Ginseng Polysaccharide Enhances the Humoral and Cellular Immune Responses to SARS-CoV-2 RBD Protein Subunit Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Protein Expression and Purification
2.3. BAL Collection
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Lymphocyte Preparation
2.6. IFN-γ Enzyme Linked Immunospot Assay
2.7. Flow Cytometry
2.8. Intracellular Cytokine Staining
2.9. Statistical Analysis
3. Results
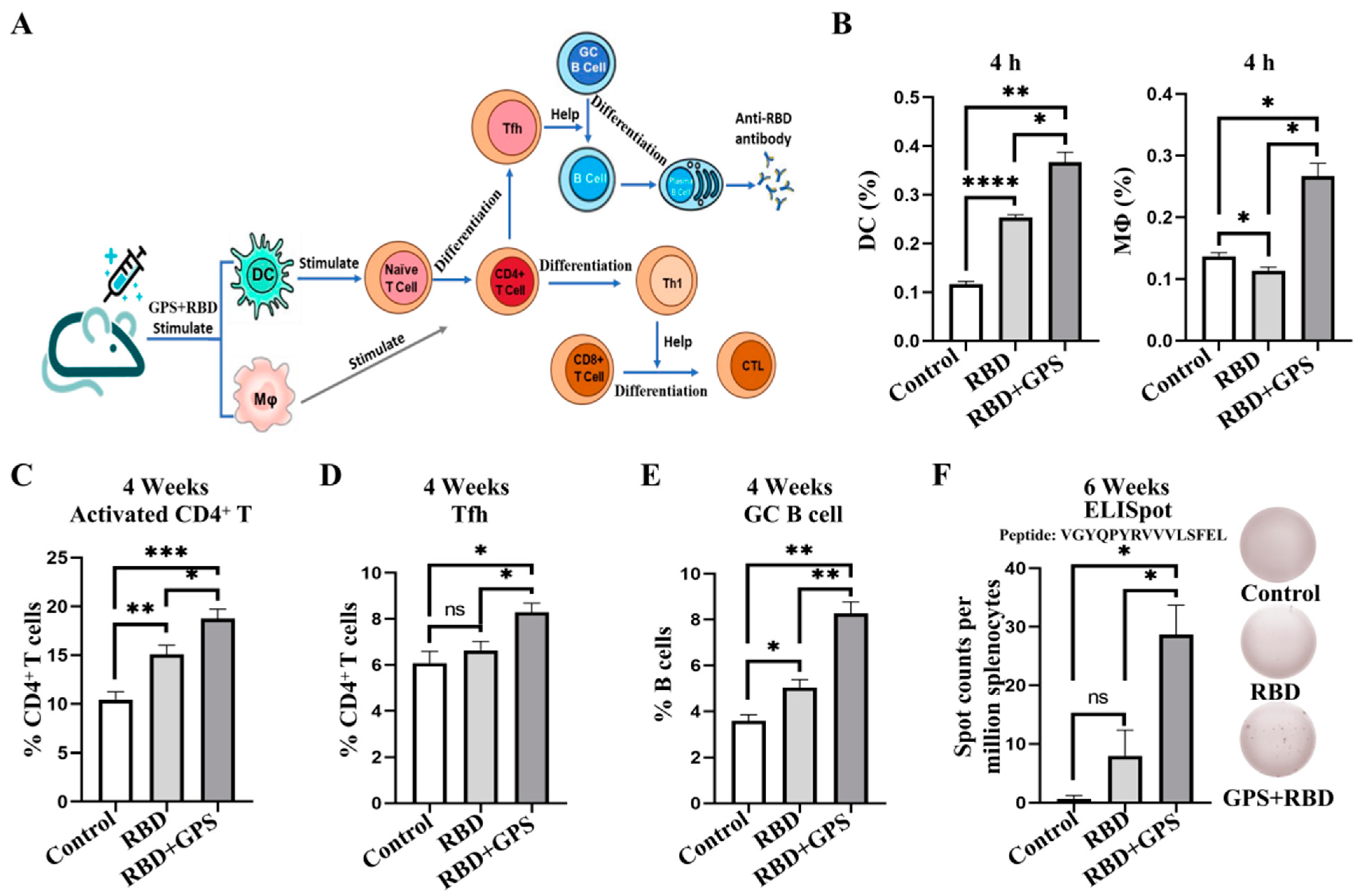
3.1. Ginseng Polysaccharide-Assisted RBD Protein More Effectively Initiated Immune Response
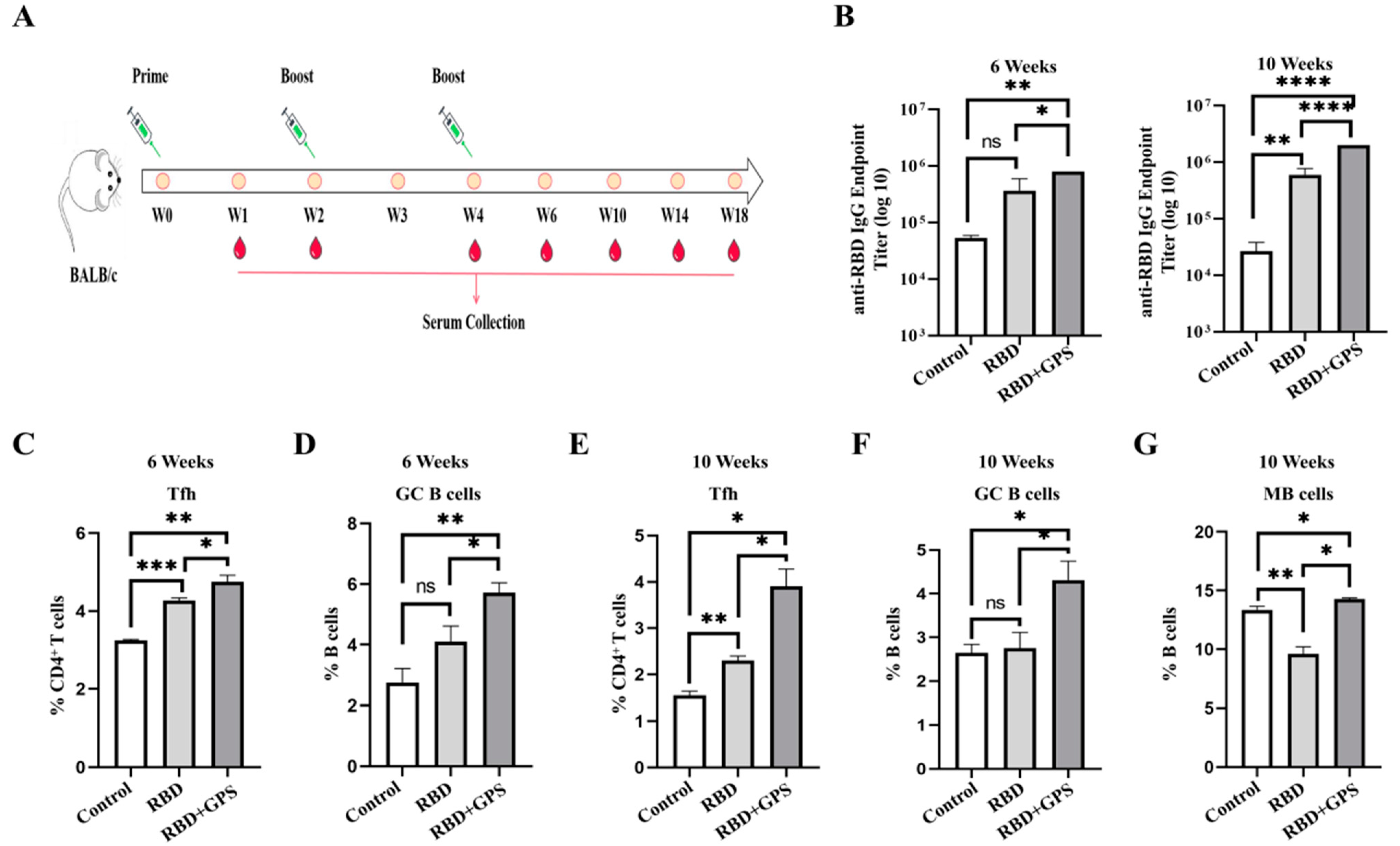
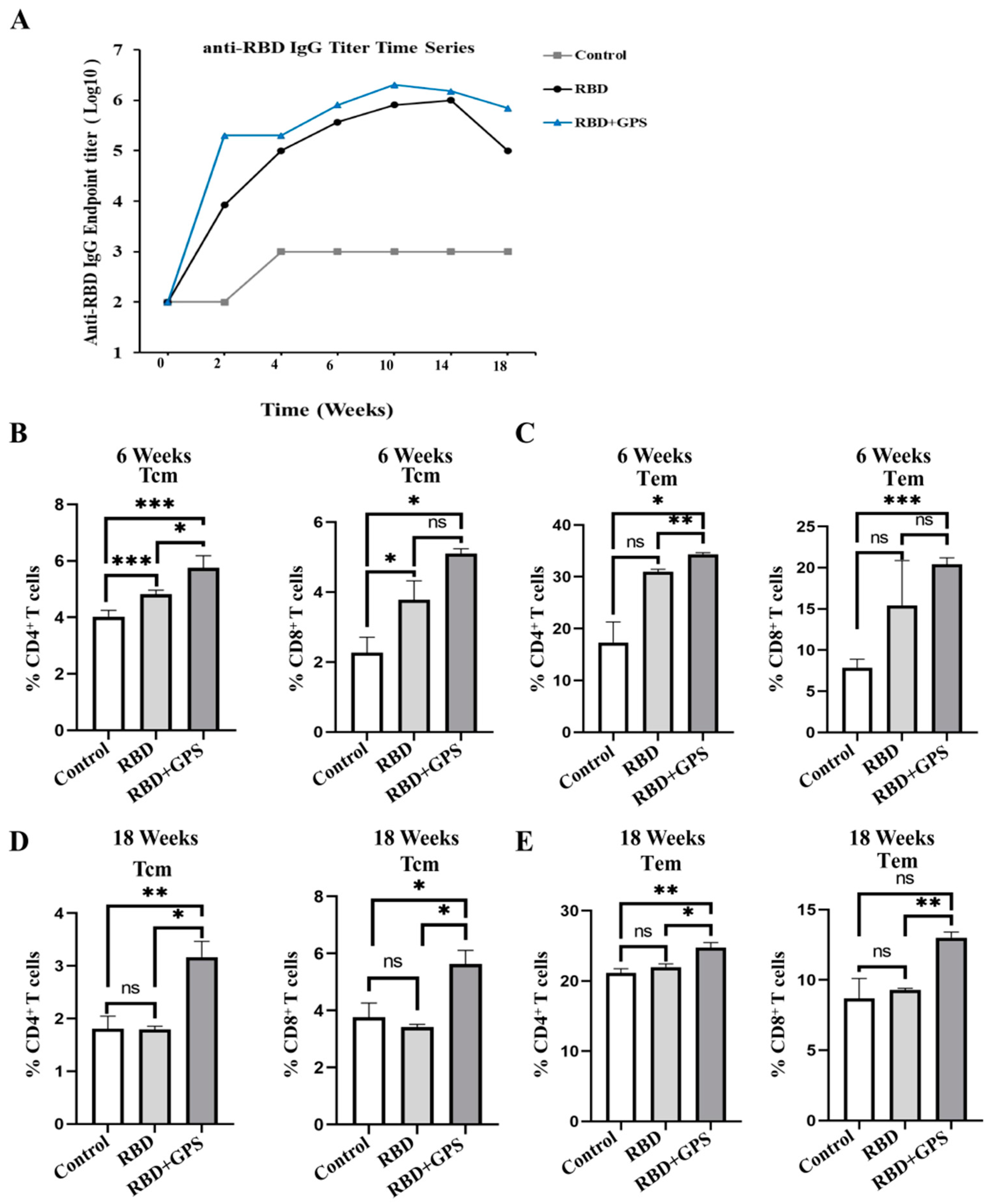
3.2. Ginseng Polysaccharides Enhanced Humoral Immune Responses in RBD Protein
3.3. Pulmonary Biodistribution of SARS-CoV-2 RBD Reactive IgG and IgA
3.4. GPS Improved RBD Protein Cellular Immune Responses in Mice
3.5. Ginseng Polysaccharides Enhanced Long-Term Protective Memory in RBD Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, S. Timely development of vaccines against SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yuan, M.; Powers, J.M.; Hu, M.; Munt, J.E.; Arunachalam, P.S.; Leist, S.R.; Bellusci, L.; Kim, J.; Sprouse, K.R.; et al. Broadly neutralizing antibodies against sarbecoviruses generated by immunization of macaques with an AS03-adjuvanted COVID-19 vaccine. Sci. Transl. Med. 2023, 15, eadg7404. [Google Scholar] [CrossRef] [PubMed]
- Chappell, K.J.; Mordant, F.L.; Li, Z.; Wijesundara, D.K.; Ellenberg, P.; Lackenby, J.A.; Cheung, S.T.M.; Modhiran, N.; Avumegah, M.S.; Henderson, C.L.; et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2021, 21, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: First Approval of the Protein-Based Adjuvanted Nuvaxovid (NVX-CoV2373) Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide Immune Protection from Viral Variants. Med. Sci. Monit. 2022, 28, e936523. [Google Scholar] [CrossRef] [PubMed]
- Seenappa, L.M.; Jakubowski, A.; Steinbuck, M.P.; Palmer, E.; Haqq, C.M.; Carter, C.; Fontenot, J.; Villinger, F.; McNeil, L.K.; DeMuth, P.C. Amphiphile-CpG vaccination induces potent lymph node activation and COVID-19 immunity in mice and non-human primates. NPJ Vaccines 2022, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Perelman, M.; Hinchcliffe, M. Chitosan: A promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccin. Immunother. 2014, 10, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Gan, F.; Zhang, Z.; Hu, J.; Chen, X.; Huang, K. Astragalus polysaccharides inhibits PCV2 replication by inhibiting oxidative stress and blocking NF-κB pathway. Int. J. Biol. Macromol. 2015, 81, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Zhuo, C.; He, Z.; Wang, H.; Liu, W.; Zhang, R.; Wang, Y. Anti-herpes simplex virus activity of polysaccharides from Eucheuma gelatinae. World J. Microbiol. Biotechnol. 2015, 31, 453–460. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.J.; Lim, J.H.; Yang, J.W.; Shin, J.C.; Lee, S.W.; Suh, J.W.; Hwang, S.B. Triterpenoid Saponins Isolated from Platycodon grandiflorum Inhibit Hepatitis C Virus Replication. Evid.-Based Complement. Altern. Med. 2013, 2013, 560417. [Google Scholar] [CrossRef]
- Shimizu, J.F.; Lima, C.S.; Pereira, C.M.; Bittar, C.; Batista, M.N.; Nazaré, A.C.; Polaquini, C.R.; Zothner, C.; Harris, M.; Rahal, P.; et al. Flavonoids from Pterogyne nitens Inhibit Hepatitis C Virus Entry. Sci. Rep. 2017, 7, 16127. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Zhao, W. The Adjuvant Effects on Vaccine and the Immunomodulatory Mechanisms of Polysaccharides from Traditional Chinese Medicine. Front. Mol. Biosci. 2021, 8, 655570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lv, C.; Lu, J. Natural occurring polysaccharides from Panax ginseng C. A. Meyer: A review of isolation, structures, and bioactivities. Int. J. Biol. Macromol. 2019, 133, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Shao, S.; Wang, D.; Zhao, D.; Wang, M. Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 2021, 12, 494–518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhang, F.; Pan, W.; Yang, Y.F.; Jiang, X.Y. Clinical potentials of ginseng polysaccharide for treating gestational diabetes mellitus. World J. Clin. Cases 2021, 9, 4959–4979. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Hwang, S.H.; Yoon, T.J.; Kim, S.H.; Shin, K.S. Polysaccharides from ginseng leaves inhibit tumor metastasis via macrophage and NK cell activation. Int. J. Biol. Macromol. 2017, 103, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Ye, Y.P.; Pan, H.J.; Pan, Y.J. Adjuvant effect of Panax notoginseng saponins on the immune responses to ovalbumin in mice. Vaccine 2004, 22, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Concha, C.; Lin, F.; Waller, K.P. Adjuvant effect of ginseng extracts on the immune responses to immunisation against Staphylococcus aureus in dairy cattle. Vet. Immunol. Immunopathol. 2003, 91, 29–37. [Google Scholar] [CrossRef]
- Rivera, E.; Hu, S.; Concha, C. Ginseng and aluminium hydroxide act synergistically as vaccine adjuvants. Vaccine 2003, 21, 1149–1157. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Huang, Y.; Li, X.; Feng, J.; Li, Y.; Zhang, R. A conserved N protein nano-vaccine of COVID-19 exerts potent and cross-reactive humoral and cellular immune responses in mice. J. Med. Virol. 2023, 95, e29115. [Google Scholar] [CrossRef]
- Kedzierska, K.; Thomas, P.G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Rep. Med. 2022, 3, 100562. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.T.; Sbrana, E.; Iwata-Yoshikawa, N.; Newman, P.C.; Garron, T.; Atmar, R.L.; Peters, C.J.; Couch, R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE 2012, 7, e35421. [Google Scholar] [CrossRef]
- Lin, L.; Ting, S.; Yufei, H.; Wendong, L.; Yubo, F.; Jing, Z. Epitope-based peptide vaccines predicted against novel coronavirus disease caused by SARS-CoV-2. Virus Res. 2020, 288, 198082. [Google Scholar] [CrossRef] [PubMed]
- Ashik, A.I.; Hasan, M.; Tasnim, A.T.; Chowdhury, M.B.; Hossain, T.; Ahmed, S. An immunoinformatics study on the spike protein of SARS-CoV-2 revealing potential epitopes as vaccine candidates. Heliyon 2020, 6, e04865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.e2417. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Walls, A.C.; Golden, N.; Atyeo, C.; Fischinger, S.; Li, C.; Aye, P.; Navarro, M.J.; Lai, L.; Edara, V.V.; et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 2021, 594, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, S.P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Meng, J.; Meng, Y.; Liang, Z.; Du, L.; Zhang, Z.; Hu, X.; Shan, F. Phenotypic and functional analysis of the modification of murine bone marrow dendritic cells (BMDCs) induced by neutral Ginseng polysaccharides (NGP). Hum. Vaccin. Immunother. 2013, 9, 233–241. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Primorac, D.; Vrdoljak, K.; Brlek, P.; Pavelić, E.; Molnar, V.; Matišić, V.; Ivkošić, E.; Parčina, M. Adaptive Immune Responses and Immunity to SARS-CoV-2. Front. Immunol. 2022, 13, 848582. [Google Scholar] [CrossRef] [PubMed]
- Çölkesen, F.; Kandemir, B.; Arslan, Ş.; Çölkesen, F.; Yıldız, E.; Korkmaz, C.; Vatansev, H.; Evcen, R.; Aykan, F.S.; Kılınç, M.; et al. Relationship between Selective IgA Deficiency and COVID-19 Prognosis. Jpn. J. Infect. Dis. 2022, 75, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Olivo, A.; Lécuroux, C.; Bitu, M.; Avettand-Fenoel, V.; Boufassa, F.; Essat, A.; Meyer, L.; Doisne, J.M.; Favier, B.; Vaslin, B.; et al. CXCR3 and CXCR5 are highly expressed in HIV-1-specific CD8 central memory T cells from infected patients. Eur. J. Immunol. 2021, 51, 2040–2050. [Google Scholar] [CrossRef]
- Mintz, M.A.; Cyster, J.G. T follicular helper cells in germinal center B cell selection and lymphomagenesis. Immunol. Rev. 2020, 296, 48–61. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Bhargava, B. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A dou-ble-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Dotiwala, F.; Upadhyay, A.K. A comprehensive review of BBV152 vaccine development, effectiveness, safety, challenges, and prospects. Front. Immunol. 2022, 13, 940715. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, Y.; Zhang, X.; Zhao, T.; Xu, J.; Han, R. Ginseng polysaccharide inhibits MDA-MB-231 cell proliferation by activating the inflammatory response. Exp. Ther. Med. 2020, 20, 229. [Google Scholar] [CrossRef]
- Ma, J.; Liu, H.; Wang, X. Effect of ginseng polysaccharides and dendritic cells on the balance of Th1/Th2 T helper cells in patients with non-small cell lung cancer. J. Tradit. Chin. Med. 2014, 34, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Son, H.J.; Kim, K.S. A 14-week randomized, placebo-controlled, double-blind clinical trial to evaluate the efficacy and safety of ginseng polysaccharide (Y-75). J. Transl. Med. 2014, 12, 283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Predy, G.N.; Goel, V.; Lovlin, R.; Donner, A.; Stitt, L.; Basu, T.K. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: A randomized controlled trial. CMAJ 2005, 173, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Singleton, K.L.; Joffe, A.; Leitner, W.W. Review: Current trends, challenges, and success stories in adjuvant research. Front. Immunol. 2023, 14, 1105655. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Feng, J.; Huang, Y.; Zhou, B.; Li, B.; Zhang, R. Ginseng Polysaccharide Enhances the Humoral and Cellular Immune Responses to SARS-CoV-2 RBD Protein Subunit Vaccines. Vaccines 2023, 11, 1833. https://doi.org/10.3390/vaccines11121833
Zhang J, Feng J, Huang Y, Zhou B, Li B, Zhang R. Ginseng Polysaccharide Enhances the Humoral and Cellular Immune Responses to SARS-CoV-2 RBD Protein Subunit Vaccines. Vaccines. 2023; 11(12):1833. https://doi.org/10.3390/vaccines11121833
Chicago/Turabian StyleZhang, Jing, Jing Feng, Yang Huang, Boyan Zhou, Bing Li, and Rongxin Zhang. 2023. "Ginseng Polysaccharide Enhances the Humoral and Cellular Immune Responses to SARS-CoV-2 RBD Protein Subunit Vaccines" Vaccines 11, no. 12: 1833. https://doi.org/10.3390/vaccines11121833
APA StyleZhang, J., Feng, J., Huang, Y., Zhou, B., Li, B., & Zhang, R. (2023). Ginseng Polysaccharide Enhances the Humoral and Cellular Immune Responses to SARS-CoV-2 RBD Protein Subunit Vaccines. Vaccines, 11(12), 1833. https://doi.org/10.3390/vaccines11121833





