Baseline Pneumococcal IgG Levels and Response to 23-Valent Pneumococcal Polysaccharide Vaccine among Adults from Beijing, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Clinical Samples
2.2. Vaccine
2.3. S. pneumoniae Serotype-Specific IgG Detection
2.4. Statistical Analysis
3. Results
3.1. Study Population
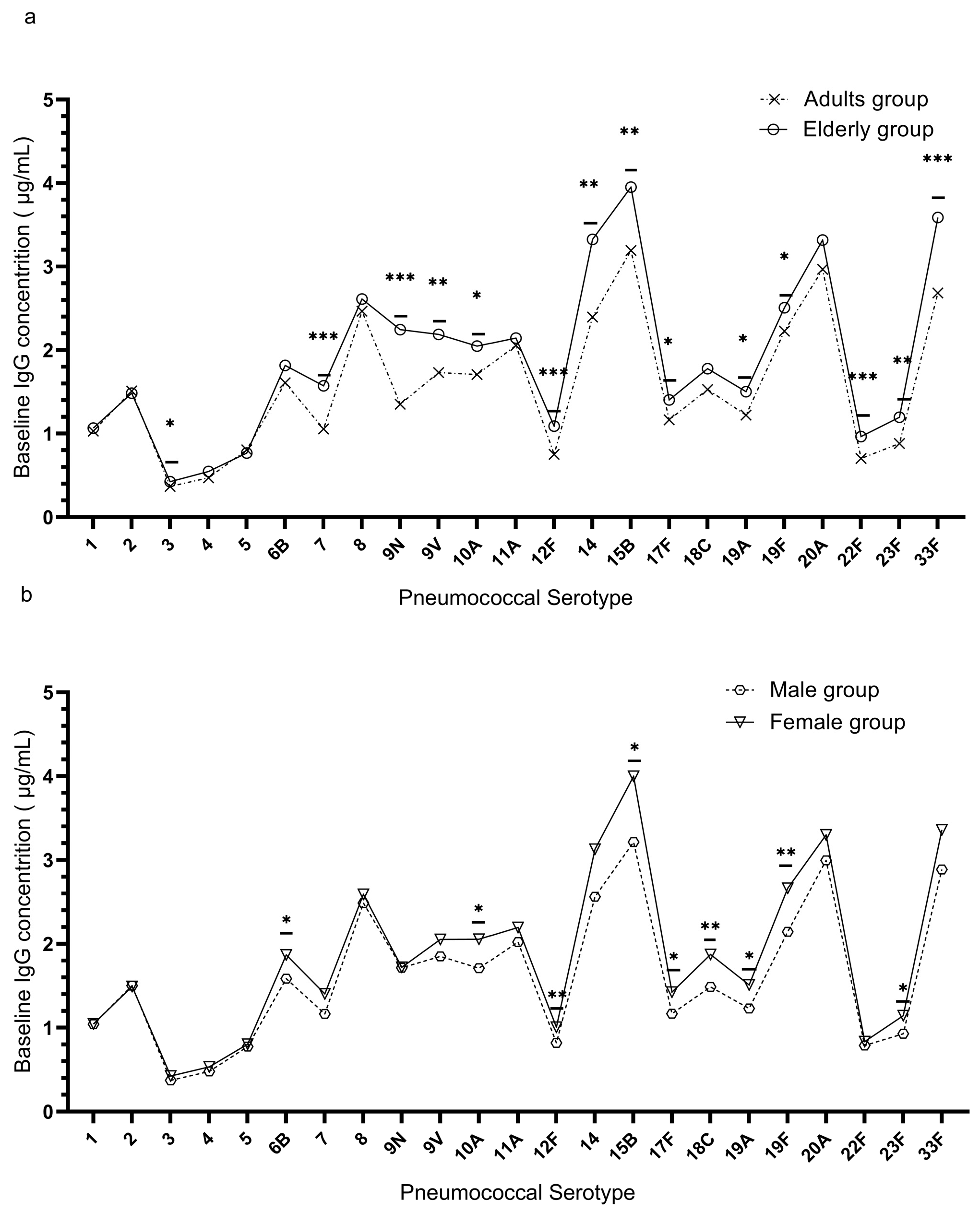
3.2. Distribution of Pre-Vaccination (Baseline) IgG Antibodies
3.3. Antibody Response to PPSV23
3.4. Relationship between Baseline IgG Concentration and Seroconversion Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Waz, N.T.; Oliveira, S.; Girardello, R.; Lincopan, N.; Barazzone, G.; Parisotto, T.; Hakansson, A.P.; Converso, T.R.; Darrieux, M. Influence of the Polysaccharide Capsule on the Bactericidal Activity of Indolicidin on Streptococcus pneumoniae. Front. Microbiol. 2022, 13, 898815. [Google Scholar] [CrossRef]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef]
- Hyams, C.; Challen, R.; Hettle, D.; Amin-Chowdhury, Z.; Grimes, C.; Ruffino, G.; Conway, R.; Heath, R.; North, P.; Malin, A.; et al. Serotype Distribution and Disease Severity in Adults Hospitalized with Streptococcus pneumoniae Infection, Bristol and Bath, UK, 2006–2022. Emerg. Infect. Dis. 2023, 29, 1953–1964. [Google Scholar] [CrossRef]
- Meeting of the Immunization Strategic Advisory Group of Experts, November 2007—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2008, 83, 1–15.
- Dhoubhadel, B.G.; Morimoto, K. Prevention of pneumococcal diseases: The challenge remains. Lancet Glob. Health. 2022, 10, e1375–e1376. [Google Scholar] [CrossRef]
- Deb, A.; Podmore, B.; Barnett, R.; Beier, D.; Galetzka, W.; Qizilbash, N.; Haeckl, D.; Mihm, S.; Johnson, K.D.; Weiss, T. Clinical and economic burden of pneumococcal disease among individuals aged 16 years and older in Germany. Epidemiol. Infect. 2022, 150, e204. [Google Scholar] [CrossRef]
- Adler, H.; German, E.L.; Mitsi, E.; Nikolaou, E.; Pojar, S.; Hales, C.; Robinson, R.; Connor, V.; Hill, H.; Hyder-Wright, A.D. Experimental Human Pneumococcal Colonization in Older Adults Is Feasible and Safe, Not Immunogenic. Am. J. Resp. Crit. Care Med. 2021, 203, 604–613. [Google Scholar] [CrossRef]
- Davies, L.R.L.; Cizmeci, D.; Guo, W.; Luedemann, C.; Alexander-Parrish, R.; Grant, L.; Isturiz, R.; Theilacker, C.; Jodar, L.; Gessner, B.D.; et al. Polysaccharide and conjugate vaccines to Streptococcus pneumoniae generate distinct humoral responses. Sci. Transl. Med. 2022, 14, eabm4065. [Google Scholar] [CrossRef]
- Cripps, A.W.; Folaranmi, T.; Johnson, K.D.; Musey, L.; Niederman, M.S.; Buchwald, U.K. Immunogenicity following revaccination or sequential vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV23) in older adults and those at increased risk of pneumococcal disease: A review of the literature. Exp. Rev. Vacc. 2021, 20, 257–267. [Google Scholar] [CrossRef]
- Lai, X.; Lyu, Y.; Zhang, H.; Feng, H.; Fang, H. PPSV-23 recommendation and vaccination coverage in China: A cross-sectional survey among healthcare workers, older adults and chronic disease patients. Exp. Rev. Vacc. 2022, 21, 1343–1353. [Google Scholar] [CrossRef]
- LaFon, D.C.; Nahm, M.H. Measuring immune responses to pneumococcal vaccines. J. Immunol. Methods. 2018, 461, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.W.; van Kessel, D.A.; Rijkers, G.T. Impact of Using Different Response Criteria for Pneumococcal Polysaccharide Vaccination for Assessment of Humoral Immune Status. J. Clin. Immunol. 2018, 38, 149–152. [Google Scholar] [CrossRef]
- Hare, N.D.; Smith, B.J.; Ballas, Z.K. Antibody response to pneumococcal vaccination as a function of preimmunization titer. J. Allergy Clin. Immunol. 2009, 123, 195–200. [Google Scholar] [CrossRef]
- Hosking, L.M.; Perrett, K.P.; Czajko, C.; Clark, M.; Flynn, S.; Richards, S.; Choo, S. Pneumococcal IgG Antibody Responses to 23vPPV in Healthy Controls Using an Automated ELISA. J. Clin. Immunol. 2022, 42, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.S.; Mitsi, E.; Jones, S.; Jochems, S.P.; Roalfe, L.; Thindwa, D.; Meiring, J.E.; Msefula, J.; Bonomali, F.; Makhaza Jere, T.; et al. Quality of antibody responses by adults and young children to 13-valent pneumococcal conjugate vaccination and Streptococcus pneumoniae colonisation. Vaccine 2022, 40, 7201–7210. [Google Scholar] [CrossRef]
- Ciprero, K.; Zykov, K.A.; Briko, N.I.; Shekar, T.; Sterling, T.M.; Bitieva, E.; Stek, J.E.; Musey, L. Safety and immunogenicity of a single dose 23-valent pneumococcal polysaccharide vaccine in Russian subjects. Human Vacc. Immunother. 2016, 12, 2142–2147. [Google Scholar] [CrossRef]
- Renaud, L.; Schraen, S.; Fouquet, G.; Guidez, S.; Demarquette, H.; Nudel, M.; Cayssials, E.; Bories, C.; Herbaux, C.; Systchenko, T. Response to pneumococcal vaccination in multiple myeloma. Cancer Med. 2019, 8, 3822–3830. [Google Scholar] [CrossRef]
- Musher, D.M.; Manof, S.B.; Liss, C.; McFetridge, R.D.; Marchese, R.D.; Bushnell, B.; Alvarez, F.; Painter, C.; Blum, M.D.; Silber, J.L. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J. Infect. Dis. 2010, 201, 516–524. [Google Scholar] [CrossRef]
- Park, M.A.; Jenkins, S.M.; Smith, C.Y.; Pyle, R.C.; Sacco, K.A.; Ryu, E.; Hagan, J.B.; Joshi, A.Y.; Snyder, M.R.; Abraham, R.S. Pneumococcal serotype-specific cut-offs based on antibody responses to pneumococcal polysaccharide vaccination in healthy adults. Vaccine 2021, 39, 2850–2856. [Google Scholar] [CrossRef]
- Adler, H.; Ferreira, D.M.; Gordon, S.B.; Rylance, J. Pneumococcal Capsular Polysaccharide Immunity in the Elderly. Clin. Vaccine Immunol. 2017, 24, e00004–e00017. [Google Scholar] [CrossRef]
- Ramos-Sevillano, E.; Ercoli, G.; Brown, J.S. Mechanisms of Naturally Acquired Immunity to Streptococcus pneumoniae. Front. Immunol. 2019, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; Ballow, M.; Stiehm, E.R.; Ballas, Z.K.; Chinen, J.; De La Morena, M.; Kumararatne, D.; Harville, T.O.; Hesterberg, P.; Koleilat, M. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2012, 130, S1–S24. [Google Scholar] [PubMed]
- Goldblatt, D.; Tan, C.Y.; Burbidge, P.; McElhiney, S.; McLaughlin, L.; Tucker, R.; Rauh, M.; Sidhu, M.; Giardina, P.C. Assignment of Weight-Based Antibody Units for Seven Additional Serotypes to a Human Pneumococcal Standard Reference Serum, 007sp. Clin. Vaccine Immunol. 2015, 22, 1154–1159. [Google Scholar] [CrossRef]
- Plikaytis, B.D.; Turner, S.H.; Gheesling, L.L.; Carlone, G.M. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1991, 29, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Ridda, I.; Macintyre, C.R.; Lindley, R.; Gao, Z.; Sullivan, J.S.; Yuan, F.F.; McIntyre, P.B. Immunological responses to pneumococcal vaccine in frail older people. Vaccine 2009, 27, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Gaultier, G.N.; Nix, E.B.; Thorgrimson, J.; Boreham, D.; McCready, W.; Ulanova, M. Naturally acquired antibodies against 7 Streptococcus pneumoniae serotypes in Indigenous and non-Indigenous adults. PLoS ONE 2022, 17, e0267051. [Google Scholar] [CrossRef] [PubMed]
- Swarthout, T.D.; Henrion, M.Y.R.; Thindwa, D.; Meiring, J.E.; Mbewe, M.; Kalizang’Oma, A.; Brown, C.; Msefula, J.; Moyo, B.; Mataya, A.A.; et al. Waning of antibody levels induced by a 13-valent pneumococcal conjugate vaccine, using a 3 + 0 schedule, within the first year of life among children younger than 5 years in Blantyre, Malawi: An observational, population-level, serosurveillance study. Lancet Infect. Dis. 2022, 22, 1737–1747. [Google Scholar] [CrossRef]
- Mitsi, E.; McLenaghan, D.; Wolf, A.S.; Jones, S.; Collins, A.M.; Hyder-Wright, A.D.; Goldblatt, D.; Heyderman, R.S.; Gordon, S.B.; Ferreira, D.M. Thirteen-Valent Pneumococcal Conjugate Vaccine-Induced Immunoglobulin G (IgG) Responses in Serum Associated with Serotype-Specific IgG in the Lung. J. Infect. Dis. 2022, 225, 1626–1631. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Musher, D.M.; Groover, J.E.; Reichler, M.R.; Riedo, F.X.; Schwartz, B.; Watson, D.A.; Baughn, R.E.; Breiman, R.F. Emergence of antibody to capsular polysaccharides of Streptococcus pneumoniae during outbreaks of pneumonia: Association with nasopharyngeal colonization. Clin. Infect. Dis. 1997, 24, 441–446. [Google Scholar] [CrossRef]
- Song, C.H.; Estevez, D.; Chernikova, D.; Hernandez, F.; Sakai-Bizmark, R.; Stiehm, R. Low Baseline Pneumococcal Antibody Titers Predict Specific Antibody Deficiency, Increased Upper Respiratory Infections, and Allergy Sensitization. Allergy Rhinol. (Providence) 2020, 11, 2152656719900338. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Hussain, M.; Andrews, N.; Ashton, L.; Virta, C.; Melegaro, A.; Pebody, R.; George, R.; Soininen, A.; Edmunds, J.; et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: A longitudinal household study. J. Infect. Dis. 2005, 192, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, S.; Zhou, S.; Zhao, W.; Li, Q.; Lv, M.; Zhang, P.; Zhang, H.; Lan, W.; Kang, Y.; et al. Immunogenicity and safety of 7-valent pneumococcal conjugate vaccine (PCV7) in children aged 2–5 years in China. Vaccine 2021, 39, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Krone, C.L.; van de Groep, K.; Trzcinski, K.; Sanders, E.A.; Bogaert, D. Immunosenescence and pneumococcal disease: An imbalance in host-pathogen interactions. Lancet Respir. Med. 2014, 2, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Simell, B.; Vuorela, A.; Ekström, N.; Palmu, A.; Reunanen, A.; Meri, S.; Käyhty, H.; Väkeväinen, M. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine. 2011, 29, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper. Wkly. Epidemiol. Rec. 2019, 94, 85–103. [Google Scholar]
- Li, M.C.; Wang, Y.; Zhang, H.; Liu, Y.; Chen, X.J.; Yang, H.W.; Ma, P.; Wang, D.C.; Zhang, B.C.; Dong, A.Y.; et al. Serotype distribution and clinical characteristics associated with streptococcus pneumoniae among Chinese children and adults with invasive pneumococcal disease: A multicenter observational study. Human Vacc. Immunother. 2021, 17, 146–156. [Google Scholar] [CrossRef]
- Lyu, S.; Hu, H.L.; Yang, Y.H.; Yao, K.H. A systematic review about Streptococcus Pneumoniae serotype distribution in children in mainland of China before the PCV13 was licensed. Exp. Rev. Vacc. 2017, 16, 997–1006. [Google Scholar] [CrossRef]
- Cavaliere, F.M.; Graziani, S.; Del Duca, E.; Bilotta, C.; Sgrulletti, M.; Quinti, I.; Moschese, V. IgM, IgA and IgG response to conjugate polysaccharides in children with recurrent respiratory infections. Scand. J. Immunol. 2021, 93, e12955. [Google Scholar] [CrossRef]
- Nolan, K.M.; Zhang, Y.; Antonello, J.M.; Howlett, A.H.; Bonhomme, C.J.; Greway, R.; Green, T.; de Gorguette d’Argoeuves, P.; Goldblatt, D.; Murphy, R.D. Enhanced antipneumococcal antibody electrochemiluminescence assay: Validation and bridging to the WHO reference ELISA. Bioanalysis 2020, 12, 1363–1375. [Google Scholar] [CrossRef]


| Serotype | Pre-Vaccination IgG < 1.3 µg/mL | Pre-Vaccination IgG ≥ 1.3 µg/mL | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Number | Seroconversion Rate | GMFR | Number | Seroconversion Rate | GMFR | P1 a | P2 b | |
| 1 | 317 | 92.74 | 11.09 | 210 | 88.10 | 6.59 | NS | <0.05 |
| 2 | 271 | 95.57 | 13.18 | 313 | 87.86 | 7.30 | <0.05 | <0.05 |
| 3 | 484 | 68.18 | 3.77 | 80 | 26.25 | 1.44 | <0.05 | <0.05 |
| 4 | 435 | 85.52 | 7.09 | 82 | 71.95 | 3.58 | <0.05 | <0.05 |
| 5 | 412 | 87.38 | 7.39 | 172 | 73.84 | 4.26 | <0.05 | <0.05 |
| 6B | 212 | 79.72 | 5.17 | 351 | 72.36 | 3.72 | NS | <0.05 |
| 7 | 280 | 92.86 | 11.44 | 283 | 86.93 | 6.21 | <0.05 | <0.05 |
| 8 | 118 | 94.07 | 10.70 | 439 | 82.69 | 5.15 | <0.05 | <0.05 |
| 9N | 226 | 95.58 | 12.44 | 358 | 89.39 | 7.02 | <0.05 | <0.05 |
| 9V | 193 | 87.56 | 5.91 | 383 | 81.46 | 4.23 | NS | <0.05 |
| 10A | 195 | 84.62 | 7.24 | 337 | 90.21 | 7.61 | NS | NS |
| 11A | 168 | 88.69 | 6.20 | 385 | 74.55 | 3.58 | <0.05 | <0.05 |
| 12F | 391 | 78.01 | 4.48 | 186 | 58.06 | 2.49 | <0.05 | <0.05 |
| 14 | 152 | 91.45 | 11.19 | 416 | 70.19 | 4.44 | <0.05 | <0.05 |
| 15B | 85 | 95.29 | 9.35 | 484 | 83.88 | 5.11 | <0.05 | <0.05 |
| 17F | 283 | 90.81 | 9.02 | 263 | 86.69 | 5.40 | NS | <0.05 |
| 18C | 213 | 92.49 | 8.34 | 334 | 82.63 | 4.43 | <0.05 | <0.05 |
| 19A | 274 | 79.56 | 6.20 | 294 | 81.29 | 5.60 | NS | NS |
| 19F | 128 | 88.28 | 4.96 | 433 | 72.98 | 3.62 | <0.05 | <0.05 |
| 20A | 82 | 74.39 | 3.87 | 478 | 63.60 | 2.78 | NS | <0.05 |
| 22F | 334 | 87.72 | 7.87 | 135 | 71.11 | 4.35 | <0.05 | <0.05 |
| 23F | 358 | 85.75 | 6.84 | 209 | 67.94 | 3.17 | <0.05 | <0.05 |
| 33F | 93 | 93.55 | 13.50 | 466 | 92.06 | 7.42 | NS | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Lv, M.; Bai, S.; Chen, W.; Zhao, W.; Wang, J.; Zhang, A.; Li, J.; Xie, H.; Gao, Y.; et al. Baseline Pneumococcal IgG Levels and Response to 23-Valent Pneumococcal Polysaccharide Vaccine among Adults from Beijing, China. Vaccines 2023, 11, 1780. https://doi.org/10.3390/vaccines11121780
Zhou S, Lv M, Bai S, Chen W, Zhao W, Wang J, Zhang A, Li J, Xie H, Gao Y, et al. Baseline Pneumococcal IgG Levels and Response to 23-Valent Pneumococcal Polysaccharide Vaccine among Adults from Beijing, China. Vaccines. 2023; 11(12):1780. https://doi.org/10.3390/vaccines11121780
Chicago/Turabian StyleZhou, Shanshan, Min Lv, Shuang Bai, Weixin Chen, Wei Zhao, Jian Wang, Ao Zhang, Jing Li, Hui Xie, Yanqing Gao, and et al. 2023. "Baseline Pneumococcal IgG Levels and Response to 23-Valent Pneumococcal Polysaccharide Vaccine among Adults from Beijing, China" Vaccines 11, no. 12: 1780. https://doi.org/10.3390/vaccines11121780
APA StyleZhou, S., Lv, M., Bai, S., Chen, W., Zhao, W., Wang, J., Zhang, A., Li, J., Xie, H., Gao, Y., Li, D., & Wu, J. (2023). Baseline Pneumococcal IgG Levels and Response to 23-Valent Pneumococcal Polysaccharide Vaccine among Adults from Beijing, China. Vaccines, 11(12), 1780. https://doi.org/10.3390/vaccines11121780






