; Esposito, G.; Curcio, A.; Indolfi, C. Inhibition of MiR-92a Increases Endothelial Proliferation and Migration in Vitro as Well as Reduces Neointimal Proliferation in Vivo after Vascular Injury. Basic Res. Cardiol. 2012, 107, 296. [Google Scholar] [CrossRef]
- Jeong, D.; Yoo, J.; Lee, P.; Kepreotis, S.V.; Lee, A.; Wahlquist, C.; Brown, B.D.; Kho, C.; Mercola, M.; Hajjar, R.J. MiR-25 Tough Decoy Enhances Cardiac Function in Heart Failure. Mol. Ther. 2018, 26, 718–729. [Google Scholar] [CrossRef]
- Dirkx, E.; Gladka, M.M.; Philippen, L.E.; Armand, A.-S.; Kinet, V.; Leptidis, S.; El Azzouzi, H.; Salic, K.; Bourajjaj, M.; da Silva, G.J.J.; et al. Nfat and MiR-25 Cooperate to Reactivate the Transcription Factor Hand2 in Heart Failure. Nat. Cell Biol. 2013, 15, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.P.; Wu, J.; Wang, X.; Sartor, M.A.; Jones, K.; Qian, J.; Nicolaou, P.; Pritchard, T.J.; Fan, G.C. MicroRNA-320 Is Involved in the Regulation of Cardiac Ischemia/Reperfusion Injury by Targeting Heat-Shock Protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The MiRNA-212/132 Family Regulates Both Cardiac Hypertrophy and Cardiomyocyte Autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Merk, D.R.; Deng, A.; Chin, J.T.; Raaz, U.; Schoelmerich, A.M.; Raiesdana, A.; Leeper, N.J.; et al. Inhibition of MicroRNA-29b Reduces Murine Abdominal Aortic Aneurysm Development. J. Clin. Investig. 2012, 122, 497–506. [Google Scholar] [CrossRef]
- Boon, R.A.; Seeger, T.; Heydt, S.; Fischer, A.; Hergenreider, E.; Horrevoets, A.; Vinciguerra, M.; Rosenthal, N.; Sciacca, S.; Pilato, M.; et al. MicroRNA-29 in Aortic Dilation:Implications for Aneurysm Formation. Circ. Res. 2011, 109, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.L.; Hullinger, T.G.; Semus, H.M.; Dickinson, B.A.; Seto, A.G.; Lynch, J.M.; Stack, C.; Latimer, P.A.; Olson, E.N.; van Rooij, E. Therapeutic Inhibition of MiR-208a Improves Cardiac Function and Survival during Heart Failure. Circulation 2011, 124, 1537–1547. [Google Scholar] [CrossRef]
- Wahlestedt, C.; Salmi, P.; Good, L.; Kela, J.; Johnsson, T.; Hökfelt, T.; Broberger, C.; Porreca, F.; Lai, J.; Ren, K.; et al. Potent and Nontoxic Antisense Oligonucleotides Containing Locked Nucleic Acids. Proc. Natl. Acad. Sci. USA 2000, 97, 5633–5638. [Google Scholar] [CrossRef]
- Mangos, M.M.; Min, K.-L.; Viazovkina, E.; Galarneau, A.; Elzagheid, M.I.; Parniak, M.A.; Damha, M.J. Efficient RNase H-Directed Cleavage of RNA Promoted by Antisense DNA or 2’F-ANA Constructs Containing Acyclic Nucleotide Inserts. J. Am. Chem. Soc. 2003, 125, 654–661. [Google Scholar] [CrossRef]
- Krieg, A.M. Targeting LDL Cholesterol with LNA. Mol. Ther. Nucleic Acids 2012, 1, e6. [Google Scholar] [CrossRef] [PubMed]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zörnig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long Noncoding RNA MALAT1 Regulates Endothelial Cell Function and Vessel Growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.-T.; Gupta, S.K.; Viereck, J.; Foinquinos, A.; Samolovac, S.; Kramer, F.L.; Garg, A.; Remke, J.; Zimmer, K.; Batkai, S.; et al. Inhibition of the Cardiac Fibroblast-Enriched LncRNA Meg3 Prevents Cardiac Fibrosis and Diastolic Dysfunction. Circ. Res. 2017, 121, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cai, J.; Han, Y.; Chen, J.; Huang, Z.-P.; Chen, C.; Cai, Y.; Huang, H.; Yang, Y.; Liu, Y.; et al. LincRNA-P21 Regulates Neointima Formation, Vascular Smooth Muscle Cell Proliferation, Apoptosis, and Atherosclerosis by Enhancing P53 Activity. Circulation 2014, 130, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, C.-Y.; Zhou, L.-Y.; Wang, J.-X.; Wang, M.; Zhao, B.; Zhao, W.-K.; Xu, S.-J.; Fan, L.-H.; Zhang, X.-J.; et al. APF LncRNA Regulates Autophagy and Myocardial Infarction by Targeting MiR-188-3p. Nat. Commun. 2015, 6, 6779. [Google Scholar] [CrossRef] [PubMed]
- Burel, S.A.; Hart, C.E.; Cauntay, P.; Hsiao, J.; Machemer, T.; Katz, M.; Watt, A.; Bui, H.-H.; Younis, H.; Sabripour, M.; et al. Hepatotoxicity of High Affinity Gapmer Antisense Oligonucleotides Is Mediated by RNase H1 Dependent Promiscuous Reduction of Very Long Pre-MRNA Transcripts. Nucleic Acids Res. 2016, 44, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, L.; Song, J.; Wang, Z.; Huang, X.; Guo, Z.; Chen, F.; Zhao, X. Long Noncoding RNA MALAT1 Mediates Cardiac Fibrosis in Experimental Postinfarct Myocardium Mice Model. J. Cell. Physiol. 2019, 234, 2997–3006. [Google Scholar] [CrossRef]
- Qu, X.; Du, Y.; Shu, Y.; Gao, M.; Sun, F.; Luo, S.; Yang, T.; Zhan, L.; Yuan, Y.; Chu, W.; et al. MIAT Is a Pro-Fibrotic Long Non-Coding RNA Governing Cardiac Fibrosis in Post-Infarct Myocardium. Sci. Rep. 2017, 7, 42657. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.-J.; Zhang, H.-S. LncRNA-CARl in a Rat Model of Myocardial Infarction. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4332–4340. [Google Scholar] [CrossRef]
- Wang, K.; Liu, F.; Zhou, L.-Y.; Long, B.; Yuan, S.-M.; Wang, Y.; Liu, C.-Y.; Sun, T.; Zhang, X.-J.; Li, P.-F. The Long Noncoding RNA CHRF Regulates Cardiac Hypertrophy by Targeting MiR-489. Circ. Res. 2014, 114, 1377–1388. [Google Scholar] [CrossRef]
- Viereck, J.; Kumarswamy, R.; Foinquinos, A.; Xiao, K.; Avramopoulos, P.; Kunz, M.; Dittrich, M.; Maetzig, T.; Zimmer, K.; Remke, J.; et al. Long Noncoding RNA Chast Promotes Cardiac Remodeling. Sci. Transl. Med. 2016, 8, 326ra22. [Google Scholar] [CrossRef]
- Han, P.; Li, W.; Lin, C.-H.; Yang, J.; Shang, C.; Nuernberg, S.T.; Jin, K.K.; Xu, W.; Lin, C.-Y.; Lin, C.-J.; et al. A Long Noncoding RNA Protects the Heart from Pathological Hypertrophy. Nature 2014, 514, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Hobuß, L.; Bär, C.; Thum, T. Long Non-Coding RNAs: At the Heart of Cardiac Dysfunction? Front. Physiol. 2019, 10, 30. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Huang, X.; Guo, X.; Liu, Y.; Zhong, J.; Yuan, J. STAT3-Induced Upregulation of LncRNA MEG3 Regulates the Growth of Cardiac Hypertrophy through MiR-361-5p/HDAC9 Axis. Sci. Rep. 2019, 9, 460. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, M.; Wang, H.; Zhao, S.; Zhao, D.; Yang, Y.; Wang, Z.M.; Wang, F.; Yang, Z.J.; Lu, X.; et al. Association of Polymorphisms in Long Noncoding RNA H19 with Coronary Artery Disease Risk in a Chinese Population. Mutat. Res. 2015, 772, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Breckwoldt, K.; Remmele, C.W.; Hartmann, D.; Dittrich, M.; Pfanne, A.; Just, A.; Xiao, K.; Kunz, M.; Müller, T.; et al. Development of Long Noncoding RNA Based Strategies to Modulate Tissue Vascularization. J. Am. Coll. Cardiol. 2015, 66, 2005–2015. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Erratum: Knocking down Barriers: Advances in SiRNA Delivery. Nat. Rev. Drug Discov. 2010, 9, 412. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Bumcrot, D.; Manoharan, M.; Koteliansky, V.; Sah, D.W.Y. RNAi Therapeutics: A Potential New Class of Pharmaceutical Drugs. Nat. Chem. Biol. 2006, 2, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Santel, A.; Aleku, M.; Keil, O.; Endruschat, J.; Esche, V.; Fisch, G.; Dames, S.; Löffler, K.; Fechtner, M.; Arnold, W.; et al. A Novel SiRNA-Lipoplex Technology for RNA Interference in the Mouse Vascular Endothelium. Gene Ther. 2006, 13, 1222–1234. [Google Scholar] [CrossRef]
- Van de Water, F.M.; Boerman, O.C.; Wouterse, A.C.; Peters, J.G.P.; Russel, F.G.M.; Masereeuw, R. Intravenously Administered Short Interfering RNA Accumulates in the Kidney and Selectively Suppresses Gene Function in Renal Proximal Tubules. Drug Metab. Dispos. 2006, 34, 1393–1397. [Google Scholar] [CrossRef]
- Takakura, Y.; Mahato, R.I.; Hashida, M. Extravasation of Macromolecules. Adv. Drug Deliv. Rev. 1998, 34, 93–108. [Google Scholar] [CrossRef]
- Li, W.; Szoka, F.C., Jr. Lipid-Based Nanoparticles for Nucleic Acid Delivery. Pharm. Res. 2007, 24, 438–449. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Poh, M.-Z.; Insin, N.; Bawendi, M.G.; Fukumura, D.; Munn, L.L.; Jain, R.K. Diffusion of Particles in the Extracellular Matrix: The Effect of Repulsive Electrostatic Interactions. Biophys. J. 2010, 99, 1342–1349. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated Portals of Entry into the Cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Swanson, J.A.; Watts, C. Macropinocytosis. Trends Cell Biol. 1995, 5, 424–428. [Google Scholar] [CrossRef]
- Bareford, L.M.; Swaan, P.W. Endocytic Mechanisms for Targeted Drug Delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Partlow, K.C.; Lanza, G.M.; Wickline, S.A. Exploiting Lipid Raft Transport with Membrane Targeted Nanoparticles: A Strategy for Cytosolic Drug Delivery. Biomaterials 2008, 29, 3367–3375. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Masuda, T.; Katayama, K.; Yoshikawa, T.; Tsutsumi, Y.; Akashi, M.; Mayumi, T.; Nakagawa, S. Fusogenic Liposome Delivers Encapsulated Nanoparticles for Cytosolic Controlled Gene Release. J. Control. Release 2005, 105, 344–353. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, W.; Jin, H.; Lovell, J.F.; Yang, M.; Ding, L.; Chen, J.; Corbin, I.; Luo, Q.; Zheng, G. Biomimetic Nanocarrier for Direct Cytosolic Drug Delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 9171–9175. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A Combinatorial Library of Lipid-like Materials for Delivery of RNAi Therapeutics. Nat. Biotechnol. 2008, 26, 561–569. [Google Scholar] [CrossRef]
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M.; et al. Mechanisms and Optimization of in Vivo Delivery of Lipophilic SiRNAs. Nat. Biotechnol. 2007, 25, 1149–1157. [Google Scholar] [CrossRef]
- Morrissey, D.V.; Lockridge, J.A.; Shaw, L.; Blanchard, K.; Jensen, K.; Breen, W.; Hartsough, K.; Machemer, L.; Radka, S.; Jadhav, V.; et al. Potent and Persistent in Vivo Anti-HBV Activity of Chemically Modified SiRNAs. Nat. Biotechnol. 2005, 23, 1002–1007. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational Design of Cationic Lipids for SiRNA Delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef]
- Truong, N.P.; Gu, W.; Prasadam, I.; Jia, Z.; Crawford, R.; Xiao, Y.; Monteiro, M.J. An Influenza Virus-Inspired Polymer System for the Timed Release of SiRNA. Nat. Commun. 2013, 4, 1902. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Z.; Dou, S.; Wang, Y.-C.; Long, H.-Y.; Xiong, M.-H.; Mao, C.-Q.; Yao, Y.-D.; Wang, J. Single-Step Assembly of Cationic Lipid-Polymer Hybrid Nanoparticles for Systemic Delivery of SiRNA. ACS Nano 2012, 6, 4955–4965. [Google Scholar] [CrossRef]
- Dunn, S.S.; Tian, S.; Blake, S.; Wang, J.; Galloway, A.L.; Murphy, A.; Pohlhaus, P.D.; Rolland, J.P.; Napier, M.E.; DeSimone, J.M. Reductively Responsive SiRNA-Conjugated Hydrogel Nanoparticles for Gene Silencing. J. Am. Chem. Soc. 2012, 134, 7423–7430. [Google Scholar] [CrossRef] [PubMed]
- Endo-Takahashi, Y.; Negishi, Y.; Nakamura, A.; Suzuki, D.; Ukai, S.; Sugimoto, K.; Moriyasu, F.; Takagi, N.; Suzuki, R.; Maruyama, K.; et al. PDNA-Loaded Bubble Liposomes as Potential Ultrasound Imaging and Gene Delivery Agents. Biomaterials 2013, 34, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Suma, T.; Miyata, K.; Anraku, Y.; Watanabe, S.; Christie, R.J.; Takemoto, H.; Shioyama, M.; Gouda, N.; Ishii, T.; Nishiyama, N.; et al. Smart Multilayered Assembly for Biocompatible SiRNA Delivery Featuring Dissolvable Silica, Endosome-Disrupting Polycation, and Detachable PEG. ACS Nano 2012, 6, 6693–6705. [Google Scholar] [CrossRef]
- McMahon, K.M.; Mutharasan, R.K.; Tripathy, S.; Veliceasa, D.; Bobeica, M.; Shumaker, D.K.; Luthi, A.J.; Helfand, B.T.; Ardehali, H.; Mirkin, C.A.; et al. Biomimetic High Density Lipoprotein Nanoparticles for Nucleic Acid Delivery. Nano Lett. 2011, 11, 1208–1214. [Google Scholar] [CrossRef]
- Qi, L.; Gao, X. Quantum Dot-Amphipol Nanocomplex for Intracellular Delivery and Real-Time Imaging of SiRNA. ACS Nano 2008, 2, 1403–1410. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, K.; Moon, S.H.; Lee, Y.; Park, T.G.; Cheon, J. All-in-One Target-Cell-Specific Magnetic Nanoparticles for Simultaneous Molecular Imaging and SiRNA Delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 4174–4179. [Google Scholar] [CrossRef]
- Jiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-Coated Iron Oxide Nanoparticles for Efficient DNA and SiRNA Delivery. Nano Lett. 2013, 13, 1059–1064. [Google Scholar] [CrossRef]
- Posadas, I.; Guerra, F.J.; Ceña, V. Nonviral Vectors for the Delivery of Small Interfering RNAs to the CNS. Nanomedicine 2010, 5, 1219–1236. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Gherardini, L.; Bardi, G.; Nunes, A.; Guo, C.; Bussy, C.; Herrero, M.A.; Bianco, A.; Prato, M.; Kostarelos, K.; et al. Functional Motor Recovery from Brain Ischemic Insult by Carbon Nanotube-Mediated SiRNA Silencing. Proc. Natl. Acad. Sci. USA 2011, 108, 10952–10957. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lytton-Jean, A.K.R.; Chen, Y.; Love, K.T.; Park, A.I.; Karagiannis, E.D.; Sehgal, A.; Querbes, W.; Zurenko, C.S.; Jayaraman, M.; et al. Molecularly Self-Assembled Nucleic Acid Nanoparticles for Targeted in Vivo SiRNA Delivery. Nat. Nanotechnol. 2012, 7, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. MiR-15 and MiR-16 Induce Apoptosis by Targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef]
- Hermeking, H. The MiR-34 Family in Cancer and Apoptosis. Cell Death Differ. 2010, 17, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Knight, R.A. MiR-34: From Bench to Bedside. Oncotarget 2014, 5, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and MRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, C.; Paunovska, K.; Hatit, M.Z.C.; Lokugamage, M.P.; Dahlman, J.E. Therapeutic RNA Delivery for COVID and Other Diseases. Adv. Healthc. Mater. 2021, 10, e2002022. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Lipid Bilayers and Vesicles. Biochim. Biophys. Acta 1977, 470, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Darjuan, M.M.; Mercer, J.E.; Chen, S.; van der Meel, R.; Thewalt, J.L.; Tam, Y.Y.C.; Cullis, P.R. On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and SiRNA. ACS Nano 2018, 12, 4787–4795. [Google Scholar] [CrossRef]
- Herrera, M.; Kim, J.; Eygeris, Y.; Jozic, A.; Sahay, G. Illuminating Endosomal Escape of Polymorphic Lipid Nanoparticles That Boost MRNA Delivery. Biomater. Sci. 2021, 9, 4289–4300. [Google Scholar] [CrossRef]
- Malamas, A.S.; Gujrati, M.; Kummitha, C.M.; Xu, R.; Lu, Z.-R. Design and Evaluation of New PH-Sensitive Amphiphilic Cationic Lipids for SiRNA Delivery. J. Control. Release 2013, 171, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Altınoglu, S.; Wang, M.; Xu, Q. Combinatorial Library Strategies for Synthesis of Cationic Lipid-like Nanoparticles and Their Potential Medical Applications. Nanomedicine 2015, 10, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef]
- Love, K.T.; Mahon, K.P.; Levins, C.G.; Whitehead, K.A.; Querbes, W.; Dorkin, J.R.; Qin, J.; Cantley, W.; Qin, L.L.; Racie, T.; et al. Lipid-like Materials for Low-Dose, in Vivo Gene Silencing. Proc. Natl. Acad. Sci. USA 2010, 107, 1864–1869. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Lee, A.C.H.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-Mediated Gene Silencing in Non-Human Primates. Nature 2006, 441, 111–114. [Google Scholar] [CrossRef]
- Dong, Y.; Love, K.T.; Dorkin, J.R.; Sirirungruang, S.; Zhang, Y.; Chen, D.; Bogorad, R.L.; Yin, H.; Chen, Y.; Vegas, A.J.; et al. Lipopeptide Nanoparticles for Potent and Selective SiRNA Delivery in Rodents and Nonhuman Primates. Proc. Natl. Acad. Sci. USA 2014, 111, 3955–3960. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the Potency of SiRNA Lipid Nanoparticles for Hepatic Gene Silencing in Vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8529–8533. [Google Scholar] [CrossRef]
- Paunovska, K.; Da Silva Sanchez, A.J.; Sago, C.D.; Gan, Z.; Lokugamage, M.P.; Islam, F.Z.; Kalathoor, S.; Krupczak, B.R.; Dahlman, J.E. Nanoparticles Containing Oxidized Cholesterol Deliver MRNA to the Liver Microenvironment at Clinically Relevant Doses. Adv. Mater. 2019, 31, e1807748. [Google Scholar] [CrossRef]
- Kauffman, K.J. Rapid, Single-Cell Analysis and Discovery of Vectored MRNA Transfection in Vivo with a LoxP-Flanked Tdtomato Reporter Mouse. Mol. Ther. Nucleic Acids 2018, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; DeRosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for MRNA Delivery in Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef] [PubMed]
- Sedic, M. Safety Evaluation of Lipid Nanoparticleformulated Modified MRNA in the Sprague-Dawley Rat and Cynomolgus Monkey. Vet. Pathol. 2018, 55, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent in Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]
- Benenato, K.E.; Kumarasinghe, E.S.; Cornebise, M. Compounds and Compositions for Intracellular Delivery of Therapeutic Agents. WO2017049245A3, 18 May 2017. [Google Scholar]
- Zhang, X.; Zhao, W.; Nguyen, G.N.; Zhang, C.; Zeng, C.; Yan, J.; Du, S.; Hou, X.; Li, W.; Jiang, J.; et al. Functionalized Lipid-like Nanoparticles for in Vivo MRNA Delivery and Base Editing. Sci. Adv. 2020, 6, 34. [Google Scholar] [CrossRef]
- Musunuru, K.; Chadwick, A.C.; Mizoguchi, T.; Garcia, S.P.; DeNizio, J.E.; Reiss, C.W.; Wang, K.; Iyer, S.; Dutta, C.; Clendaniel, V.; et al. In Vivo CRISPR Base Editing of PCSK9 Durably Lowers Cholesterol in Primates. Nature 2021, 593, 429–434. [Google Scholar] [CrossRef]
- Rothgangl, T.; Dennis, M.K.; Lin, P.J.C.; Oka, R.; Witzigmann, D.; Villiger, L.; Qi, W.; Hruzova, M.; Kissling, L.; Lenggenhager, D.; et al. In Vivo Adenine Base Editing of PCSK9 in Macaques Reduces LDL Cholesterol Levels. Nat. Biotechnol. 2021, 39, 949–957. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Investor Overview. Beam Therapeutics. Available online: https://investors.beamtx.com/ (accessed on 26 December 2022).
- Luigetti, M.; Romano, A.; Di Paolantonio, A.; Bisogni, G.; Sabatelli, M. Diagnosis and Treatment of Hereditary Transthyretin Amyloidosis (hATTR) Polyneuropathy: Current Perspectives on Improving Patient Care. Ther. Clin. Risk Manag. 2020, 16, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid Ther. 2018, 28, 146–157. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The Role of Helper Lipids in Lipid Nanoparticles (LNPs) Designed for Oligonucleotide Delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 129–137. [Google Scholar] [CrossRef]
- Dahlman, J.E.; Barnes, C.; Khan, O.; Thiriot, A.; Jhunjunwala, S.; Shaw, T.E.; Xing, Y.; Sager, H.B.; Sahay, G.; Speciner, L.; et al. In Vivo Endothelial SiRNA Delivery Using Polymeric Nanoparticles with Low Molecular Weight. Nat. Nanotechnol. 2014, 9, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Khan, O.F.; Kowalski, P.S.; Doloff, J.C.; Tsosie, J.K.; Bakthavatchalu, V.; Winn, C.B.; Haupt, J.; Jamiel, M.; Langer, R.; Anderson, D.G. Endothelial SiRNA Delivery in Nonhuman Primates Using Ionizable Low–Molecular Weight Polymeric Nanoparticles. Sci. Adv. 2018, 4, eaar8409. [Google Scholar] [CrossRef]
- Sago, C.D.; Lokugamage, M.P.; Islam, F.Z.; Krupczak, B.R.; Sato, M.; Dahlman, J.E. Nanoparticles That Deliver RNA to Bone Marrow Identified by in Vivo Directed Evolution. J. Am. Chem. Soc. 2018, 140, 17095–17105. [Google Scholar] [CrossRef]
- Paunovska, K.; Gil, C.J.; Lokugamage, M.P.; Sago, C.D.; Sato, M.; Lando, G.N.; Gamboa Castro, M.; Bryksin, A.V.; Dahlman, J.E. Analyzing 2000 in Vivo Drug Delivery Data Points Reveals Cholesterol Structure Impacts Nanoparticle Delivery. ACS Nano 2018, 12, 8341–8349. [Google Scholar] [CrossRef]
- Sago, C.D.; Lokugamage, M.P.; Paunovska, K.; Vanover, D.A.; Monaco, C.M.; Shah, N.N.; Gamboa Castro, M.; Anderson, S.E.; Rudoltz, T.G.; Lando, G.N.; et al. High-Throughput in Vivo Screen of Functional MRNA Delivery Identifies Nanoparticles for Endothelial Cell Gene Editing. Proc. Natl. Acad. Sci. USA 2018, 115, E9944–E9952. [Google Scholar] [CrossRef]
- Lokugamage, M.P.; Vanover, D.; Beyersdorf, J.; Hatit, M.Z.C.; Rotolo, L.; Echeverri, E.S.; Peck, H.E.; Ni, H.; Yoon, J.-K.; Kim, Y.; et al. Optimization of Lipid Nanoparticles for the Delivery of Nebulized Therapeutic MRNA to the Lungs. Nat. Biomed. Eng. 2021, 5, 1059–1068. [Google Scholar] [CrossRef]
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.C.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of SiRNA Lipid Nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139. [Google Scholar] [CrossRef] [PubMed]
- Ryals, R.C.; Patel, S.; Acosta, C.; McKinney, M.; Pennesi, M.E.; Sahay, G. The Effects of PEGylation on LNP Based MRNA Delivery to the Eye. PLoS ONE 2020, 15, e0241006. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Eygeris, Y.; Patel, S.; Jozic, A.; Sahay, G. Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett. 2020, 20, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Cheng, Q. Selective Organ Targeting (SORT) Nanoparticles for Tissue-Specific MRNA Delivery and CRISPR-Cas Gene Editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Intellia Therapeutics Presents Preclinical Proof of Concept for CRISPR-Based In Vivo Editing of Bone Marrow at Keystone eSymposium—Intellia Therapeutics. Available online: https://ir.intelliatx.com/news-releases/news-release-details/intellia-therapeutics-presents-preclinical-proof-concept-crispr (accessed on 15 January 2023).
- Zhou, G.; Hu, T.; Du, Q.; Huang, W.; Yao, C. Nanoparticle-Delivered MicroRNA-153-3p Alleviates Myocardial Infarction-Induced Myocardial Injury in a Rat Model. ACS Biomater. Sci. Eng. 2022, 8, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric Nanoparticles: A Study on the Preparation Variables and Characterization Methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Z.; Viennois, E.; Kang, Y.; Zhang, M.; Han, M.K.; Chen, J.; Merlin, D. Combination Therapy for Ulcerative Colitis: Orally Targeted Nanoparticles Prevent Mucosal Damage and Relieve Inflammation. Theranostics 2016, 6, 2250–2266. [Google Scholar] [CrossRef] [PubMed]
- Koenig, O.; Walker, T.; Perle, N.; Zech, A.; Neumann, B.; Schlensak, C.; Wendel, H.-P.; Nolte, A. New Aspects of Gene-Silencing for the Treatment of Cardiovascular Diseases. Pharmaceuticals 2013, 6, 881–914. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Lintermans, L.L.; Morselt, H.W.M.; Leus, N.G.J.; Ruiters, M.H.J.; Molema, G.; Kamps, J.A.A.M. Anti-VCAM-1 and Anti-E-Selectin SAINT-O-Somes for Selective Delivery of SiRNA into Inflammation-Activated Primary Endothelial Cells. Mol. Pharm. 2013, 10, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Ku, S.H.; Lee, M.S.; Jeong, J.H.; Mok, H.; Choi, D.; Kim, S.H. Cardiac RNAi Therapy Using RAGE SiRNA/Deoxycholic Acid-Modified Polyethylenimine Complexes for Myocardial Infarction. Biomaterials 2014, 35, 7562–7573. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, Y.; Zhang, R.; Shen, M.; Zhu, Y.; Zhang, Q.; Liu, H.; Han, D.; Shi, X.; Zhang, J. Inhibition of Cardiomyocyte Apoptosis Post-Acute Myocardial Infarction through the Efficient Delivery of MicroRNA-24 by Silica Nanoparticles. Nanoscale Adv. 2021, 3, 6379–6385. [Google Scholar] [CrossRef]
- Yang, H.; Qin, X.; Wang, H.; Zhao, X.; Liu, Y.; Wo, H.-T.; Liu, C.; Nishiga, M.; Chen, H.; Ge, J.; et al. An in Vivo MiRNA Delivery System for Restoring Infarcted Myocardium. ACS Nano 2019, 13, 9880–9894. [Google Scholar] [CrossRef]
- Bheri, S.; Davis, M.E. Nanoparticle-Hydrogel System for Post-Myocardial Infarction Delivery of MicroRNA. ACS Nano 2019, 13, 9702–9706. [Google Scholar] [CrossRef]
- Bejerano, T.; Etzion, S.; Elyagon, S.; Etzion, Y.; Cohen, S. Nanoparticle Delivery of MiRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018, 18, 5885–5891. [Google Scholar] [CrossRef]
- Sun, T.; Simmons, R.; Huo, D.; Pang, B.; Zhao, X.; Kim, C.W.; Jo, H.; Xia, Y. Targeted Delivery of Anti-MiR-712 by VCAM1-Binding Au Nanospheres for Atherosclerosis Therapy. ChemNanoMat 2016, 2, 400–406. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Kim, C.W.; Seo, J.W.; Kumar, S.; Son, D.J.; Gagnon, M.K.J.; Ingham, E.S.; Ferrara, K.W.; Jo, H. Multifunctional Nanoparticles Facilitate Molecular Targeting and MiRNA Delivery to Inhibit Atherosclerosis in ApoE(−/−) Mice. ACS Nano 2015, 9, 8885–8897. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.-C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in Vitro and in Vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.-M.; Penzkofer, D.; Teske, R.; Dutzmann, J.; Koch, A.; Bielenberg, W.; Bonauer, A.; Boon, R.A.; Fischer, A.; Bauersachs, J.; et al. Inhibition of MiR-92a Improves Re-Endothelialization and Prevents Neointima Formation Following Vascular Injury. Cardiovasc. Res. 2014, 103, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Deuse, T.; Stubbendorff, M.; Chernogubova, E.; Erben, R.G.; Eken, S.M.; Jin, H.; Li, Y.; Busch, A.; Heeger, C.-H.; et al. Local MicroRNA Modulation Using a Novel Anti-MiR-21-Eluting Stent Effectively Prevents Experimental in-Stent Restenosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1945–1953. [Google Scholar] [CrossRef]
- Lucas, T.; Dimmeler, S. RNA Therapeutics for Treatment of Cardiovascular Diseases: Promises and Challenges: Promises and Challenges. Circ. Res. 2016, 119, 794–797. [Google Scholar] [CrossRef]
- Eulalio, A.; Mano, M.; Dal Ferro, M.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional Screening Identifies MiRNAs Inducing Cardiac Regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 Controls Cardiac Hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Desai, A.S.; Barnard, D.; Bouchard, A.; Jaski, B.; Lyon, A.R.; et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Patients with Cardiac Disease (CUPID 2): A Randomised, Multinational, Double-Blind, Placebo-Controlled, Phase 2b Trial. Lancet 2016, 387, 1178–1186. [Google Scholar] [CrossRef]
- Buchholz, C.J.; Friedel, T.; Büning, H. Surface-Engineered Viral Vectors for Selective and Cell Type-Specific Gene Delivery. Trends Biotechnol. 2015, 33, 777–790. [Google Scholar] [CrossRef]
- Muik, A.; Reul, J.; Friedel, T.; Muth, A.; Hartmann, K.P.; Schneider, I.C.; Münch, R.C.; Buchholz, C.J. Covalent Coupling of High-Affinity Ligands to the Surface of Viral Vector Particles by Protein Transsplicing Mediates Cell Type-Specific Gene Transfer. Biomaterials 2017, 144, 84–94. [Google Scholar] [CrossRef]
- Lucas, T.; Schäfer, F.; Müller, P.; Eming, S.A.; Heckel, A.; Dimmeler, S. Lightinducible AntimiR-92a as a Therapeutic Strategy to Promote Skin Repair in Healing-Impaired Diabetic Mice. Nat. Commun. 2017, 8, 15162. [Google Scholar] [CrossRef]
- Rohde, J.-H.; Weigand, J.E.; Suess, B.; Dimmeler, S. A Universal Aptamer Chimera for the Delivery of Functional MicroRNA-126. Nucleic Acid Ther. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Schober, A.; Nazari-Jahantigh, M.; Wei, Y.; Bidzhekov, K.; Gremse, F.; Grommes, J.; Megens, R.T.A.; Heyll, K.; Noels, H.; Hristov, M.; et al. MicroRNA-126-5p Promotes Endothelial Proliferation and Limits Atherosclerosis by Suppressing Dlk1. Nat. Med. 2014, 20, 368–376. [Google Scholar] [CrossRef]
- Sun, X.; He, S.; Wara, A.K.M.; Icli, B.; Shvartz, E.; Tesmenitsky, Y.; Belkin, N.; Li, D.; Blackwell, T.S.; Sukhova, G.K.; et al. Systemic Delivery of MicroRNA-181b Inhibits Nuclear Factor-ΚB Activation, Vascular Inflammation, and Atherosclerosis in Apolipoprotein E-Deficient Mice. Circ. Res. 2014, 114, 32–40. [Google Scholar] [CrossRef]
- Lesizza, P.; Prosdocimo, G.; Martinelli, V.; Sinagra, G.; Zacchigna, S.; Giacca, M. Single-Dose Intracardiac Injection of pro-Regenerative MicroRNAs Improves Cardiac Function after Myocardial Infarction. Circ. Res. 2017, 120, 1298–1304. [Google Scholar] [CrossRef]
- Bader, A.G. MiR-34—A MicroRNA Replacement Therapy Is Headed to the Clinic. Front. Genet. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P.; Graham, M.J.; Yu, J.; Carty, R.; Low, A.; Chappell, A.; Schmidt, K.; Zhao, C.; Aghajan, M.; Murray, H.F.; et al. Targeted Delivery of Antisense Oligonucleotides to Hepatocytes Using Triantennary N-Acetyl Galactosamine Improves Potency 10-Fold in Mice. Nucleic Acids Res. 2014, 42, 8796–8807. [Google Scholar] [CrossRef]
- Fernandez-Prado, R.; Perez-Gomez, M.V.; Ortiz, A. Pelacarsen for Lowering Lipoprotein(a): Implications for Patients with Chronic Kidney Disease. Clin. Kidney J. 2020, 13, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.A.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV Infection by Targeting MicroRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S.; Wancewicz, E.; Matson, J.; Pearce, M.; Siwkowski, A.; Swayze, E.; Bennett, F. Effect of Dose and Plasma Concentration on Liver Uptake and Pharmacologic Activity of a 2’-Methoxyethyl Modified Chimeric Antisense Oligonucleotide Targeting PTEN. Biochem. Pharmacol. 2009, 78, 284–291. [Google Scholar] [CrossRef]
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X.-H. Cellular Uptake and Trafficking of Antisense Oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237. [Google Scholar] [CrossRef]
- Wagenaar, T.R.; Tolstykh, T.; Shi, C.; Jiang, L.; Zhang, J.; Li, Z.; Yu, Q.; Qu, H.; Sun, F.; Cao, H.; et al. Identification of the Endosomal Sorting Complex Required for Transport-I (ESCRT-I) as an Important Modulator of Anti-MiR Uptake by Cancer Cells. Nucleic Acids Res. 2015, 43, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Chau, N.; Bhat, B.; Gupta, S.K.; Linsley, P.S.; Bauersachs, J.; Engelhardt, S. Comparison of Different MiR-21 Inhibitor Chemistries in a Cardiac Disease Model. J. Clin. Investig. 2011, 121, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Berman, C.L.; Barros, S.A.; Galloway, S.M.; Kasper, P.; Oleson, F.B.; Priestley, C.C.; Sweder, K.S.; Schlosser, M.J.; Sobol, Z. OSWG Recommendations for Genotoxicity Testing of Novel Oligonucleotide-Based Therapeutics. Nucleic Acid Ther. 2016, 26, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Peacock, H.; Fucini, R.V.; Jayalath, P.; Ibarra-Soza, J.M.; Haringsma, H.J.; Flanagan, W.M.; Willingham, A.; Beal, P.A. Nucleobase and Ribose Modifications Control Immunostimulation by a MicroRNA-122-Mimetic RNA. J. Am. Chem. Soc. 2011, 133, 9200–9203. [Google Scholar] [CrossRef]
- Monteith, D.K.; Geary, R.S.; Leeds, J.M.; Johnston, J.; Monia, B.P.; Levin, A.A. Preclinical Evaluation of the Effects of a Novel Antisense Compound Targeting C-Raf Kinase in Mice and Monkeys. Toxicol. Sci. 1998, 46, 365–375. [Google Scholar] [CrossRef]
- Levin, A.A. A Review of Issues in the Pharmacokinetics and Toxicology of Phosphorothioate Antisense Oligonucleotides. Biochim. Biophys. Acta Gene Struct. Expr. 1999, 1489, 69–84. [Google Scholar] [CrossRef]
- Henry, S.P.; Beattie, G.; Yeh, G.; Chappel, A.; Giclas, P.; Mortari, A.; Jagels, M.A.; Kornbrust, D.J.; Levin, A.A. Complement Activation Is Responsible for Acute Toxicities in Rhesus Monkeys Treated with a Phosphorothioate Oligodeoxynucleotide. Int. Immunopharmacol. 2002, 2, 1657–1666. [Google Scholar] [CrossRef]
- Flierl, U.; Nero, T.L.; Lim, B.; Arthur, J.F.; Yao, Y.; Jung, S.M.; Gitz, E.; Pollitt, A.Y.; Zaldivia, M.T.K.; Jandrot-Perrus, M.; et al. Phosphorothioate Backbone Modifications of Nucleotide-Based Drugs Are Potent Platelet Activators. J. Exp. Med. 2015, 212, 129–137. [Google Scholar] [CrossRef]
- Sewing, S.; Roth, A.B.; Winter, M.; Dieckmann, A.; Bertinetti-Lapatki, C.; Tessier, Y.; McGinnis, C.; Huber, S.; Koller, E.; Ploix, C.; et al. Assessing Single-Stranded Oligonucleotide Drug-Induced Effects in Vitro Reveals Key Risk Factors for Thrombocytopenia. PLoS ONE 2017, 12, e0187574. [Google Scholar] [CrossRef]
- Swayze, E.E.; Siwkowski, A.M.; Wancewicz, E.V.; Migawa, M.T.; Wyrzykiewicz, T.K.; Hung, G.; Monia, B.P.; Bennett, C.F. Antisense Oligonucleotides Containing Locked Nucleic Acid Improve Potency but Cause Significant Hepatotoxicity in Animals. Nucleic Acids Res. 2007, 35, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Burdick, A.D.; Sciabola, S.; Mantena, S.R.; Hollingshead, B.D.; Stanton, R.; Warneke, J.A.; Zeng, M.; Martsen, E.; Medvedev, A.; Makarov, S.S.; et al. Sequence Motifs Associated with Hepatotoxicity of Locked Nucleic Acid-Modified Antisense Oligonucleotides. Nucleic Acids Res. 2014, 42, 4882–4891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goel, V.; Robbie, G.J. Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients with Hereditary Transthyretin-Mediated Amyloidosis. J. Clin. Pharmacol. 2020, 60, 573–585. [Google Scholar] [CrossRef]
- Zhang, X.; Goel, V.; Attarwala, H.; Sweetser, M.T.; Clausen, V.A.; Robbie, G.J. Patisiran Pharmacokinetics, Pharmacodynamics, and Exposure-Response Analyses in the Phase 3 APOLLO Trial in Patients with Hereditary Transthyretin-Mediated (HATTR) Amyloidosis. J. Clin. Pharmacol. 2020, 60, 37–49. [Google Scholar] [CrossRef]
- MULTI-DISCIPLINE REVIEW. Summary Review Office Director cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210922Orig1s000MultiR.pdf (accessed on 26 December 2022).
- Parums, D.V. Editorial: First Full Regulatory Approval of a COVID-19 Vaccine, the BNT162b2 Pfizer-BioNTech Vaccine, and the Real-World Implications for Public Health Policy. Med. Sci. Monit. 2021, 27, e934625. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable Lipids Enabling Rapidly Eliminated Lipid Nanoparticles for Systemic Delivery of RNAi Therapeutics. Mol. Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-Based Analysis of Lipid Nanoparticle-Mediated SiRNA Delivery, Intracellular Trafficking and Endosomal Escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Ai, A.; Liu, X.; Hamar, P.; Trifonova, R.; Charisse, K.; Manoharan, M.; Kirchhausen, T.; Lieberman, J. Visualizing Lipid-Formulated SiRNA Release from Endosomes and Target Gene Knockdown. Nat. Biotechnol. 2015, 33, 870–876. [Google Scholar] [CrossRef]
- Alberer, M. Safety and Immunogenicity of a MRNA Rabies Vaccine in Healthy Adults: An Open-Label, Non-Randomised, Prospective, First-in-Human Phase 1 Clinical Trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-Term Storage of Lipid-like Nanoparticles for MRNA Delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Gerhardt, A.; Voigt, E.; Archer, M.; Reed, S.; Larson, E.; Van Hoeven, N.; Kramer, R.; Fox, C.; Casper, C. A Thermostable, Flexible RNA Vaccine Delivery Platform for Pandemic Response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Besin, G.; Milton, J.; Sabnis, S.; Howell, R.; Mihai, C.; Burke, K.; Benenato, K.E.; Stanton, M.; Smith, P.; Senn, J.; et al. Accelerated Blood Clearance of Lipid Nanoparticles Entails a Biphasic Humoral Response of B-1 Followed by B-2 Lymphocytes to Distinct Antigenic Moieties. ImmunoHorizons 2019, 3, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.T.; Turner, T.; Visseren, F.L.J.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Krempf, M. ODYSSEY LONG TERM Investigators. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Raal, F.J.; Santos, R.D.; Blom, D.J.; Marais, A.D.; Charng, M.-J.; Cromwell, W.C.; Lachmann, R.H.; Gaudet, D.; Tan, J.L.; Chasan-Taber, S.; et al. Mipomersen, an Apolipoprotein B Synthesis Inhibitor, for Lowering of LDL Cholesterol Concentrations in Patients with Homozygous Familial Hypercholesterolaemia: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2010, 375, 998–1006. [Google Scholar] [CrossRef]
- Boada, C.; Sukhovershin, R.; Pettigrew, R.; Cooke, J.P. RNA Therapeutics for Cardiovascular Disease. Curr. Opin. Cardiol. 2021, 36, 256–263. [Google Scholar] [CrossRef]
- Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Bär, C.; Borchert, T.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. CDR132L Improves Systolic and Diastolic Function in a Large Animal Model of Chronic Heart Failure. Eur. Heart J. 2021, 42, 192–201. [Google Scholar] [CrossRef]
- Bejar, N.; Tat, T.T.; Kiss, D.L. RNA Therapeutics: The next Generation of Drugs for Cardiovascular Diseases. Curr. Atheroscler. Rep. 2022, 24, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, M.W.; Elmén, J.; Fisker, N.; Hansen, H.F.; Persson, R.; Møller, M.R.; Rosenbohm, C.; Ørum, H.; Straarup, E.M.; Koch, T. PCSK9 LNA Antisense Oligonucleotides Induce Sustained Reduction of LDL Cholesterol in Nonhuman Primates. Mol. Ther. 2012, 20, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, C.; Freemantle, N.; Hughes, S.G.; Furniss, S.; Sulke, N. The Effect of C-Reactive Protein Reduction with a Highly Specific Antisense Oligonucleotide on Atrial Fibrillation Assessed Using Beat-to-Beat Pacemaker Holter Follow-Up. J. Interv. Card. Electrophysiol. 2015, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wolska, A.; Yang, Z.-H.; Remaley, A.T. Hypertriglyceridemia: New Approaches in Management and Treatment: New Approaches in Management and Treatment. Curr. Opin. Lipidol. 2020, 31, 331–339. [Google Scholar] [CrossRef]
- Krychtiuk, K.A.; Rader, D.J.; Granger, C.B. RNA-Targeted Therapeutics in Cardiovascular Disease: The Time Is Now. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 9, 94–99. [Google Scholar] [CrossRef]
- Koren, M.J.; Moriarty, P.M.; Baum, S.J.; Neutel, J.; Hernandez-Illas, M.; Weintraub, H.S.; Florio, M.; Kassahun, H.; Melquist, S.; Varrieur, T.; et al. Preclinical Development and Phase 1 Trial of a Novel SiRNA Targeting Lipoprotein(a). Nat. Med. 2022, 28, 96–103. [Google Scholar] [CrossRef]
- A Study of LY3561774 in Participants with Dyslipidemia. Available online: https://clinicaltrials.gov/ct2/show/NCT04644809?term=LY+3561774&draw=2&rank=1 (accessed on 26 December 2022).
- Bhatia, H.S.; Wilkinson, M.J. Lipoprotein(a): Evidence for Role as a Causal Risk Factor in Cardiovascular Disease and Emerging Therapies. J. Clin. Med. 2022, 11, 6040. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Michael, O.S.; Rathee, S.; Singh, K.R.B.; Ajayi, O.O.; Adetunji, J.B.; Ojha, A.; Singh, J.; Singh, R.P. Potentialities of Nanomaterials for the Management and Treatment of Metabolic Syndrome: A New Insight. Mater. Today Adv. 2022, 13, 100198. [Google Scholar] [CrossRef]
- Laggerbauer, B.; Engelhardt, S. MicroRNAs as Therapeutic Targets in Cardiovascular Disease. J. Clin. Investig. 2022, 132, e159179. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long Non-Coding RNAs: From Disease Code to Drug Role. Acta Pharm. Sin. B 2021, 11, 340–354. [Google Scholar] [CrossRef]
- Kalwa, M.; Hänzelmann, S.; Otto, S.; Kuo, C.-C.; Franzen, J.; Joussen, S.; Fernandez-Rebollo, E.; Rath, B.; Koch, C.; Hofmann, A.; et al. The lncRNA HOTAIR Impacts on Mesenchymal Stem Cellsviatriple Helix Formation. Nucleic Acids Res. 2016, 44, 10631–10643. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Q.; Mao, J.; Zhang, J.; Li, L. The Roles of lncRNA in Myocardial Infarction: Molecular Mechanisms, Diagnosis Biomarkers, and Therapeutic Perspectives. Front. Cell Dev. Biol. 2021, 9, 680713. [Google Scholar] [CrossRef]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.-M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-Coding RNAs in Cardiovascular Diseases: Diagnostic and Therapeutic Perspectives. Eur. Heart J. 2017, 39, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.M.; Echeverria, D.; Hassler, M.; Ly, S.; Khvorova, A. Cell Type Impacts Accessibility of mRNA to Silencing by RNA Interference. Mol. Ther. Nucleic Acids 2020, 21, 384–393. [Google Scholar] [CrossRef]
- Translate Bio Announces Results from Second Interim Data Analysis from Ongoing Phase 1/2 Clinical Trial of MRT5005 in Patients with Cystic Fibrosis (CF). Available online: https://www.globenewswire.com/en/news-release/2021/03/17/2194916/0/en/Translate-Bio-Announces-Results-from-Second-Interim-Data-Analysis-from-Ongoing-Phase-1-2-Clinical-Trial-of-MRT5005-in-Patients-with-Cystic-Fibrosis-CF.html (accessed on 15 January 2023).
- Orlandini von Niessen, A.G.; Poleganov, M.A.; Rechner, C.; Plaschke, A.; Kranz, L.M.; Fesser, S.; Diken, M.; Löwer, M.; Vallazza, B.; Beissert, T.; et al. Improving mRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3′ UTRs Identified by Cellular Library Screening. Mol. Ther. 2019, 27, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Frederick, J.P.; Huang, E.Y.; Burke, K.E.; Mauger, D.M.; Andrianova, E.A.; Farlow, S.J.; Siddiqui, S.; Pimentel, J.; Cheung-Ong, K.; et al. MicroRNAs Enable mRNA Therapeutics to Selectively Program Cancer Cells to Self-Destruct. Nucleic Acid Ther. 2018, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]

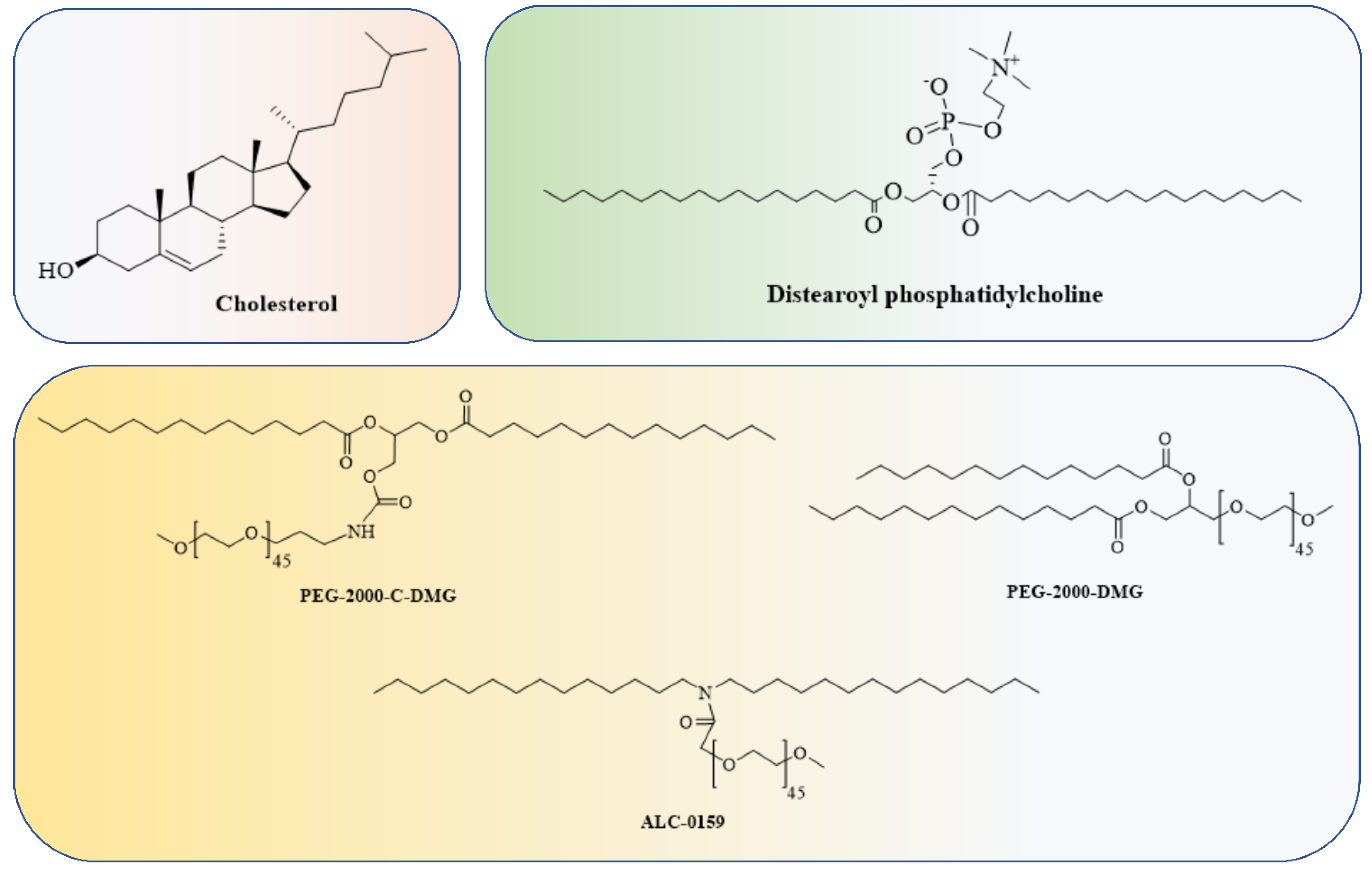

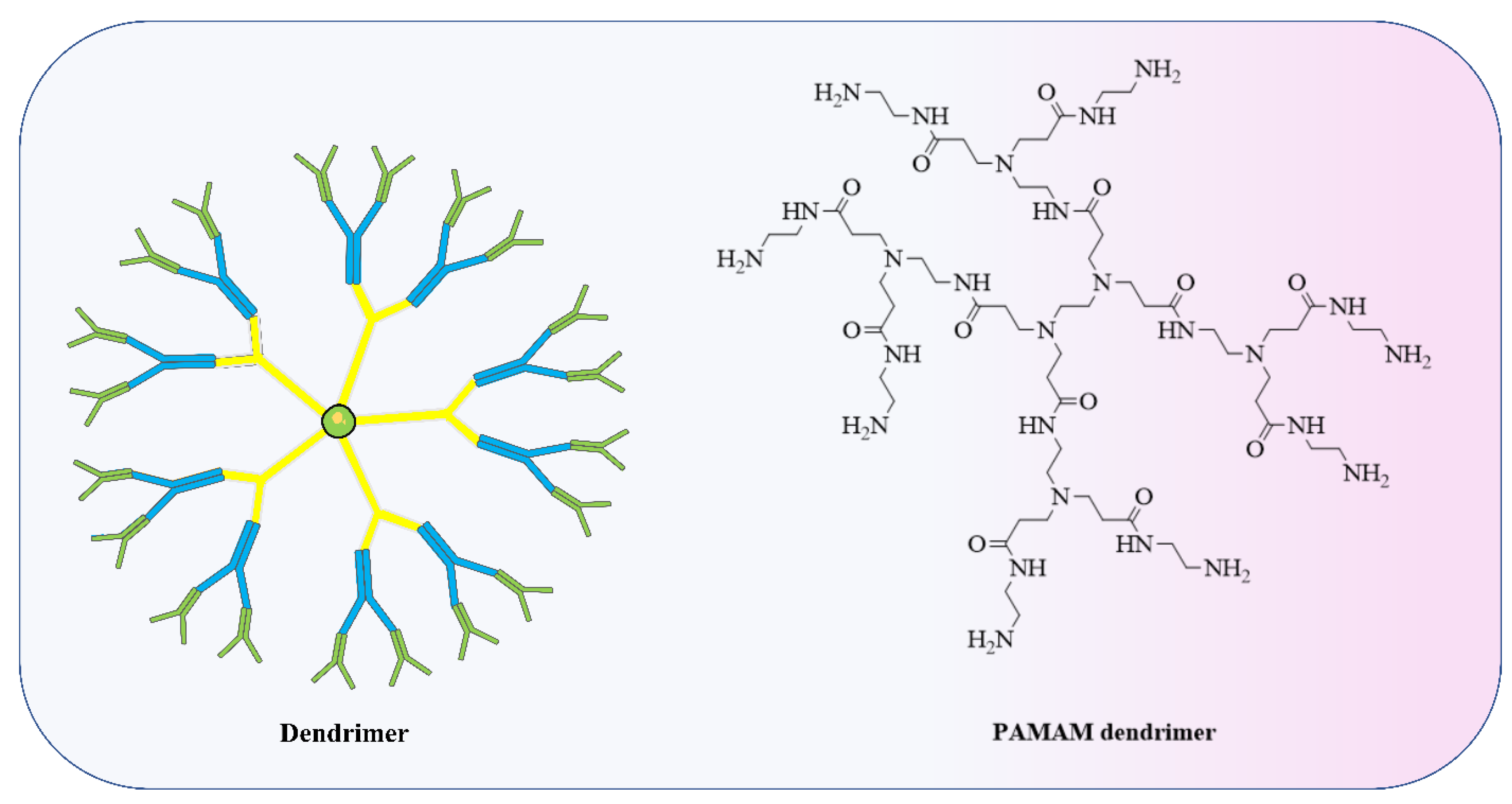


| Sr No | Mi RNAs | Disease Implicated in | Model | Mechanisms | Ref. |
|---|---|---|---|---|---|
| 1 | miR-15 | MI | Injury caused by ischemia/reperfusion in pigs and mice | Members of the microRNA-15 family have been shown to regulate mitochondrial activity by targeting proteins such as pyruvate dehydrogenase lipoamide kinase isozyme 4 and checkpoint kinase 1. | [10,11] |
| 2 | miR-24 | MI | MI mouse model | The miR-24 family member Bcl2 is repressed. One characteristic of miRNA-mediated regulation of critical cellular events is exemplified by the BIM protein. It has been found that miR-24 inhibits apoptosis in cardiomyocytes in a cell-independent manner. | [12] |
| 3 | miR-34 | MI | Mice | Through its regulation of suppression of the target gene PNUTS (protein phosphatase 1 nuclear-targeting subunit), miR-34a promotes telomere degradation and, by extension, age-related induction of cardiomyocyte cell death. | [13,14,15] |
| 4 | miR-92a | MI | Inhibits angiogenesis by reducing integrin α5 and sirtuin 1 expression. | [16,17,18] | |
| 5 | miR-199a | MI | Pig model | Improves cardiac function by stimulating endogenous myocardial repair mechanisms. | [19] |
| 6 | miR-320 | MI | Ischemia/reperfusion injury | Increased miR-320-3p expression and decreased Akt3 expression were seen in cardiomyocytes after H/R damage. | [20] |
| 7 | miR-590 | MI | Patient | Regulating SOX4 can help restore the cell cycle process, which in turn boosts cardiomyocyte proliferation and reduces the severity of AMI. | [21] |
| 8 | miR-29 | Cardiac fibrosis | Adult male and female C57BL/6 mice | Several matrix proteins are targeted by overexpression to reduce fibrosis. | [22] |
| 9 | miR-21 | Cardiac fibrosis | Mice | Reduces the function of Spry1 (sprouty homologue 1), hence promoting the profibrotic ERK-MAP kinase signalling pathway. | [23] |
| 10 | miR-1, | Hypertrophy and heart failure | Male Sprague–Dawley rats | Its expression went down by a lot, as did the amount of collagen in the body and the activity of key profibrotic factors can also be involved in regulating fibrosis by going after Fbln2, a secreted ECM protein that plays a crucial part in tissue remodelling in diseased condition. | [24] |
| 11 | miR-133, | Hypertrophy and heart failure | When miR-133a is misregulated, it inhibits the expression of NFATc4, a mediator of hypertrophy. | [25] | |
| 12 | miR-208, | Hypertrophy and heart failure | Mice | Overexpression of miR-208 inhibits the muscle-wasting proteins THAP1 and myostatin. | [26] |
| 13 | miR-25 | Hypertrophy and heart failure | TAC model | Blocking microRNA-25a, cardiac function was restored by inhibiting the calcium uptake pump SERCA2a (sarco/endoplasmic reticulum Ca2+-ATPase 2a), which improved calcium management. | [27] |
| 14 | miR-212/132 | Hypertrophy and heart failure | TAC mouse model | Inhibits the GFP/SERCA2a-3′-UTR expression. | [28] |
| 15 | miR-92a | Atherosclerosis and vascular remodelling | Mice | Inhibiting miR-92a, which functions as a proinflammatory regulator in endothelial cells by activating inflammatory cytokines and chemokines and augmenting monocyte adhesion, protects against endothelial dysfunction. | [29] |
| 16 | miR-126 | Atherosclerosis and vascular remodelling | Female C57/BL6 mice | Through an indirect pathway mediated by apoptosis and VCAM-1 expression, suppression of stromal cell-derived factor-1/CXCL12 expression may attenuate leukocyte homing from blood circulation through the endothelium in vivo. | [30] |
| 17 | miR-146 | Atherosclerosis and vascular remodelling | Patients with aortic valve stenosis | Downstream TLR4 signalling was controlled by a negative-feedback loop that included IL-1 receptor associated kinase 1 (IRAK1) and TNF-receptor associated factor 6 (TRAF6). | [31] |
| 18 | miR-181 | Atherosclerosis and vascular remodelling | ApoE−/− mice | IB kinase (IKK) complex regulatory/scaffold subunits TAB2 and NEMO are critical for inflammation-induced canonical NF-κB activation, which leads to the phosphorylation and degradation of IBs and the nuclear translocation of p65, thereby inhibiting vascular inflammation. | [32] |
| Sr No | lnc RNAs | Disease Implicated in | Model | Mechanisms | Ref. |
|---|---|---|---|---|---|
| 1 | Malat-1 | MI | Postinfarct myocardium mice model | Post-MI, induced endothelial cell proliferation and ischemia-induced revascularization promoted by miR-145 regulation of TGF-1 expression cause cardiac fibrosis and decrease cardiac function. | [58,63] |
| 2 | lincRNA-p21 | Atherosclerosis | Carotid artery injury model | Modulator of cell death by inhibiting p53 transcription during atherosclerosis is inhibited by lentivirus-mediated siRNA release targeting lincRNA-p21, causing neointima hyperplasia. | [60] |
| 3 | MIAT | MI | Mice | A molecular sponge for miR-15097 and a target gene modulator for the fibrosis-related factors miR-24, furin, and TGF-β1. | [58,64] |
| 4 | CARL | MI | Rat | Block particular microRNAs to control cardiomyocyte cell death. | [65] |
| 5 | CHRF | Cardiac hypertrophy | Transgenic mice that overexpress miR-489 in the heart | Directly bind miR-489 and control expression of MyD88 and hypertrophy. | [66] |
| 6 | Chast | Cardiac hypertrophy | TAC-operated mice | Repression of the pleckstrin homology domain containing protein family M member 1 promotes hypertrophy and prevents autophagy in cardiomyocytes. | [67] |
| 7 | Mhrt | Cardiac hypertrophy | TAC-operated mice | Mhrt activity is inhibited under pathological stress situations including pressure overload–induced hypertrophy and interferes with chromatin remodelling factor Brg1, regulating its target genes Myh6, Myh7, and osteopontin. | [68,69] |
| 8 | Meg3 | Cardiac hypertrophy | C57BL6 mice | MMP-2 upregulation in cardiac fibroblasts induced cardiac fibrosis and diastolic dysfunction. | [59,70]. |
| 9 | lncRNA H19 | Coronary artery disease | Mice | Induced through vascular damage and human atherosclerotic plaques. | [71] |
| 10 | linc00323-003 | Atherosclerosis | Inhibit the transcription of GATA2 (GATA-binding protein 2), a critical endothelial transcription factor that may control cell sproliferation and tube formation. | [72] |
| Vaccines | Type | Clinical Trial | Ref | |
|---|---|---|---|---|
| TQJ230 (AKCEA-APO(a)-LRx), | Lipoprotein mRNA-specific ASO | Lowering of lipoprotein | Phase II | [181] |
| Volanesorsen | ASO | This ASO, which can be given subcutaneously, inhibits APOC3 mRNA stability by binding to it. Apoc-II was reduced by 80%, triglycerides by 71%, and HDL-C by 46%. | Phase II | [213] |
| CDR 132L | ASO | Heart failure | Phase I | [214] |
| MRG-110 | ASO | Heart failure and ischemia; wounds (NDR) | Phase I (no development reported) (NDR) | [215] |
| SPC 4955 | ASO | Hypercholesterolemia | Discontinued | [215] |
| SPC 5001 | ASO | Hypercholesterolemia | Discontinued | [216] |
| ISIS CRPRx | ASO | Atrial fibrillation | Discontinued | [217] |
| ARO APOC3 | siRNA | Dyslipidemias; hypertriglyceridemia (II); hyperlipoproteinemia type I (III) | Phase III; phase II | [218] |
| Zilebesiran | siRNA | Hypertension (II); preeclampsia (NDR) | Phase II; no development reported | [219] |
| Olpasiran | siRNA | CVD | Phase II | [220] |
| LY 3,561,774 | siRNA | CVD; dyslipidaemias; metabolic disorders | Phase I | [221] |
| LY 3,819,469 | siRNA | CVD; metabolic disorders | Phase I | [222] |
| SLN 360 | siRNA | CVD (Pre); dyslipidaemias; hyperlipidaemia(I) | Preclinical; phase I | [223] |
| miR132-3p-inhibitor (CDR132L) | miR | Stable heart failure | Phase I | [224] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaharyar, M.A.; Bhowmik, R.; Al-Abbasi, F.A.; AlGhamdi, S.A.; Alghamdi, A.M.; Sarkar, A.; Kazmi, I.; Karmakar, S. Vaccine Formulation Strategies and Challenges Involved in RNA Delivery for Modulating Biomarkers of Cardiovascular Diseases: A Race from Laboratory to Market. Vaccines 2023, 11, 241. https://doi.org/10.3390/vaccines11020241
Shaharyar MA, Bhowmik R, Al-Abbasi FA, AlGhamdi SA, Alghamdi AM, Sarkar A, Kazmi I, Karmakar S. Vaccine Formulation Strategies and Challenges Involved in RNA Delivery for Modulating Biomarkers of Cardiovascular Diseases: A Race from Laboratory to Market. Vaccines. 2023; 11(2):241. https://doi.org/10.3390/vaccines11020241
Chicago/Turabian StyleShaharyar, Md. Adil, Rudranil Bhowmik, Fahad A. Al-Abbasi, Shareefa A. AlGhamdi, Amira M. Alghamdi, Arnab Sarkar, Imran Kazmi, and Sanmoy Karmakar. 2023. "Vaccine Formulation Strategies and Challenges Involved in RNA Delivery for Modulating Biomarkers of Cardiovascular Diseases: A Race from Laboratory to Market" Vaccines 11, no. 2: 241. https://doi.org/10.3390/vaccines11020241
APA StyleShaharyar, M. A., Bhowmik, R., Al-Abbasi, F. A., AlGhamdi, S. A., Alghamdi, A. M., Sarkar, A., Kazmi, I., & Karmakar, S. (2023). Vaccine Formulation Strategies and Challenges Involved in RNA Delivery for Modulating Biomarkers of Cardiovascular Diseases: A Race from Laboratory to Market. Vaccines, 11(2), 241. https://doi.org/10.3390/vaccines11020241







