The Application of Mesenchymal Stem Cells in Future Vaccine Synthesis
Abstract
1. Introduction
1.1. Viral Vector Vaccines
1.2. Subunit Vaccines
1.2.1. Protein Vaccines
1.2.2. Toxoid Vaccines
1.2.3. Polysaccharide Vaccines
1.2.4. Nucleic Acid Vaccines
2. Characteristics of MSCs
2.1. High Proliferation Potential Makes MSCs Cost-Effective
2.2. MSCs Stimulate the Immune System in a Unique Way
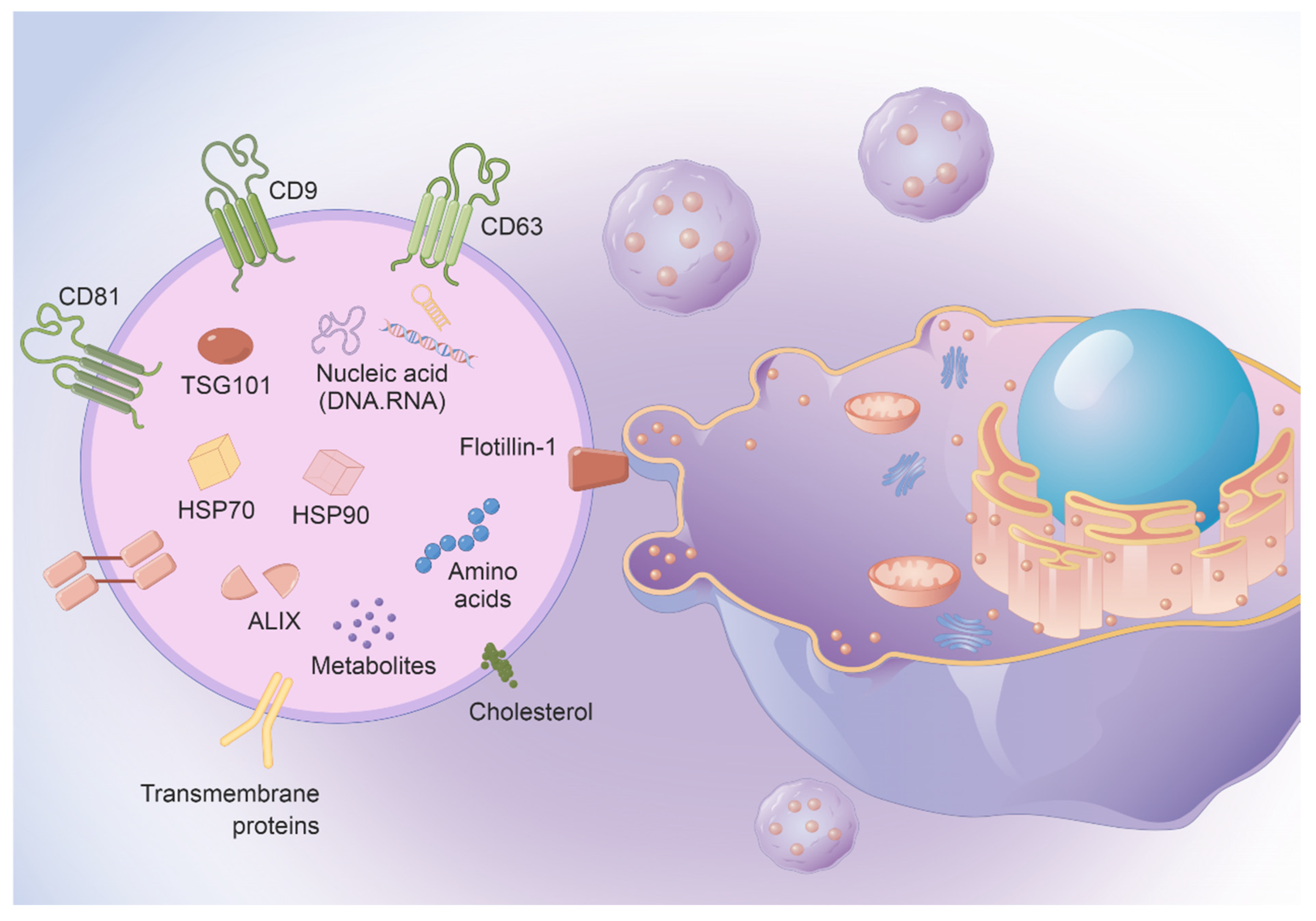
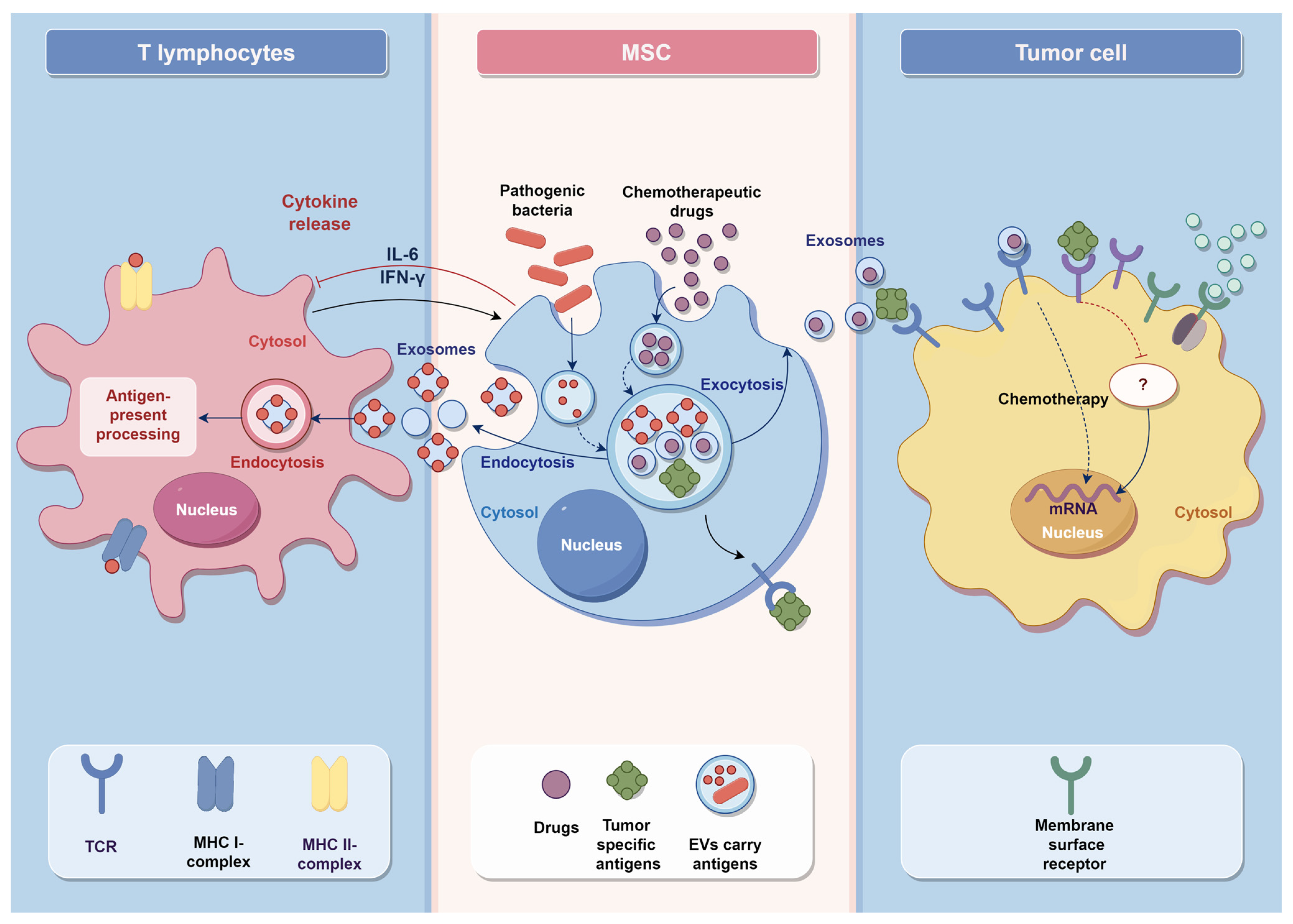
2.3. EVs Secreted by MSCs Are Also an Important Part
3. MSCs Vaccine Synthesis Application
3.1. Anti-Cancer Vaccines
3.2. Anti-Virus Vaccines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masalova, O.V.; Lesnova, E.I.; Klimova, R.R.; Momotyuk, E.D.; Kozlov, V.V.; Ivanova, A.M.; Payushina, O.V.; Butorina, N.N.; Zakirova, N.F.; Narovlyansky, A.N.; et al. Genetically Modified Mouse Mesenchymal Stem Cells Expressing Non-Structural Proteins of Hepatitis C Virus Induce Effective Immune Response. Vaccines 2020, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2018, 50, 110–120. [Google Scholar] [CrossRef]
- Nayereh, K.G.; Khadem, G. Preventive and Therapeutic Vaccines against Human Papillomaviruses Associated Cervical Cancers. Iran J. Basic Med. Sci. 2012, 15, 585–601. [Google Scholar] [PubMed]
- Su, P.; Wu, Y.; Xie, F.; Zheng, Q.; Chen, L.; Liu, Z.; Meng, X.; Zhou, F.; Zhang, L. A Review of Extracellular Vesicles in COVID-19 Diagnosis, Treatment, and Prevention. Adv. Sci. 2023, 10, e2206095. [Google Scholar] [CrossRef]
- Burton, D.R.; Walker, L.M. Rational Vaccine Design in the Time of COVID-19. Cell Host Microbe 2020, 27, 695–698. [Google Scholar] [CrossRef]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef]
- Ruella, M.; Barrett, D.M.; Shestova, O.; Perazzelli, J.; Posey, A.D.; Hong, S.J.; Kozlowski, M.; Lacey, S.F.; Melenhorst, J.J.; June, C.H.; et al. A cellular antidote to specifically deplete anti-CD19 chimeric antigen receptor-positive cells. Blood 2020, 135, 505–509. [Google Scholar] [CrossRef]
- Qadri, F.; Wierzba, T.F.; Ali, M.; Chowdhury, F.; Khan, A.I.; Saha, A.; Khan, I.A.; Asaduzzaman, M.; Akter, A.; Khan, A.; et al. Efficacy of a Single-Dose, Inactivated Oral Cholera Vaccine in Bangladesh. N. Engl. J. Med. 2016, 374, 1723–1732. [Google Scholar] [CrossRef]
- Cordero, E.; Roca-Oporto, C.; Bulnes-Ramos, A.; Aydillo, T.; Gavaldà, J.; Moreno, A.; Torre-Cisneros, J.; Montejo, J.M.; Fortun, J.; Muñoz, P.; et al. Two Doses of Inactivated Influenza Vaccine Improve Immune Response in Solid Organ Transplant Recipients: Results of TRANSGRIPE 1-2, a Randomized Controlled Clinical Trial. Clin. Infect. Dis. 2017, 64, 829–838. [Google Scholar] [CrossRef]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.T.; Calderwood, S.B.; Qadri, F. Live attenuated oral cholera vaccines. Expert Rev. Vaccines 2006, 5, 483–494. [Google Scholar] [CrossRef]
- Belshe, R.B.; Edwards, K.M.; Vesikari, T.; Black, S.V.; Walker, R.E.; Hultquist, M.; Kemble, G.; Connor, E.M. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 2007, 356, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, I.R.; Sebastian, S. Novel viral vectors in infectious diseases. Immunology 2018, 153, 1–9. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Z.; Zhang, C.; Yi, Y.; Zhu, K.; Xu, F.; Kong, W. Development of a Stable Liquid Formulation for Live Attenuated Influenza Vaccine. J. Pharm. Sci. 2019, 108, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Anders, R.F. The case for a subunit vaccine against malaria. Trends Parasitol. 2011, 27, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zheng, B.-J.; Lu, L.; Zhou, Y.; Jiang, S.; Du, L. Advancements in the development of subunit influenza vaccines. Microbes Infect. 2015, 17, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Meng, X.-J.; Calvert, J.G.; Roof, M.; Lager, K.M. Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction. Vaccine 2015, 33, 3065–3072. [Google Scholar] [CrossRef]
- Soema, P.C.; Kompier, R.; Amorij, J.-P.; Kersten, G.F. Current and next generation influenza vaccines: Formulation and production strategies. Eur. J. Pharm. Biopharm. 2015, 94, 251–263. [Google Scholar] [CrossRef]
- Cox, M.M. Recombinant protein vaccines produced in insect cells. Vaccine 2012, 30, 1759–1766. [Google Scholar] [CrossRef]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.-W.; Peng, C.-J.; Chen, M.-C.; Hsu, M.-H.; Liang, Y.-H.; Chiu, C.-H.; Fang, J.-M.; Lee, Y.C. Constructing conjugate vaccine against Salmonella Typhimurium using lipid-A free lipopolysaccharide. J. Biomed. Sci. 2020, 27, 89. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L. Pneumococcal conjugate vaccine, polysaccharide vaccine, or both for adults? We’re not there yet. Clin. Infect. Dis. 2009, 49, 1326–1328. [Google Scholar] [CrossRef][Green Version]
- Stewart, M.C.; Stewart, A.A. Mesenchymal stem cells: Characteristics, sources, and mechanisms of action. Vet. Clin. N. Am. Equine Pr. 2011, 27, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Bochon, B.; Kozubska, M.; Surygała, G.; Witkowska, A.; Kuźniewicz, R.; Grzeszczak, W.; Wystrychowski, G. Mesenchymal Stem Cells-Potential Applications in Kidney Diseases. Int. J. Mol. Sci. 2019, 20, 2462. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Jo, C.H.; Kim, H.-R.; Hwang, Y.-I. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018, 2018, 8429042. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.; Nguyen, Q.T.; Phan, T.T.; Nguyen, G.H.; Le, P.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Sheyn, D.; Shapiro, G.; Tawackoli, W.; Jun, D.S.; Koh, Y.; Kang, K.B.; Su, S.; Da, X.; Ben-David, S.; Bez, M.; et al. PTH Induces Systemically Administered Mesenchymal Stem Cells to Migrate to and Regenerate Spine Injuries. Mol. Ther. 2016, 24, 318–330. [Google Scholar] [CrossRef]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Fang, S.-B.; Zhou, Z.-R.; Peng, Y.-Q.; Liu, X.-Q.; He, B.-X.; Chen, D.-H.; Chen, D.; Fu, Q.-L. Plasma EVs Display Antigen-Presenting Characteristics in Patients with Allergic Rhinitis and Promote Differentiation of Th2 Cells. Front. Immunol. 2021, 12, 710372. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef]
- Bartunek, J.; Davison, B.; Sherman, W.; Povsic, T.; Henry, T.D.; Gersh, B.; Metra, M.; Filippatos, G.; Hajjar, R.; Behfar, A.; et al. Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial design. Eur. J. Heart Fail. 2016, 18, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Rajan, D.; Qayed, M.; Arafat, D.; Garcia, M.; Liu, Y.; Kugathasan, S.; Anderson, L.J.; Gibson, G.; Galipeau, J. Potency Analysis of Mesenchymal Stromal Cells Using a Combinatorial Assay Matrix Approach. Cell Rep. 2018, 22, 2504–2517. [Google Scholar] [CrossRef]
- Abraham, A.; Krasnodembskaya, A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl. Med. 2020, 9, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, W.; Liu, J.; Tian, J.; Wang, S.; Rui, K. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front. Immunol. 2021, 12, 749192. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.-D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transpl. 2011, 20, 655–667. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines-How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F.; et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef]
- Van Megen, K.M.; Van’t Wout, E.J.T.; Lages Motta, J.; Dekker, B.; Nikolic, T.; Roep, B.O. Activated Mesenchymal Stromal Cells Process and Present Antigens Regulating Adaptive Immunity. Front. Immunol. 2019, 10, 694. [Google Scholar] [CrossRef]
- Liu, B.; Jin, Y.; Yang, J.; Han, Y.; Shan, H.; Qiu, M.; Zhao, X.; Liu, A.; Yin, Y. Extracellular vesicles from lung tissue drive bone marrow neutrophil recruitment in inflammation. J. Extracell. Vesicles 2022, 11, e12223. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhang, Y.S. The era of translational nanomedicine. Nano TransMed 2022, 1, e9130006. [Google Scholar] [CrossRef]
- Marcoux, G.; Laroche, A.; Hasse, S.; Bellio, M.; Mbarik, M.; Tamagne, M.; Allaeys, I.; Zufferey, A.; Lévesque, T.; Rebetz, J.; et al. Platelet EVs contain an active proteasome involved in protein processing for antigen presentation via MHC-I molecules. Blood 2021, 138, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- Heydari, R.; Koohi, F.; Rasouli, M.; Rezaei, K.; Abbasgholinejad, E.; Bekeschus, S.; Doroudian, M. Exosomes as Rheumatoid Arthritis Diagnostic Biomarkers and Therapeutic Agents. Vaccines 2023, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-C.; Gao, J.-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef]
- Tsai, S.J.; Guo, C.; Atai, N.A.; Gould, S.J. Exosome-Mediated mRNA Delivery For SARS-CoV-2 Vaccination. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, e2005709. [Google Scholar] [CrossRef]
- Lam, S.M.; Huang, X.; Shui, G. Neurological aspects of SARS-CoV-2 infection: Lipoproteins and exosomes as Trojan horses. Trends Endocrinol. Metab. 2022. [Google Scholar] [CrossRef]
- Wu, L.; Xie, W.; Li, Y.; Ni, Q.; Timashev, P.; Lyu, M.; Xia, L.; Zhang, Y.; Liu, L.; Yuan, Y.; et al. Biomimetic Nanocarriers Guide Extracellular ATP Homeostasis to Remodel Energy Metabolism for Activating Innate and Adaptive Immunity System. Adv. Sci. 2022, 9, e2105376. [Google Scholar] [CrossRef]
- Wang, W.D.; Sun, Z.J. Evoking pyroptosis with nanomaterials for cancer immunotherapy: Current boom and novel outlook. Nano TransMed 2022, 1, e9130001. [Google Scholar]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin. Immunother. 2020, 16, 1232–1238. [Google Scholar] [CrossRef]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiang, J.; Chen, X. Mesenchymal stem cell-based cellular vaccine: An efficient immunotherapeutic strategy for human malignancies. Med. Hypotheses 2011, 76, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Kim, H.K.; Cho, J.; Kim, E.; Kim, J.; Yang, J.S.; Kim, K.C.; Lee, J.Y.; Shin, Y.; Palomera, L.F.; Park, J.; et al. Engineered small extracellular vesicles displaying ACE2 variants on the surface protect against SARS-CoV-2 infection. J. Extracell. Vesicles 2022, 11, e12179. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.A.; Figueroa, F.E.; Djouad, F.; Luz-Crawford, P. Mesenchymal Stem Cells Regulate the Innate and Adaptive Immune Responses Dampening Arthritis Progression. Stem Cells Int. 2016, 2016, 3162743. [Google Scholar] [CrossRef]
- Chan, J.L.; Tang, K.C.; Patel, A.P.; Bonilla, L.M.; Pierobon, N.; Ponzio, N.M.; Rameshwar, P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood 2006, 107, 4817–4824. [Google Scholar] [CrossRef]
- Wei, H.-J.; Wu, A.T.; Hsu, C.-H.; Lin, Y.-P.; Cheng, W.-F.; Su, C.-H.; Chiu, W.-T.; Whang-Peng, J.; Douglas, F.L.; Deng, W.-P. The development of a novel cancer immunotherapeutic platform using tumor-targeting mesenchymal stem cells and a protein vaccine. Mol. Ther. 2011, 19, 2249–2257. [Google Scholar] [CrossRef]
- Gebler, A.; Zabel, O.; Seliger, B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol. Med. 2012, 18, 128–134. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal stem cells: Key players in cancer progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Li, X.; Qian, Y. Vaccination efficacy with marrow mesenchymal stem cell against cancer was enhanced under simulated microgravity. Biochem. Biophys. Res. Commun. 2017, 485, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Volarevic, A.; Djonov, V.G.; Jovicic, N.; Volarevic, V. Mesenchymal Stem Cell: A Friend or Foe in Anti-Tumor Immunity. Int. J. Mol. Sci. 2021, 22, 12429. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; He, Z. Mesenchymal stem cell carriers enhance anti-tumor efficacy of oncolytic virotherapy. Oncol. Lett. 2021, 21, 238. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Liu, L.; Moore, C.; Hsu, E.; Zhang, A.; Ren, Z.; Sun, Z.; Wang, X.; Zhu, J.; Shen, J.; et al. IL-2 delivery by engineered mesenchymal stem cells re-invigorates CD8+ T cells to overcome immunotherapy resistance in cancer. Nat. Cell Biol. 2022, 24, 1754–1765. [Google Scholar] [CrossRef]
- Tehrani, R.M.; Verdi, J.; Noureddini, M.; Salehi, R.; Salarinia, R.; Mosalaei, M.; Simonian, M.; Alani, B.; Ghiasi, M.R.; Jaafari, M.R.; et al. Mesenchymal stem cells: A new platform for targeting suicide genes in cancer. J. Cell. Physiol. 2018, 233, 3831–3845. [Google Scholar] [CrossRef]
- Lu, J.-H.; Peng, B.-Y.; Chang, C.-C.; Dubey, N.K.; Lo, W.-C.; Cheng, H.-C.; Wang, J.R.; Wei, H.-J.; Deng, W.-P. Tumor-Targeted Immunotherapy by Using Primary Adipose-Derived Stem Cells and an Antigen-Specific Protein Vaccine. Cancers 2018, 10, 446. [Google Scholar] [CrossRef]
- hasempour, E.; Hesami, S.; Movahed, E.; Keshel, S.H.; Doroudian, M. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy in the brain tumors. Stem Cell Res. Ther. 2022, 13, 527. [Google Scholar] [CrossRef]
- GTsai, S.J.; Atai, N.A.; Cacciottolo, M.; Nice, J.; Salehi, A.; Guo, C.; Sedgwick, A.; Kanagavelu, S.; Gould, S.J. Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity. J. Biol. Chem. 2021, 297, 101266. [Google Scholar]
- Fernández-Messina, L.; Rodríguez-Galán, A.; de Yébenes, V.G.; Gutiérrez-Vázquez, C.; Tenreiro, S.; Seabra, M.C.; Ramiro, A.R.; Sánchez-Madrid, F. Transfer of extracellular vesicle-microRNA controls germinal center reaction and antibody production. EMBO Rep. 2020, 21, e48925. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, M.E.; Hokke, C.H.; Smits, H.H.; Hoen, E.N.M.N. Pathogen-Derived Extracellular Vesicle-Associated Molecules That Affect the Host Immune System: An Overview. Front. Microbiol. 2018, 9, 2182. [Google Scholar] [CrossRef]
- de Castilla, P.E.M.; Tong, L.; Huang, C.; Sofias, A.M.; Pastorin, G.; Chen, X.; Storm, G.; Schiffelers, R.M.; Wang, J.W. Extracellular vesicles as a drug delivery system: A systematic review of preclinical studies. Adv. Drug Deliv. Rev. 2021, 175, 113801. [Google Scholar] [CrossRef] [PubMed]

| Vaccine Type | Benefits | Limitations | Examples |
|---|---|---|---|
| Viral vector vaccines | |||
| Inactivated vaccines | High production High stability No risk of toxicity recovery | Limited immunogenicity Multiple doses required Adjuvants may be required | Hepatitis A Cholera |
| Live attenuated vaccines | Mimic natural disease process and immune response | Less stable over time Possible reverse inherent toxicity Prohibited for immune deficiency persons | Measles Influenza Yellow fever |
| Subunit vaccines | |||
| Protein vaccines | Non-infectious Low reactogenicity | Limited innate defense triggered Reduced immunogenicity | Influenza Hepatitis B Malaria |
| Toxoid vaccines | Non-infectious | Short term protection | Tetanus Diphtheria |
| Polysaccharide vaccines | Easily identifiable | Weak immunogenicity Short term protection | Pneumococci Meningococci |
| Nucleic acid vaccines | |||
| DNA vaccine | Store at room temperature Mass-production | Work in the nucleus Weak immunogenicity | Avian influenza |
| mRNA vaccine | Rapid development Low production cost | Low stability Safety lacks long-term observation | COVID-19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Duan, X.; Liu, Y.; Xu, J.; Al-bashari, A.A.G.; Ye, P.; Ye, Q.; He, Y. The Application of Mesenchymal Stem Cells in Future Vaccine Synthesis. Vaccines 2023, 11, 1631. https://doi.org/10.3390/vaccines11111631
Zhang R, Duan X, Liu Y, Xu J, Al-bashari AAG, Ye P, Ye Q, He Y. The Application of Mesenchymal Stem Cells in Future Vaccine Synthesis. Vaccines. 2023; 11(11):1631. https://doi.org/10.3390/vaccines11111631
Chicago/Turabian StyleZhang, Rui, Xingxiang Duan, Ye Liu, Jia Xu, Abdullkhaleg Ali Ghaleb Al-bashari, Peng Ye, Qingsong Ye, and Yan He. 2023. "The Application of Mesenchymal Stem Cells in Future Vaccine Synthesis" Vaccines 11, no. 11: 1631. https://doi.org/10.3390/vaccines11111631
APA StyleZhang, R., Duan, X., Liu, Y., Xu, J., Al-bashari, A. A. G., Ye, P., Ye, Q., & He, Y. (2023). The Application of Mesenchymal Stem Cells in Future Vaccine Synthesis. Vaccines, 11(11), 1631. https://doi.org/10.3390/vaccines11111631






