The Societal Value of Vaccines: Expert-Based Conceptual Framework and Methods Using COVID-19 Vaccines as a Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Targeted Literature Review (TLR)
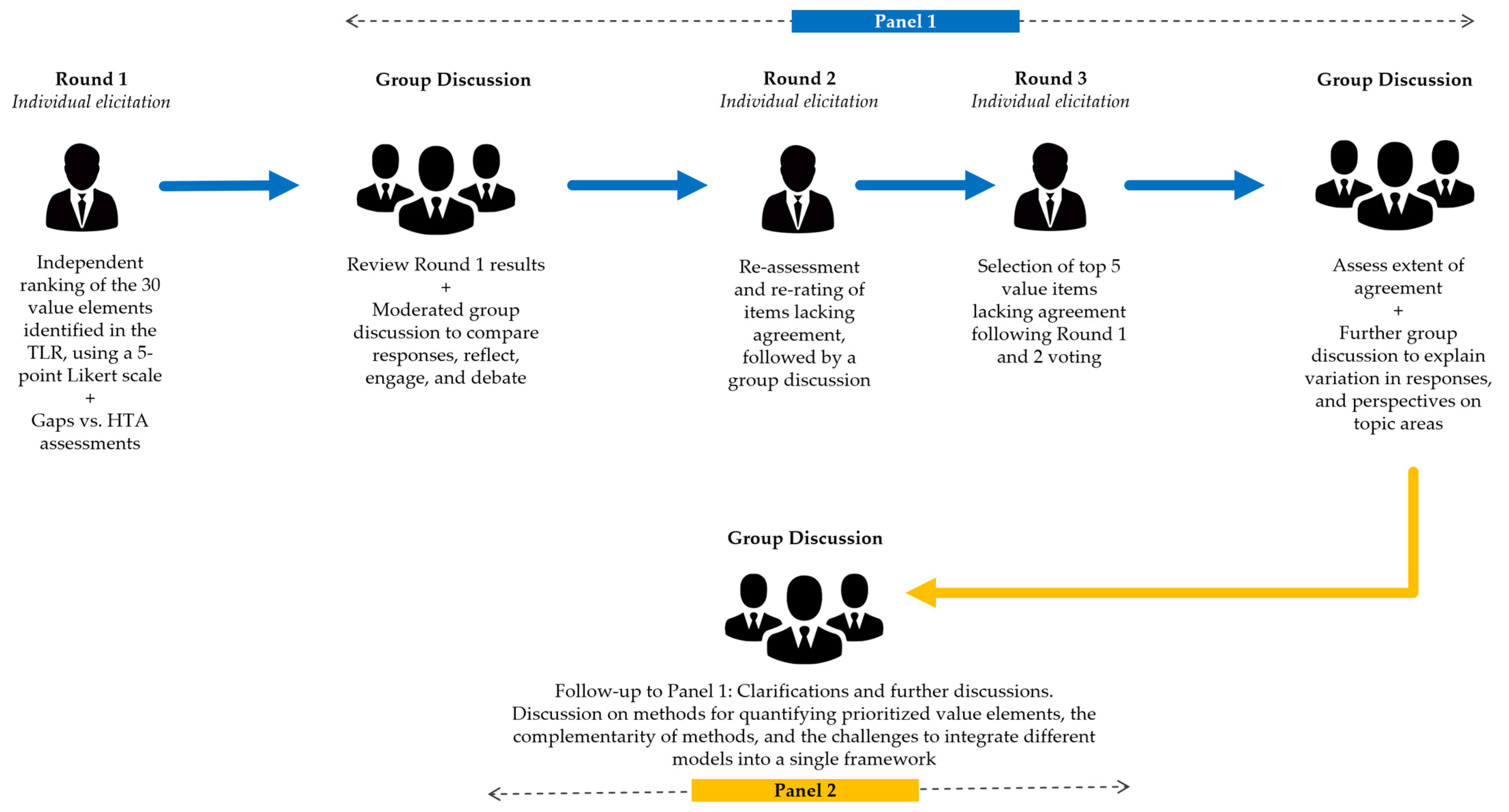
2.2. Expert Elicitation
2.2.1. Round 1: Individual Expert Elicitation
2.2.2. Panel 1 (Rounds 2 and 3)
2.2.3. Panel 2
3. Results
3.1. Findings of the Targeted Literature Review
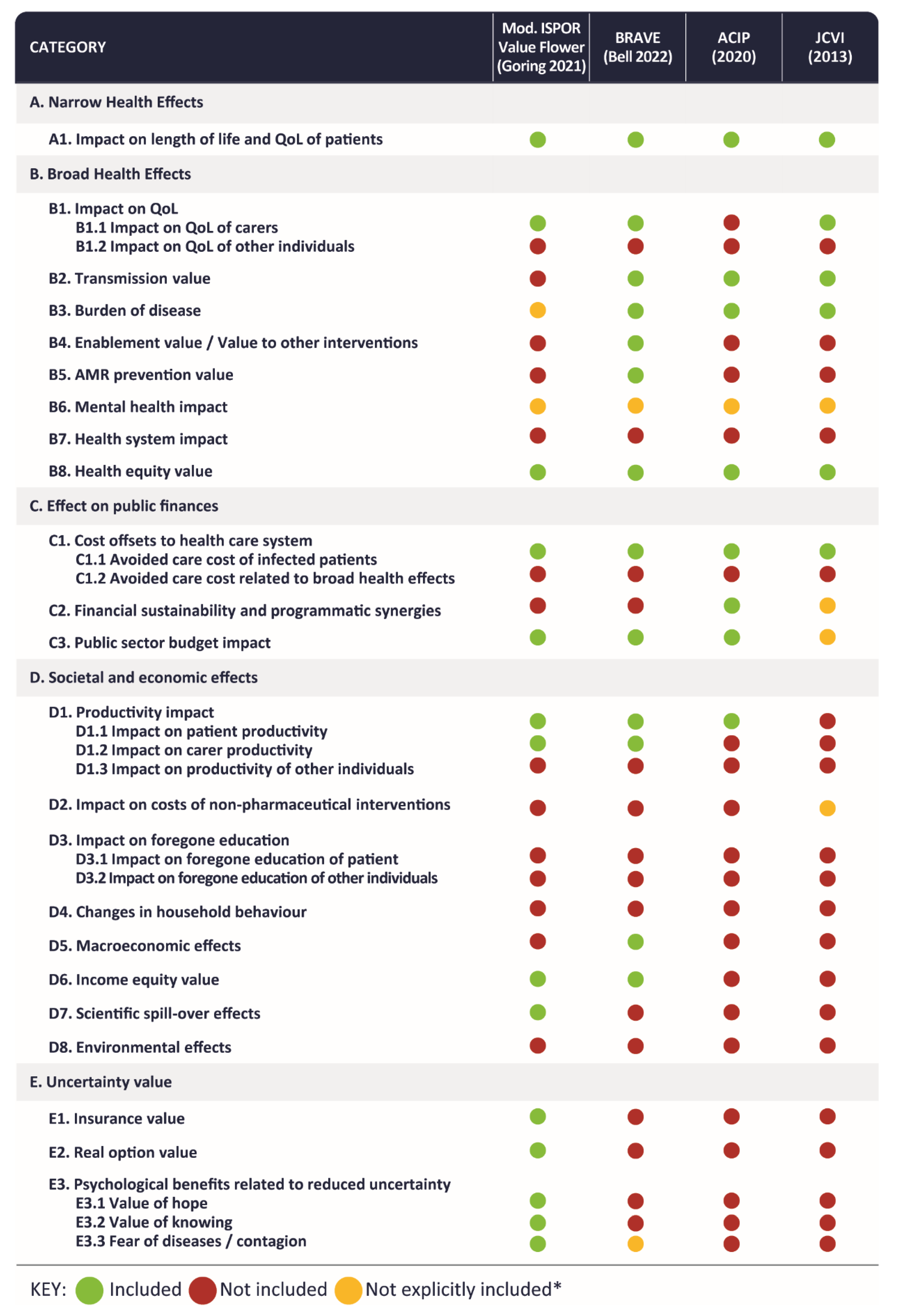
3.1.1. Identified Value Elements
3.1.2. Identified Quantification Methods
3.2. Results of the Expert Elicitations
3.2.1. Panel Characteristics
3.2.2. Conceptual Value Framework and Considerations
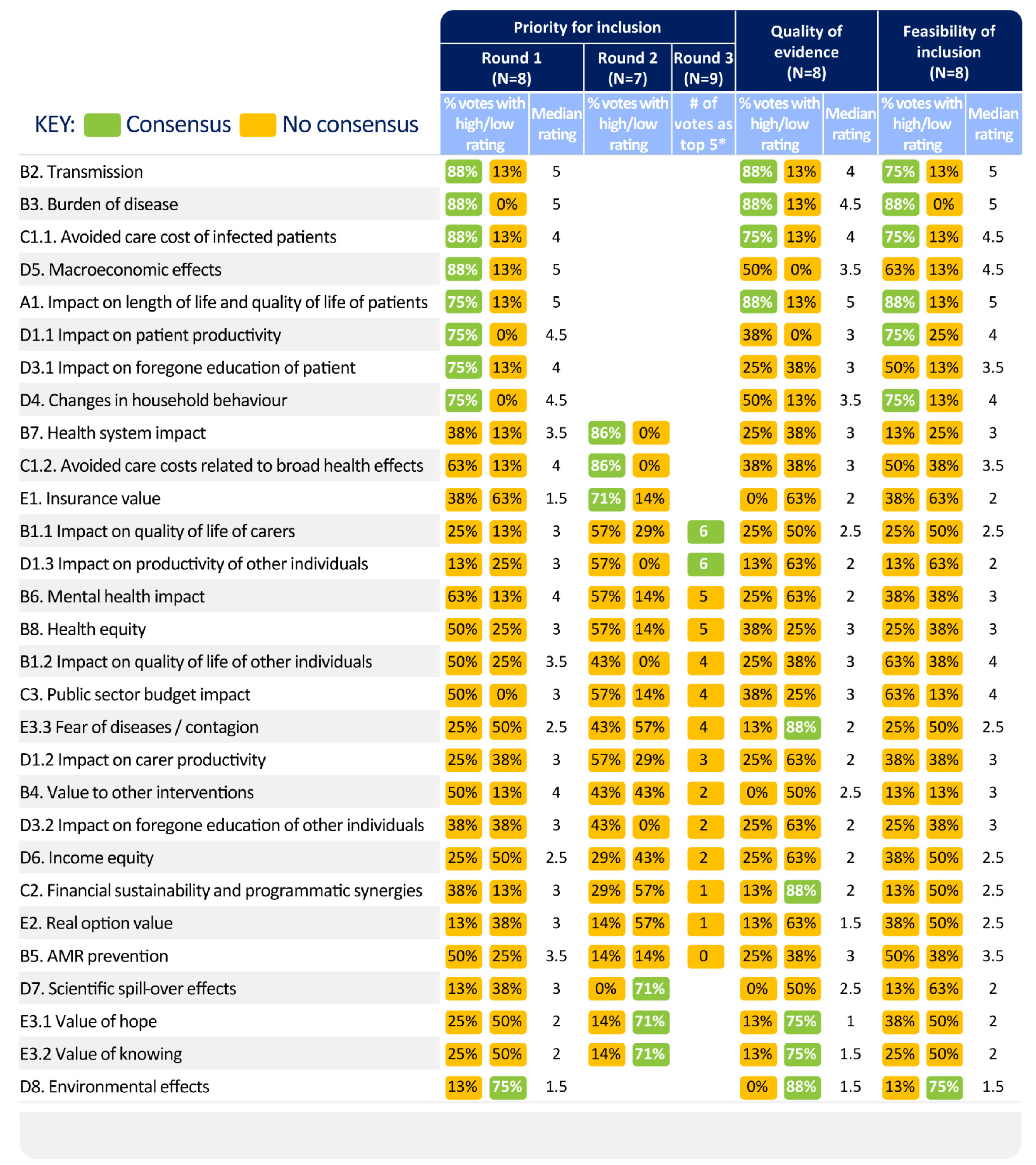
3.2.3. Prioritisation of Value Elements for COVID-19 Vaccine Evaluations
3.2.4. Evaluation of Identified Quantification Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mofijur, M.; Fattah, I.M.R.; Alam, M.A.; Islam, A.B.M.S.; Ong, H.C.; Rahman, S.M.A.; Najafi, G.; Ahmed, S.F.; Uddin, M.A.; Mahlia, T.M.I. Impact of COVID-19 on the social, economic, environmental and energy domains: Lessons learnt from a global pandemic. Sustain. Prod. Consum. 2021, 26, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; Patterson, B.J.; Yektashenas, B.; Mesa-Frias, M.; Tronczynski, K.; et al. Economic Burden of COVID-19: A Systematic Review. Clin. Outcomes Res. 2022, 14, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Okeagu, C.N.; Pham, A.D.; Silva, R.A.; Hurley, J.J.; Arron, B.L.; Sarfraz, N.; Lee, H.N.; Ghali, G.E.; Gamble, J.W.; et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Saladino, V.; Algeri, D.; Auriemma, V. The Psychological and Social Impact of Covid-19: New Perspectives of Well-Being. Front. Psychol. 2020, 11, 2550. [Google Scholar] [CrossRef] [PubMed]
- Hartley, D.M.; Perencevich, E.N. Public Health Interventions for COVID-19: Emerging Evidence and Implications for an Evolving Public Health Crisis. JAMA 2020, 323, 1908–1909. [Google Scholar] [CrossRef]
- Ayouni, I.; Maatoug, J.; Dhouib, W.; Zammit, N.; Fredj, S.B.; Ghammam, R.; Ghannem, H. Effective public health measures to mitigate the spread of COVID-19: A systematic review. BMC Public Health 2021, 21, 1015. [Google Scholar] [CrossRef]
- Neville, F.G.; Templeton, A.; Smith, J.R.; Louis, W.R. Social norms, social identities and the COVID-19 pandemic: Theory and recommendations. Soc. Personal. Psychol. Compass 2021, 15, e12596. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.; Liu, Y.; Wilbanks, D.; Jackson, J.C.; Mu, Y. Perception of strong social norms during the COVID-19 pandemic is linked to positive psychological outcomes. BMC Public Health 2022, 22, 1403. [Google Scholar] [CrossRef]
- Advisory Committee on Immunization Practice (ACIP). ACIP Evidence to Recommendation User’s Guide. Available online: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed on 23 June 2022).
- Joint Committee on Vaccination and Immunisation (JCVI). Code of Practice June 2013. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/224864/JCVI_Code_of_Practice_revision_2013_-_final.pdf (accessed on 23 June 2022).
- National Institute for Health and Care Excellence (NICE). Guide to the Methods of Technology Appraisal 2013; NICE: London, UK, 2013. [Google Scholar]
- Bell, E.; Neri, M.; Steuten, L. Towards a Broader Assessment of Value in Vaccines: The BRAVE Way Forward. Appl. Health Econ. Health Policy 2022, 20, 105–117. [Google Scholar] [CrossRef]
- Asukai, Y.; Briggs, A.; Garrison, L.P.; Geisler, B.P.; Neumann, P.J.; Ollendorf, D.A. Principles of Economic Evaluation in a Pandemic Setting: An Expert Panel Discussion on Value Assessment During the Coronavirus Disease 2019 Pandemic. Pharmacoeconomics 2021, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Barnighausen, T.; Bloom, D.E.; Cafiero-Fonseca, E.T.; O’Brien, J.C. Valuing vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12313–12319. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Brenzel, L.; Cadarette, D.; Sullivan, J. Moving beyond traditional valuation of vaccination: Needs and opportunities. Vaccine 2017, 35 (Suppl. 1), A29–A35. [Google Scholar] [CrossRef] [PubMed]
- Brassel, S.; Neri, M.; Steuten, L. Realising the Value of Vaccines in the UK: Ready for Prime Time? OHE Consulting Report. Office of Health Economics: London, UK. Available online: https://www.ohe.org/publications/realising-broader-value-vaccines-uk-ready-prime-time (accessed on 3 July 2022).
- Brassel, S.; Neri, M.; Schirrmacher, H.; Steuten, L. The Value of Vaccines in Maintaining Health System Capacity in England; Office of Health Economics: London, UK, 2021. [Google Scholar]
- Brassel, S.; Steuten, L. The Broader Value of Vaccines: The Return on Investment from A Governmental Perspective; Office of Health Economics: London, UK, 2020. [Google Scholar]
- Deogaonkar, R.; Hutubessy, R.; van der Putten, I.; Evers, S.; Jit, M. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health 2012, 12, 878. [Google Scholar] [CrossRef]
- Department of Health and Social Care (DHSC). Cost-Effectiveness Methodology for Vaccination Programmes. Consultation on the Cost-Effectiveness Methodology for Vaccination Programmes and Procurement (CEMIPP) Report. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/707847/cemipp-consultation-document.pdf (accessed on 23 June 2022).
- Goring, S.G.L.; Jansen, J.P.; Briggs, A.H. Navigating the publishing landscape: Novel elements of the value flower: Fake or truly novel? Value Outcomes Spotlight 2021, 7, 34–39. [Google Scholar]
- Jit, M.; Hutubessy, R. Methodological Challenges to Economic Evaluations of Vaccines: Is a Common Approach Still Possible? Appl. Health Econ. Health Policy 2016, 14, 245–252. [Google Scholar] [CrossRef]
- Lakdawalla, D.N.; Doshi, J.A.; Garrison, L.P., Jr.; Phelps, C.E.; Basu, A.; Danzon, P.M. Defining Elements of Value in Health Care-A Health Economics Approach: An ISPOR Special Task Force Report [3]. Value Health 2018, 21, 131–139. [Google Scholar] [CrossRef]
- Mauskopf, J.; Standaert, B.; Connolly, M.P.; Culyer, A.J.; Garrison, L.P.; Hutubessy, R.; Jit, M.; Pitman, R.; Revill, P.; Severens, J.L. Economic Analysis of Vaccination Programs: An ISPOR Good Practices for Outcomes Research Task Force Report. Value Health 2018, 21, 1133–1149. [Google Scholar] [CrossRef]
- Postma, M.; Biundo, E.; Chicoye, A.; Devlin, N.; Doherty, T.M.; Garcia-Ruiz, A.J.; Jaros, P.; Sheikh, S.; Toumi, M.; Wasem, J.; et al. Capturing the value of vaccination within health technology assessment and health economics: Country analysis and priority value concepts. Vaccine 2022, 40, 3999–4007. [Google Scholar] [CrossRef]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef]
- Bloom, D.E.; Cadarette, D.; Ferranna, M. The Societal Value of Vaccination in the Age of COVID-19. Am. J. Public Health 2021, 111, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Standaert, B.; Sauboin, C.; DeAntonio, R.; Marijam, A.; Gomez, J.; Varghese, L.; Zhang, S. How to assess for the full economic value of vaccines? From past to present, drawing lessons for the future. J. Mark. Access Health Policy 2020, 8, 1719588. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Djaafara, B.; Haw, D.; Doohan, P.; Forchini, G.; Pianella, M.; Hauck, K. Report 51—Valuing Lives, Education and the Economy in An Epidemic: Societal Benefit of SARS-CoV-2 Booster Vaccinations in Indonesia; Imperial College London: London, UK, 2022. [Google Scholar]
- McMillan, S.S.; King, M.; Tully, M.P. How to use the nominal group and Delphi techniques. Int. J. Clin. Pharm. 2016, 38, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Aggarwal, R.; Khanna, D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin. Arthritis Rheum. 2011, 41, 95–105. [Google Scholar] [CrossRef]
- Taylor, E. We Agree, Don’t We? The Delphi Method for Health Environments Research. HERD 2020, 13, 11–23. [Google Scholar] [CrossRef]
- Grisham, T. The Delphi technique: A method for testing complex and multifaceted topics. Int. J. Manag. Proj. Bus. 2009, 2, 112–130. [Google Scholar] [CrossRef]
- Mullen, P.M. Delphi: Myths and reality. J. Health Organ. Manag. 2003, 17, 37–52. [Google Scholar] [CrossRef]
- Fitch, K.; Bernstein, S.J.; Aguilar, M.D.; Burnand, B.; LaCalle, J.R.; Lazaro, P.; van het Loo, M.; McDonnell, J.; Vader, J.; Kahan, J.P. The RAND/UCLA Appropriateness Method User’s Manual; RAND Corporation: Santa Monica, CA, USA, 2001. [Google Scholar]
- Diamond, I.R.; Grant, R.C.; Feldman, B.M.; Pencharz, P.B.; Ling, S.C.; Moore, A.M.; Wales, P.W. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J. Clin. Epidemiol. 2014, 67, 401–409. [Google Scholar] [CrossRef]
- Henderson, E.J.; Rubin, G.P. Development of a community-based model for respiratory care services. BMC Health Serv. Res. 2012, 12, 193. [Google Scholar] [CrossRef]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Standardised method for reporting exercise programmes: Protocol for a modified Delphi study. BMJ Open 2014, 4, e006682. [Google Scholar] [CrossRef]
- Cutler, D.M.; Summers, L.H. The COVID-19 Pandemic and the $16 Trillion Virus. JAMA 2020, 324, 1495–1496. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Social Care (DHSC); Office for National Statistics (ONS). Direct and Indirect Health Impacts of COVID-19 in England. Available online: https://www.gov.uk/government/publications/dhsc-direct-and-indirect-health-impacts-of-covid-19-in-england-long-paper-9-september-2021 (accessed on 23 June 2022).
- Kirson, N.; Swallow, E.; Lu, J.; Mesa-Frias, M.; Bookhart, B.; Maynard, J.; Shivdasani, Y.; Lefebvre, P. The societal economic value of COVID-19 vaccines in the United States. J. Med. Econ. 2022, 25, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Mayo, M.; Potugari, B.; Bzeih, R.; Scheidel, C.; Carrera, C.; Shellenberger, R.A. Cancer Screening During the COVID-19 Pandemic: A Systematic Review and Meta-analysis. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Buehrle, D.J.; Nguyen, M.H.; Wagener, M.M.; Clancy, C.J. Impact of the Coronavirus Disease 2019 Pandemic on Outpatient Antibiotic Prescriptions in the United States. Open Forum Infect. Dis. 2020, 7, ofaa575. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Lane Clark & Peacock (LCP). Hidden Health Needs ‘the Elephant in the NHS Waiting Room’ as Waiting List Number Could Rise to over 15 Million in 2023. Available online: https://www.lcp.uk.com/media-centre/2021/12/hidden-health-needs-the-elephant-in-the-nhs-waiting-room-as-waiting-list-number-could-rise-to-over-15-million-in-2023/ (accessed on 23 June 2022).
- Pecetta, S.; Tortorice, D.; Scorza, F.B.; Pizza, M.; Dougan, G.; Hatchett, R.; Black, S.; Bloom, D.E.; Rappuoli, R. The trillion dollar vaccine gap. Sci. Transl. Med. 2022, 14, eabn4342. [Google Scholar] [CrossRef]
- Bloom, D.E.; Fan, V.Y.; Sevilla, J.P. The broad socioeconomic benefits of vaccination. Sci. Transl. Med. 2018, 10, eaaj2345. [Google Scholar] [CrossRef]
- Ferranna, M.; Robinson, L.A.; Cadarette, D.; Eber, M.; Bloom, D.E. The Benefits and Costs of U.S. Employer COVID-19 Vaccine Mandates; National Bureau of Economic Research: Cambridge, MA, USA, 2022. [Google Scholar]
- Congressional Budget Office. Budgetary Effects of the 2020 Coronavirus Pandemic. Available online: https://www.cbo.gov/publication/56388 (accessed on 23 June 2022).
- Heald, D.; Hodges, R. The accounting, budgeting and fiscal impact of COVID-19 on the United Kingdom. J. Public Budg. Account. Financ. Manag. 2020, 32, 785–795. [Google Scholar] [CrossRef]
- Arnon, A.; Ricco, J.; Smetters, K. Epidemiological and economic effects of lockdown. Brook. Pap. Econ. Act. 2020, 2020, 61–108. [Google Scholar] [CrossRef]
- Chen, J.; Vullikanti, A.; Santos, J.; Venkatramanan, S.; Hoops, S.; Mortveit, H.; Lewis, B.; You, W.; Eubank, S.; Marathe, M. Epidemiological and Economic Impact of COVID-19 in the US. Sci. Rep. 2021, 11, 20451. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.-j.; Lee, Y. Economic consequences of the COVID-19 pandemic: Will it be a barrier to achieving sustainability? Sustainability 2022, 14, 1629. [Google Scholar] [CrossRef]
- Chudik, A.; Mohaddes, K.; Pesaran, M.H.; Raissi, M.; Rebucci, A. A counterfactual economic analysis of Covid-19 using a threshold augmented multi-country model. J. Int. Money Financ. 2021, 119, 102477. [Google Scholar] [CrossRef] [PubMed]
- Hanushek, E.A.; Woessmann, L. The Economic Impacts of Learning Losses. 2020. Available online: https://www.oecd.org/education/The-economic-impacts-of-coronavirus-covid-19-learning-losses.pdf (accessed on 23 June 2022).
- Keogh-Brown, M.R.; Jensen, H.T.; Edmunds, W.J.; Smith, R.D. The impact of Covid-19, associated behaviours and policies on the UK economy: A computable general equilibrium model. SSM Popul. Health 2020, 12, 100651. [Google Scholar] [CrossRef] [PubMed]
- Wharton, P. COVID-19 Learning Loss: Long-Run Macroeconomic Effects Update. University of Pennsylvania. Available online: https://budgetmodel.wharton.upenn.edu/issues/2021/10/27/covid-19-learning-loss-long-run-macro-effects (accessed on 23 June 2022).
- Sandmann, F.G.; Davies, N.G.; Vassall, A.; Edmunds, W.J.; Jit, M.; Sun, F.Y.; Villabona-Arenas, C.J.; Nightingale, E.S.; Showering, A.; Knight, G.M.; et al. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: A transmission model-based future scenario analysis and economic evaluation. Lancet Infect. Dis. 2021, 21, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Mishra, V. Environmental impacts of coronavirus disease 2019 (COVID-19). Bioresour. Technol. Rep. 2021, 15, 100744. [Google Scholar] [CrossRef]
- Foundation, T.H. Health and Social Care Funding to 2024/25. SLIDE Deck of Key Findings; The Health Foundation: London, UK, 2021. [Google Scholar]
- Nguyen, M. The Psychological Benefits of COVID-19 Vaccination. Adv. Public Health 2021, 2021, 1718800. [Google Scholar] [CrossRef]
- Venter, Z.S.; Aunan, K.; Chowdhury, S.; Lelieveld, J. COVID-19 lockdowns cause global air pollution declines. Proc. Natl. Acad. Sci. USA 2020, 117, 18984–18990. [Google Scholar] [CrossRef]
- Groves, H.E.; Piché-Renaud, P.-P.; Peci, A.; Farrar, D.S.; Buckrell, S.; Bancej, C.; Sevenhuysen, C.; Campigotto, A.; Gubbay, J.B.; Morris, S.K. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg. Health Am. 2021, 1, 100015. [Google Scholar] [CrossRef]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2022. [Google Scholar] [CrossRef]
- Huang, Q.S.; Wood, T.; Jelley, L.; Jennings, T.; Jefferies, S.; Daniells, K.; Nesdale, A.; Dowell, T.; Turner, N.; Campbell-Stokes, P.; et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat. Commun. 2021, 12, 1001. [Google Scholar] [CrossRef]
- van den Hout, W.B. The value of productivity: Human-capital versus friction-cost method. Ann. Rheum. Dis. 2010, 69, i89. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fusco, M.; Mendes, D.; Steuten, L.; Bloom, D.E.; Drummond, M.; Hauck, K.; Pearson-Stuttard, J.; Power, R.; Salisbury, D.; Towse, A.; et al. The Societal Value of Vaccines: Expert-Based Conceptual Framework and Methods Using COVID-19 Vaccines as a Case Study. Vaccines 2023, 11, 234. https://doi.org/10.3390/vaccines11020234
Di Fusco M, Mendes D, Steuten L, Bloom DE, Drummond M, Hauck K, Pearson-Stuttard J, Power R, Salisbury D, Towse A, et al. The Societal Value of Vaccines: Expert-Based Conceptual Framework and Methods Using COVID-19 Vaccines as a Case Study. Vaccines. 2023; 11(2):234. https://doi.org/10.3390/vaccines11020234
Chicago/Turabian StyleDi Fusco, Manuela, Diana Mendes, Lotte Steuten, David E Bloom, Michael Drummond, Katharina Hauck, Jonathan Pearson-Stuttard, Rachel Power, David Salisbury, Adrian Towse, and et al. 2023. "The Societal Value of Vaccines: Expert-Based Conceptual Framework and Methods Using COVID-19 Vaccines as a Case Study" Vaccines 11, no. 2: 234. https://doi.org/10.3390/vaccines11020234
APA StyleDi Fusco, M., Mendes, D., Steuten, L., Bloom, D. E., Drummond, M., Hauck, K., Pearson-Stuttard, J., Power, R., Salisbury, D., Towse, A., Roiz, J., Szabo, G., Yang, J., & Marczell, K. (2023). The Societal Value of Vaccines: Expert-Based Conceptual Framework and Methods Using COVID-19 Vaccines as a Case Study. Vaccines, 11(2), 234. https://doi.org/10.3390/vaccines11020234





