Summary of the Current Status of DNA Vaccination for Alzheimer Disease
Abstract
:1. Introduction
Vaccines and Neurological Diseases
2. Alzheimer Disease
Diagnosis of Alzheimer Disease
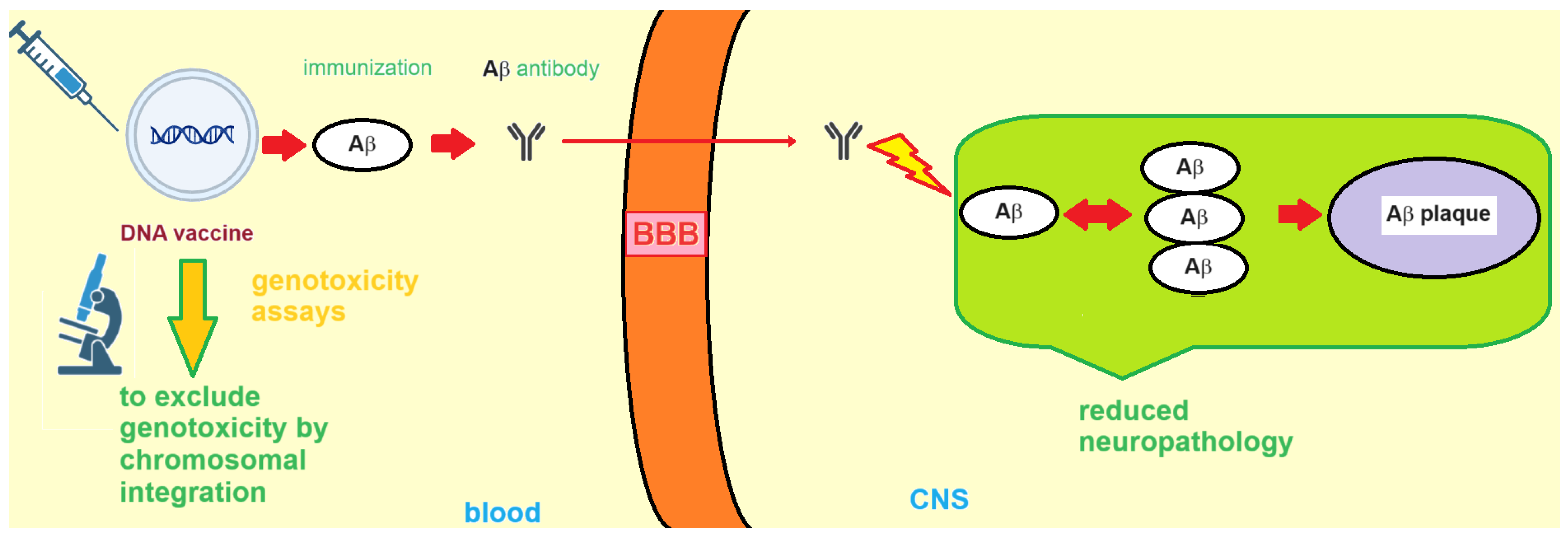
3. DNA Vaccines against AD
3.1. Formulation and Delivery of DNA-Based AD Vaccines
3.2. RNA Vaccines for Treatment of Alzheimer Disease
3.3. Nucleic Acid-Based AD Vaccines: Side Effects and Genosafety Profile
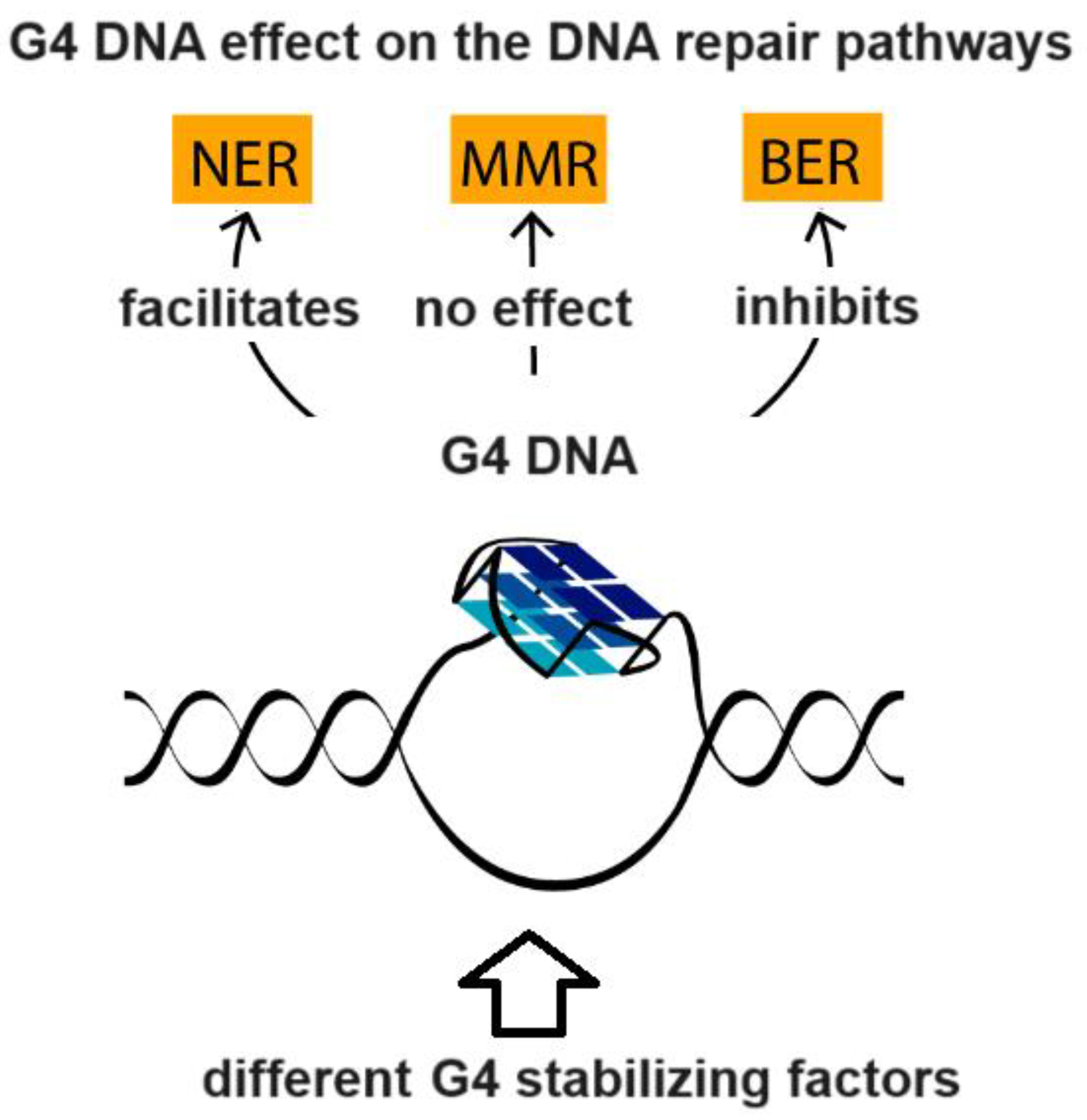
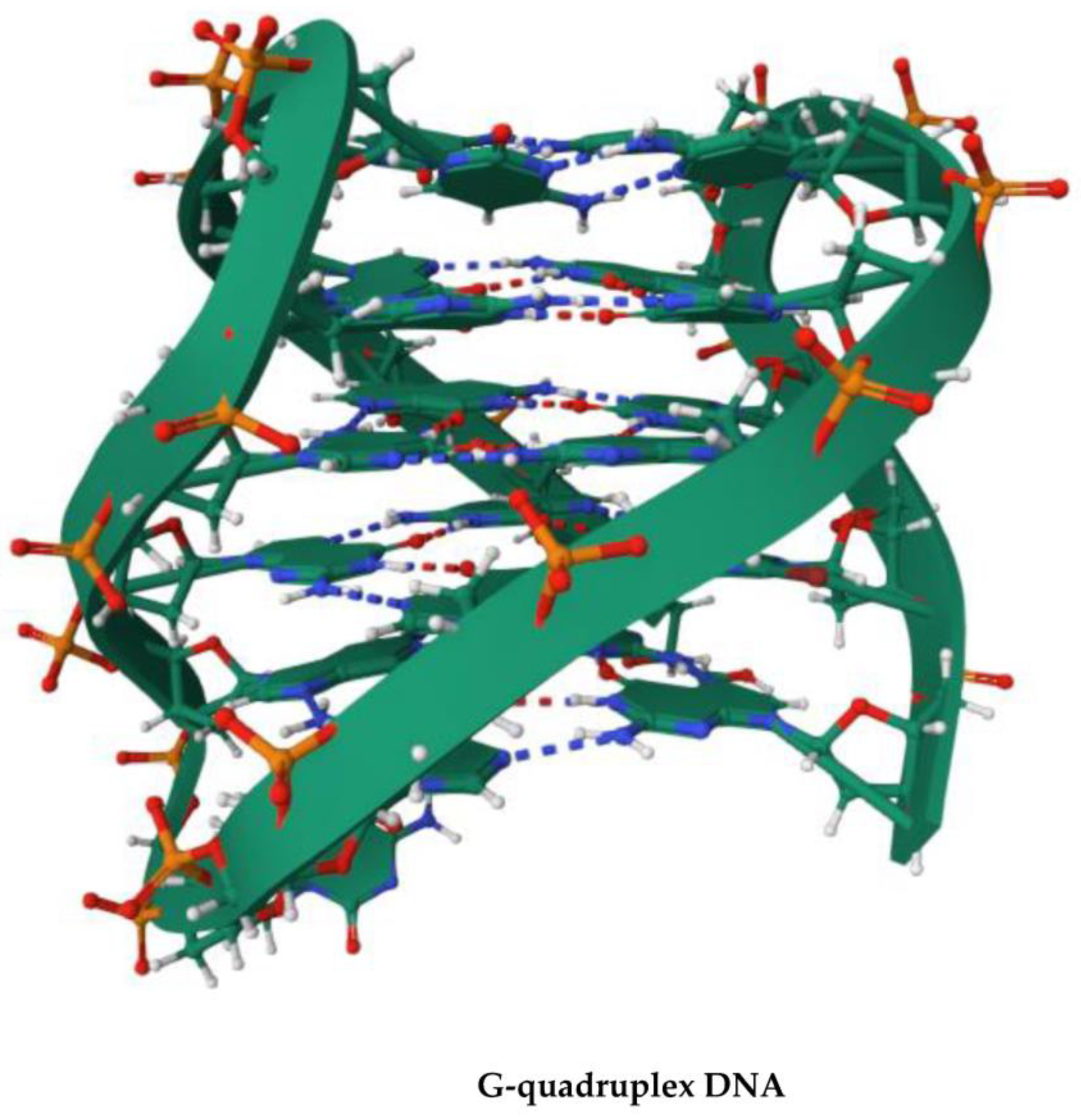
4. G-Quadruplex DNA and Alzheimer Disease: G4 Implication in AD and G4-Forming Sites in DNA Vaccines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wouk, J.; Rechenchoski, D.Z.; Rodrigues, B.C.D.; Ribelato, E.V.; Faccin-Galhardi, L.C. Viral infections and their relationship to neurological disorders. Arch. Virol. 2021, 166, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. The relationship between amyloid and tau. J. Mol. Neurosci. 2003, 20, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ferrari-Souza, J.P.; Lussier, F.Z.; Leffa, D.T.; Therriault, J.; Tissot, C.; Bellaver, B.; Ferreira, P.C.; Malpetti, M.; Wang, Y.-T.; Povala, G. APOE ε4 associates with microglial activation independently of Aβ plaques and tau tangles. Sci. Adv. 2023, 9, eade1474. [Google Scholar] [CrossRef]
- Zhang-Nunes, S.X.; Maat-Schieman, M.L.; van Duinen, S.G.; Roos, R.A.; Frosch, M.P.; Greenberg, S.M. The cerebral β-amyloid angiopathies: Hereditary and sporadic. Brain Pathol. 2006, 16, 30–39. [Google Scholar] [CrossRef]
- Dorszewska, J.; Prendecki, M.; Oczkowska, A.; Dezor, M.; Kozubski, W. Molecular basis of familial and sporadic Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 952–963. [Google Scholar] [CrossRef]
- Younger, D.S.; Younger, A.P.J.; Guttmacher, S. Childhood Vaccination. Neurol. Clin. 2016, 34, 1035–1047. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccines: The Fourth Century. Clin. Vaccine Immunol. 2009, 16, 1709–1719. [Google Scholar] [CrossRef]
- Rodrigues, C.M.C.; Pinto, M.V.; Sadarangani, M.; Plotkin, S.A. Whither vaccines? J. Infect. 2017, 74, S2–S9. [Google Scholar] [CrossRef]
- Hol, W.; Verlinde, C. Non-communicable diseases. Insulin 2006, 106, 107. [Google Scholar]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Hosseini, M.; Seyedpour, S.; Khodaei, B.; Loghman, A.-H.; Seyedpour, N.; Yazdi, M.-H.; Rezaei, N. Cancer vaccines for triple-negative breast cancer: A systematic review. Vaccines 2023, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Li, J.; Huang, M.; Cui, Q.; Liu, X.; Sun, K. Dendritic cell vaccines in breast cancer: Immune modulation and immunotherapy. Biomed. Pharmacother. 2023, 162, 114685. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; De Giglio, M.A.; Roviello, G.N. Anti-coronavirus vaccines: Past investigations on SARS-CoV-1 and MERS-CoV, the approved vaccines from BioNTech/Pfizer, Moderna, Oxford/AstraZeneca and others under Development Against SARSCoV-2 Infection. Curr. Med. Chem. 2022, 29, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Borbone, N.; Piccialli, I.; Falanga, A.P.; Piccialli, V.; Roviello, G.N.; Oliviero, G. Nucleic Acids as Biotools at the Interface between Chemistry and Nanomedicine in the COVID-19 Era. Int. J. Mol. Sci. 2022, 23, 4359. [Google Scholar] [CrossRef]
- Costanzo, V.; Roviello, G.N. The Potential Role of Vaccines in Preventing Antimicrobial Resistance (AMR): An Update and Future Perspectives. Vaccines 2023, 11, 333. [Google Scholar] [CrossRef]
- Okura, Y.; Matsumoto, Y. Recent advance in immunotherapies for Alzheimer disease, with special reference to DNA vaccination. Hum. Vaccines 2014, 5, 373–380. [Google Scholar] [CrossRef]
- Imbimbo, B.P. Toxicity of β-amyloid vaccination in patients with Alzheimer’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2002, 51, 794. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Castellani, R.J.; Rolston, R.K.; Smith, M.A. Alzheimer disease. Dis. A-Mon. 2010, 56, 484–546. [Google Scholar] [CrossRef]
- Hippius, H.; Müller, N. The work of Emil Kraepelin and his research group in München. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.A. Neuropathology of dementia disorders. CONTINUUM Lifelong Learn. Neurol. 2022, 28, 834–851. [Google Scholar] [CrossRef] [PubMed]
- Arlt, S. Non-Alzheimer’s disease—Related memory impairment and dementia. Dialogues Clin. Neurosci. 2022, 15, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Kodintsev, A.N.; Izmozherova, N.V.; Popov, A.A.; Volkova, L.I.; Antropova, I.P.; Ryabinina, A.V. Biochemical Platelet Markers of Cognitive Impairments in Alzheimer’s Disease. Neurochem. J. 2023, 17, 10–18. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Padmanabhan, P.; Yang, C.-T.; Ng, D.C.E.; Palanivel, M.; Mishra, S.; Halldin, C.; Gulyás, B. Positron emission tomographic imaging in drug discovery. Drug Discov. Today 2022, 27, 280–291. [Google Scholar] [CrossRef]
- Yan, Q.; Nho, K.; Del-Aguila, J.L.; Wang, X.; Risacher, S.L.; Fan, K.-H.; Snitz, B.E.; Aizenstein, H.J.; Mathis, C.A.; Lopez, O.L. Genome-wide association study of brain amyloid deposition as measured by Pittsburgh Compound-B (PiB)-PET imaging. Mol. Psychiatry 2021, 26, 309–321. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated tau in Alzheimer’s disease and other tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Wegmann, S.; Biernat, J.; Mandelkow, E. A current view on Tau protein phosphorylation in Alzheimer’s disease. Curr. Opin. Neurobiol. 2021, 69, 131–138. [Google Scholar] [CrossRef]
- Goedert, M.; Klug, A.; Crowther, R.A. Tau protein, the paired helical filament and Alzheimer’s disease. J. Alzheimer’s Dis. 2006, 9, 195–207. [Google Scholar] [CrossRef]
- Tzioras, M.; McGeachan, R.I.; Durrant, C.S.; Spires-Jones, T.L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 2023, 19, 19–38. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, Z.; Jiang, Y. Clinical trials of amyloid-based immunotherapy for Alzheimer’s disease: End of beginning or beginning of end? Expert Opin. Biol. Ther. 2013, 13, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, K.A.; Brown, M.E.; McLaurin, J. Reduced oligomeric and vascular amyloid-β following immunization of TgCRND8 mice with an Alzheimer’s DNA vaccine. Vaccine 2009, 27, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Okura, Y.; Miyakoshi, A.; Kohyama, K.; Park, I.-K.; Staufenbiel, M.; Matsumoto, Y. Nonviral Aβ DNA vaccine therapy against Alzheimer’s disease: Long-term effects and safety. Proc. Natl. Acad. Sci. USA 2006, 103, 9619–9624. [Google Scholar] [CrossRef] [PubMed]
- Okura, Y.; Matsumoto, Y. DNA Vaccine Therapy for Alzheimer’s Disease: Present Status and Future Direction. Rejuvenation Res. 2008, 11, 301–308. [Google Scholar] [CrossRef]
- Lambracht-Washington, D.; Qu, B.-x.; Fu, M.; Anderson, L.D.; Eagar, T.N.; Stüve, O.; Rosenberg, R.N. A peptide prime-DNA boost immunization protocol provides significant benefits as a new generation Aβ42 DNA vaccine for Alzheimer disease. J. Neuroimmunol. 2013, 254, 63–68. [Google Scholar] [CrossRef]
- Doherty, T.M.; Matsumoto, Y.; Niimi, N.; Kohyama, K. Development of a New DNA Vaccine for Alzheimer disease Targeting a Wide Range of Aβ Species and Amyloidogenic Peptides. PLoS ONE 2013, 8, e75203. [Google Scholar]
- Cribbs, D.H. Abeta DNA Vaccination for Alzheimers Disease: Focus on Disease Prevention. CNS Neurol. Disord. Drug Targets 2010, 9, 207–216. [Google Scholar] [CrossRef]
- Petrushina, I.; Hovakimyan, A.; Harahap-Carrillo, I.S.; Davtyan, H.; Antonyan, T.; Chailyan, G.; Kazarian, K.; Antonenko, M.; Jullienne, A.; Hamer, M.M.; et al. Characterization and preclinical evaluation of the cGMP grade DNA based vaccine, AV-1959D to enter the first-in-human clinical trials. Neurobiol. Dis. 2020, 139, 104823. [Google Scholar] [CrossRef]
- Valiukas, Z.; Ephraim, R.; Tangalakis, K.; Davidson, M.; Apostolopoulos, V.; Feehan, J. Immunotherapies for Alzheimer’s Disease—A Review. Vaccines 2022, 10, 1527. [Google Scholar] [CrossRef]
- Qu, L.; Sha, S.; Xing, X.-N.; Wang, T.; Li, Y.; Zhang, R.-W.; Shen, X.-L.; Cao, Y.-P. DNA vaccines targeting amyloid-β oligomer ameliorate cognitive deficits of aged APP/PS1/tau triple-transgenic mouse models of Alzheimer’s disease. Neural Regen. Res. 2022, 17, 2305. [Google Scholar] [CrossRef]
- Evans, C.F.; Davtyan, H.; Petrushina, I.; Hovakimyan, A.; Davtyan, A.; Hannaman, D.; Cribbs, D.H.; Agadjanyan, M.G.; Ghochikyan, A. Epitope-based DNA vaccine for Alzheimer’s disease: Translational study in macaques. Alzheimer’s Dement. 2013, 10, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Lambracht-Washington, D.; Fu, M.; Wight-Carter, M.; Riegel, M.; Hynan, L.S.; Rosenberg, R.N. DNA Aβ42 immunization via needle-less Jet injection in mice and rabbits as potential immunotherapy for Alzheimer’s disease. J. Neurol. Sci. 2023, 446, 120564. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kumar, S.A.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018, 80, 31–47. [Google Scholar] [CrossRef]
- Davtyan, H.; Bacon, A.; Petrushina, I.; Zagorski, K.; Cribbs, D.H.; Ghochikyan, A.; Agadjanyan, M.G. Immunogenicity of DNA-and recombinant protein-based Alzheimer disease epitope vaccines. Hum. Vaccines Immunother. 2014, 10, 1248–1255. [Google Scholar] [CrossRef]
- Sasaki, S.; Takeshita, F.; Xin, K.-Q.; Ishii, N.; Okuda, K. Adjuvant formulations and delivery systems for DNA vaccines. Methods 2003, 31, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.B.; Mason, P.W.; Geall, A.; Mandl, C.W. RNA-based vaccines. Vaccine 2012, 30, 4414–4418. [Google Scholar] [CrossRef]
- Fessel, J. A vaccine to prevent initial loss of cognition and eventual Alzheimer’s disease in elderly persons. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12126. [Google Scholar] [CrossRef]
- Panza, F.; Logroscino, G. Anti-tau vaccine in Alzheimer’s disease: A tentative step. Lancet Neurol. 2017, 16, 99–100. [Google Scholar] [CrossRef]
- Davtyan, H.; Chen, W.W.; Zagorski, K.; Davis, J.; Petrushina, I.; Kazarian, K.; Cribbs, D.H.; Agadjanyan, M.G.; Blurton-Jones, M.; Ghochikyan, A. MultiTEP platform-based DNA epitope vaccine targeting N-terminus of tau induces strong immune responses and reduces tau pathology in THY-Tau22 mice. Vaccine 2017, 35, 2015–2024. [Google Scholar] [CrossRef]
- Mardomi, A.; Mousavi, T.; Farnood, F.; Khosroshahi, H.T. Genotoxicity: A neglected but potentially critical aspect of adenoviral COVID-19 vaccines. Future Med. 2023. [Google Scholar] [CrossRef]
- Cimolai, N. Do RNA vaccines obviate the need for genotoxicity studies? Mutagenesis 2020, 35, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.P.; Terracciano, M.; Oliviero, G.; Roviello, G.N.; Borbone, N. Exploring the Relationship between G-Quadruplex Nucleic Acids and Plants: From Plant G-Quadruplex Function to Phytochemical G4 Ligands with Pharmaceutic Potential. Pharmaceutics 2022, 14, 2377. [Google Scholar] [CrossRef] [PubMed]
- Lipps, H.J.; Rhodes, D. G-quadruplex structures: In vivo evidence and function. Trends Cell Biol. 2009, 19, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Zhang, R.; Cucchiarini, A.; Mehawej, C.; Mergny, J.-L.; Mroueh, M.; Faour, W.H. G-quadruplex forming sequences in the genes coding for cytochrome P450 enzymes and their potential roles in drug metabolism. Biochimie 2023, 214, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-D.; Zhong, M.-Q.; Gao, Y.; Yang, Z.-L.; Jia, M.-H.; Hu, X.-H.; Xu, Y.; Shen, X.-C. A Unique G-Quadruplex Aptamer: A Novel Approach for Cancer Cell Recognition, Cell Membrane Visualization, and RSV Infection Detection. Int. J. Mol. Sci. 2023, 24, 14344. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef]
- Pavlova, A.V.; Kubareva, E.A.; Monakhova, M.V.; Zvereva, M.I.; Dolinnaya, N.G. Impact of G-quadruplexes on the regulation of genome integrity, DNA damage and repair. Biomolecules 2021, 11, 1284. [Google Scholar] [CrossRef]
- Vijay Kumar, M.J.; Morales, R.; Tsvetkov, A.S. G-quadruplexes and associated proteins in aging and Alzheimer’s disease. Front. Aging 2023, 4, 1164057. [Google Scholar] [CrossRef]
- Brčić, J.; Plavec, J. NMR structure of a G-quadruplex formed by four d(G4C2) repeats: Insights into structural polymorphism. Nucleic Acids Res. 2018, 46, 11605–11617. [Google Scholar] [CrossRef]
- Angeli, F.; Spanevello, A.; Reboldi, G.; Visca, D.; Verdecchia, P. SARS-CoV-2 vaccines: Lights and shadows. Eur. J. Intern. Med. 2021, 88, 1–8. [Google Scholar] [CrossRef]
- Bartas, M.; Volná, A.; Brázda, V.; Pecinka, P. G-quadruplex forming sites in DNA/RNA vaccines. In Proceedings of the 8th International Meeting on Quadruplex Nucleic Acids, Marienbad, Czech Republic, 28 June 2022; Available online: https://www.researchgate.net/publication/361813967_G-quadruplex_forming_sites_in_DNARNA_vaccines (accessed on 7 October 2023).
- Brazda, V.; Kolomaznik, J.; Mergny, J.-L.; Stastny, J. G4Killer web application: A tool to design G-quadruplex mutations. Bioinformatics 2020, 36, 3246–3247. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Roviello, G.N. Exploring the Protective Effect of Food Drugs against Viral Diseases: Interaction of Functional Food Ingredients and SARS-CoV-2, Influenza Virus, and HSV. Life 2023, 13, 402. [Google Scholar] [CrossRef] [PubMed]


| Name | Animal Model | Strengths | Weaknesses |
|---|---|---|---|
| Aβ-Fc | APP23 mice | reduced Aβ burden no excessive neuroinflammation/T cell responses | no major weaknesses noted |
| YM3711 | B6C3-Tg 85Dbo/J mice; New Zealand white rabbits; cynomolgus monkeys | significant reduction in Aβ and other amyloidogenic peptides in the brain | no major weaknesses noted |
| AV-1955 | rhesus macaques | generates long-term and potent anti-Aβ antibodies | repeated (up to five times) immunization steps needed to achieve acceptable anti-Aβ antibody levels |
| AV-1959D | Tg2576 and Tg-SwDI mice | induces strong and therapeutically potent anti-Aβ antibodies with a favorable safety profile | low-grade reactions at the injection site |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicidomini, C.; Borbone, N.; Roviello, V.; Roviello, G.N.; Oliviero, G. Summary of the Current Status of DNA Vaccination for Alzheimer Disease. Vaccines 2023, 11, 1706. https://doi.org/10.3390/vaccines11111706
Vicidomini C, Borbone N, Roviello V, Roviello GN, Oliviero G. Summary of the Current Status of DNA Vaccination for Alzheimer Disease. Vaccines. 2023; 11(11):1706. https://doi.org/10.3390/vaccines11111706
Chicago/Turabian StyleVicidomini, Caterina, Nicola Borbone, Valentina Roviello, Giovanni N. Roviello, and Giorgia Oliviero. 2023. "Summary of the Current Status of DNA Vaccination for Alzheimer Disease" Vaccines 11, no. 11: 1706. https://doi.org/10.3390/vaccines11111706
APA StyleVicidomini, C., Borbone, N., Roviello, V., Roviello, G. N., & Oliviero, G. (2023). Summary of the Current Status of DNA Vaccination for Alzheimer Disease. Vaccines, 11(11), 1706. https://doi.org/10.3390/vaccines11111706










