SARS-CoV-2 Vaccine Effectiveness in Hospitalized Patients: A Multicenter Test-Negative Case–Control Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ita, K. Coronavirus Disease (COVID-19): Current Status and Prospects for Drugs and Vaccine Development. Arch. Med. Res. 2021, 52, 15–24. [Google Scholar] [CrossRef]
- Marian, A.J. Current state of vaccine development and targeted therapies for COVID-19: Impact of basis science discoveries. Cardiovasc. Pathol. 2021, 50, 107278. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 13, 373. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- González, S.; Olszevicki, S.; Salazar, M.; Calabria, A.; Regairaz, L.; Marín, L.; Campos, P.; Varela, T.; Martínez, V.V.G.; Ceriani, L.; et al. Effectiveness of the first component of Gam-COVID-Vac (Sputnik V) on reduction of SARS-CoV-2 confirmed infections, hospitalizations and mortality in patients aged 60-79: A retrospective cohort study in Argentina. EClinicalMedicine 2021, 40, 101126. [Google Scholar] [CrossRef]
- Secretaria de Salud. Available online: http://vacunacovid.gob.mx/wordpress/calendario-vacunacion (accessed on 22 July 2022).
- 2021 [Internet]. Available online: https://transparencia.sre.gob.mx/gestion-diplomatica-vacunas-covid/ (accessed on 22 July 2022).
- Leonard, J.A.; Tedijanto, C.; Cowling, B.J.; Lipsitch, M. Measurement of vaccine direct effects under the test-negative design. Am. J. Epidemiol. 2018, 187, 2686–2697. [Google Scholar] [CrossRef]
- Public Health Surveillance for COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-SurveillanceGuidance-2022.2/ (accessed on 22 July 2022).
- World Health Organization (WHO). Cite CC BY-NC-SA3.0IGO. 2021. Sample Size Calculator to Calculate Vaccine Effectiveness (VE) for Cohort Studies. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement_tool-2021.1 (accessed on 22 July 2022).
- World Health Organization (WHO). Evaluation of COVID-19 Vaccine Effectiveness; WHO: Geneva, Switzerland, 2021; Volume 70. [Google Scholar]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Torres-Estrella, C.U.; Reyes-Montes, M.D.R.; Duarte-Escalante, E.; Martínez, M.S.; Frías-De-León, M.G.; Acosta-Altamirano, G. Vaccines Against COVID-19: A Review. Vaccines 2022, 10, 414. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. In Signal Transduction and Targeted Therapy; Springer Nature: Berlin/Heidelberg, Germany, 2020; Volume 5. [Google Scholar]
- Verani, J.R.; Baqui, A.H.; Broome, C.V.; Cherian, T.; Cohen, C.; Farrar, J.L.; Feikin, D.R.; Groome, M.J.; Hajjeh, R.A.; Johnson, H.L.; et al. Case-Control Vaccine Effectiveness Studies: Preparation, Design, and Enrollment of Cases and Controls; Vaccine; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 35, pp. 3295–3302. [Google Scholar]
- Zhou, Z.; Zhu, Y.; Chu, M. Role of COVID-19 Vaccines in SARS-CoV-2 Variants. Front. Immunol. 2022, 13, 898192. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Skowronsku, D.M.; Febriani, Y.; Ouakki, M.; Setayeshgar, S.; El Adam, S.; Zou, M.; Talbot, D.; Prystajecky, N.; Tyson, J.R.; Gilca, R.; et al. Two-Dose SARS-CoV-2 Vaccine Effectiveness with Mixed Schedules and Extended Dosing Intervals: Test-Negative Design Studies From British Columbia and Quebec, Canada. Clin. Infect. Dis. 2022, 19, ciac290. [Google Scholar] [CrossRef]
- Sheikh, A.; Robertson, C.; Taylor, B. BNT162b2 and ChAdOx1 Ncov-19 Vaccine Effectiveness Against Death from the Delta Variant. N. Engl. J. Med. 2021, 385, 2195–2197. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Miqueleiz, A.; Guevara, M.; Fernández-Huerta, M.; Burgui, C.; Casado, I.; Portillo, M.E.; Navascués, A.; Ezpeleta, C.; et al. Product-Specific COVID-19 Vaccine Effectiveness Against Secondary Infection in Close Contacts, Navarre, Spain, April to August 2021. Eurosurveillance 2021, 26, 2100894. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- 2021 [Internet]. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 22 July 2022).
- Khanam, F.; Islam, M.T.; Ahmmed, F.; Ahmed, S.U.; Hossen, M.I.; Rajib, M.N.H.; Haque, S.; Biswas, P.K.; Tauheed, I.; Zaman, K.; et al. Measuring the Effectiveness of COVID-19 Vaccines Used during a Surge of the Delta Variant of SARS-CoV-2 in Bangladesh: A Test-Negative Design Evaluation. Vaccines 2022, 10, 2069. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunization with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Eroglu, B.; Nuwarda, R.F.; Ramzan, I.; Kayser, V. A Narrative Review of COVID-19 Vaccines. Vaccines 2021, 10, 62. [Google Scholar] [CrossRef]
- Taboada, B.; Zárate, S.; García-López, R.; Muñoz-Medina, J.E.; Sanchez-Flores, A.; Herrera-Estrella, A.; Boukadida, C.; Gómez-Gil, B.; Mojica, N.S.; Rosales-Rivera, M.; et al. Dominance of Three Sublineages of the SARS-CoV-2 Delta Variant in Mexico. Viruses 2022, 14, 1165. [Google Scholar] [CrossRef]

| Cases (371) | Controls (390) | p-Value | |
|---|---|---|---|
| Age (years) | 55 ± 17 | 52 ± 18 | 0.004 |
| Masculine gender (%) | 58% (217) | 56% (217) | 0.37 |
| BMI (kg/m2) | 31 ± 23 | 27 ± 7 | 0.001 |
| At least one comorbidity (%) | 60% (224) | 72% (281) | 0.01 |
| Obesity | 39% (143) | 24% (94) | <0.001 |
| Diabetes | 34% (127) | 23% (88) | <0.001 |
| Hypertension | 35% (128) | 31% (120) | 0.27 |
| Cardiovascular disease | 4% (16) | 6% (22) | 0. 4 |
| Current or previous tobacco smoking | 36% (135) | 47% (182) | 0.006 |
| Current tobacco smoking | 8% (31) | 9% (36) | 0.49 |
| Cigarettes/day | 9 ± 17 | 9 ± 16 | 0.35 |
| Biomass smoke exposure | 26% (95) | 33% (129) | 0.03 |
| At least one COVID-19 vaccine (%) | 52% (192) | 78% (303) | <0.001 |
| Full vaccination | 38% (142) | 63% (246) | <0.001 |
| Incomplete vaccination | 13% (49) | 14% (55) | 0.71 |
| 1 dose | 18% (65) | 17% (68) | |
| 2 doses | 33% (122) | 56% (220) | |
| 3 doses | 1% (5) | 4% (15) | |
| Recent travel | 16% (59) | 21% (81) | 0.10 |
| Self-reported adherence to protective measures | 65% (242) | 75% (293) | 0.001 |
| Personal risk | 42% (155) | 45% (175) | 0.36 |
| In contact with SARS-CoV-2-positive persons | 36% (132) | 11% (44) | <0.001 |
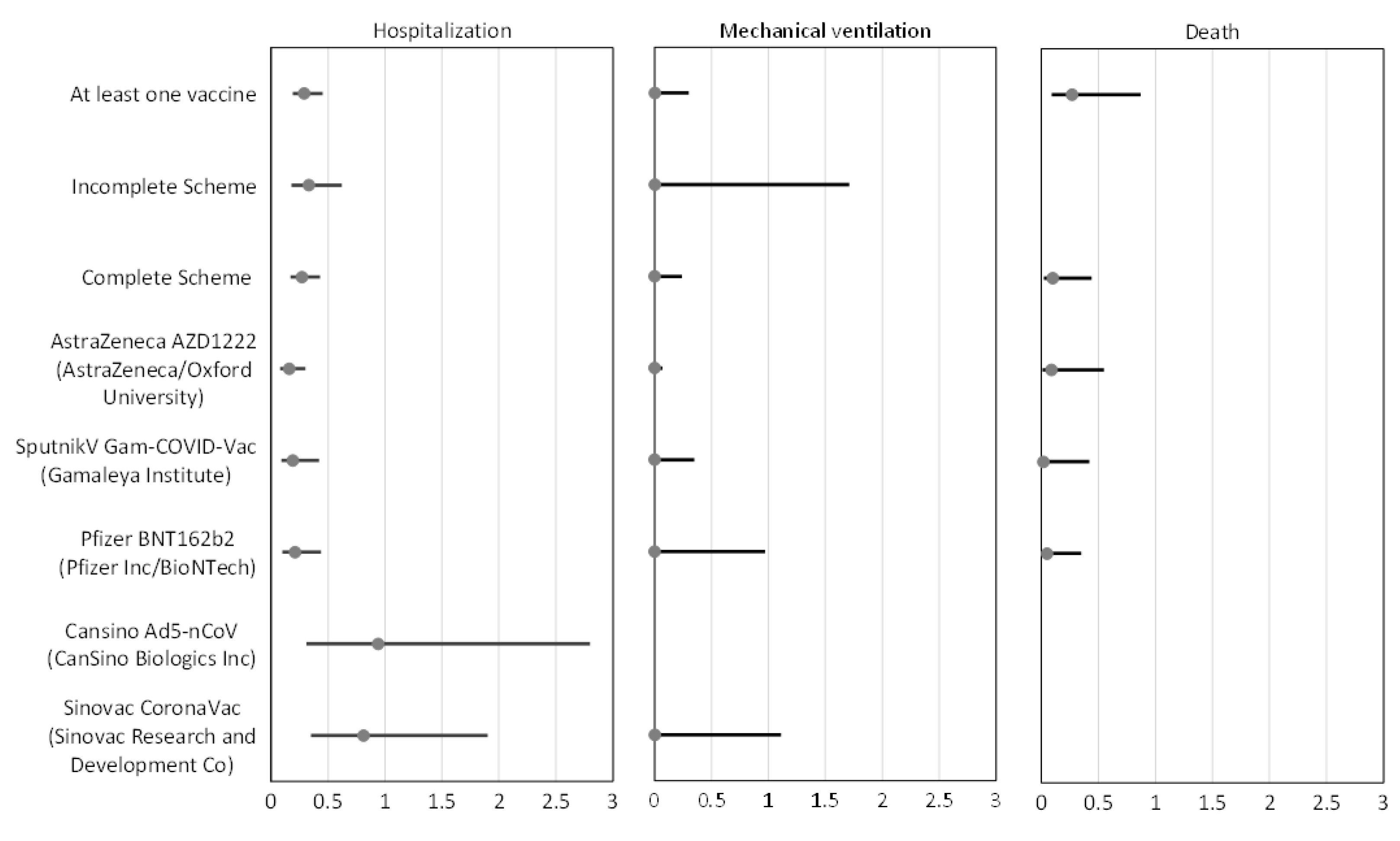
| Hospitalization | Mechanical Ventilation | Death | ||||
|---|---|---|---|---|---|---|
| Effectiveness | Adjusted OR (95%CI) | Effectiveness | Adjusted OR (95%CI) | Effectiveness | Adjusted OR (95%CI) | |
| At least one vaccine | 71% | 0.29 (0.19–0.45) | 98% | 0.02 (0.002–0.30) | 73% | 0.27 (0.09–0.87) |
| Incomplete Scheme | 67% | 0.33 (0.18–0.62) | 94% | 0.06 (0.002–1.71) | - | - |
| Complete Scheme | 73% | 0.27 (0.17–0.43) | 99% | 0.01 (0.001–0.24) | 90% | 0.10 (0.02–0.44) |
| AstraZeneca AZD1222 (AstraZeneca/Oxford University) | 84% | 0.16 (0.08–0.30) | 99% | 0.002 (0.00007–0.07) | 91% | 0.09 (0.01–0.55) |
| Sputnik V Gam-COVID-Vac (Gamaleya Institute) | 81% | 0.19 (0.09–0.42) | 99% | 0.004 (0.00005–0.35) | 98% | 0.02 (0.001–0.42) |
| Pfizer BNT162b2 (Pfizer Inc/BioNTech) | 79% | 0.21 (0.10–0.44) | 98% | 0.02 (0.0008–0.97) | 95% | 0.05 (0.008–0.35) |
| CanSino Ad5-nCoV (CanSino Biologics) | 6% | 0.94 (0.31–2.8) | - | - | - | - |
| SinoVac CoronaVac (SinoVac Research and Development Co) | 19% | 0.81 (0.35–1.9) | 98% | 0.02 (0.003–1.11) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirión-Romero, I.; Fernández-Plata, R.; Pérez-Kawabe, M.; Meza-Meneses, P.A.; Castro-Fuentes, C.A.; Rivera-Martínez, N.E.; Barrón-Palma, E.V.; Sánchez-Sandoval, A.L.; Cornejo-Juárez, P.; Sepúlveda-Delgado, J.; et al. SARS-CoV-2 Vaccine Effectiveness in Hospitalized Patients: A Multicenter Test-Negative Case–Control Study. Vaccines 2023, 11, 1779. https://doi.org/10.3390/vaccines11121779
Thirión-Romero I, Fernández-Plata R, Pérez-Kawabe M, Meza-Meneses PA, Castro-Fuentes CA, Rivera-Martínez NE, Barrón-Palma EV, Sánchez-Sandoval AL, Cornejo-Juárez P, Sepúlveda-Delgado J, et al. SARS-CoV-2 Vaccine Effectiveness in Hospitalized Patients: A Multicenter Test-Negative Case–Control Study. Vaccines. 2023; 11(12):1779. https://doi.org/10.3390/vaccines11121779
Chicago/Turabian StyleThirión-Romero, Ireri, Rosario Fernández-Plata, Midori Pérez-Kawabe, Patricia A. Meza-Meneses, Carlos Alberto Castro-Fuentes, Norma E. Rivera-Martínez, Eira Valeria Barrón-Palma, Ana Laura Sánchez-Sandoval, Patricia Cornejo-Juárez, Jesús Sepúlveda-Delgado, and et al. 2023. "SARS-CoV-2 Vaccine Effectiveness in Hospitalized Patients: A Multicenter Test-Negative Case–Control Study" Vaccines 11, no. 12: 1779. https://doi.org/10.3390/vaccines11121779
APA StyleThirión-Romero, I., Fernández-Plata, R., Pérez-Kawabe, M., Meza-Meneses, P. A., Castro-Fuentes, C. A., Rivera-Martínez, N. E., Barrón-Palma, E. V., Sánchez-Sandoval, A. L., Cornejo-Juárez, P., Sepúlveda-Delgado, J., Torres-Erazo, D. S., & Pérez-Padilla, J. R., on behalf of the Collaboration Group. (2023). SARS-CoV-2 Vaccine Effectiveness in Hospitalized Patients: A Multicenter Test-Negative Case–Control Study. Vaccines, 11(12), 1779. https://doi.org/10.3390/vaccines11121779








