Development of A Standardized Opsonophagocytosis Killing Assay for Group B Streptococcus and Assessment in an Interlaboratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Generation of Frozen Bacterial Working Stocks
2.2. Sera
2.3. Flow Cytometry
2.4. HL-60 Cells
2.5. Complement
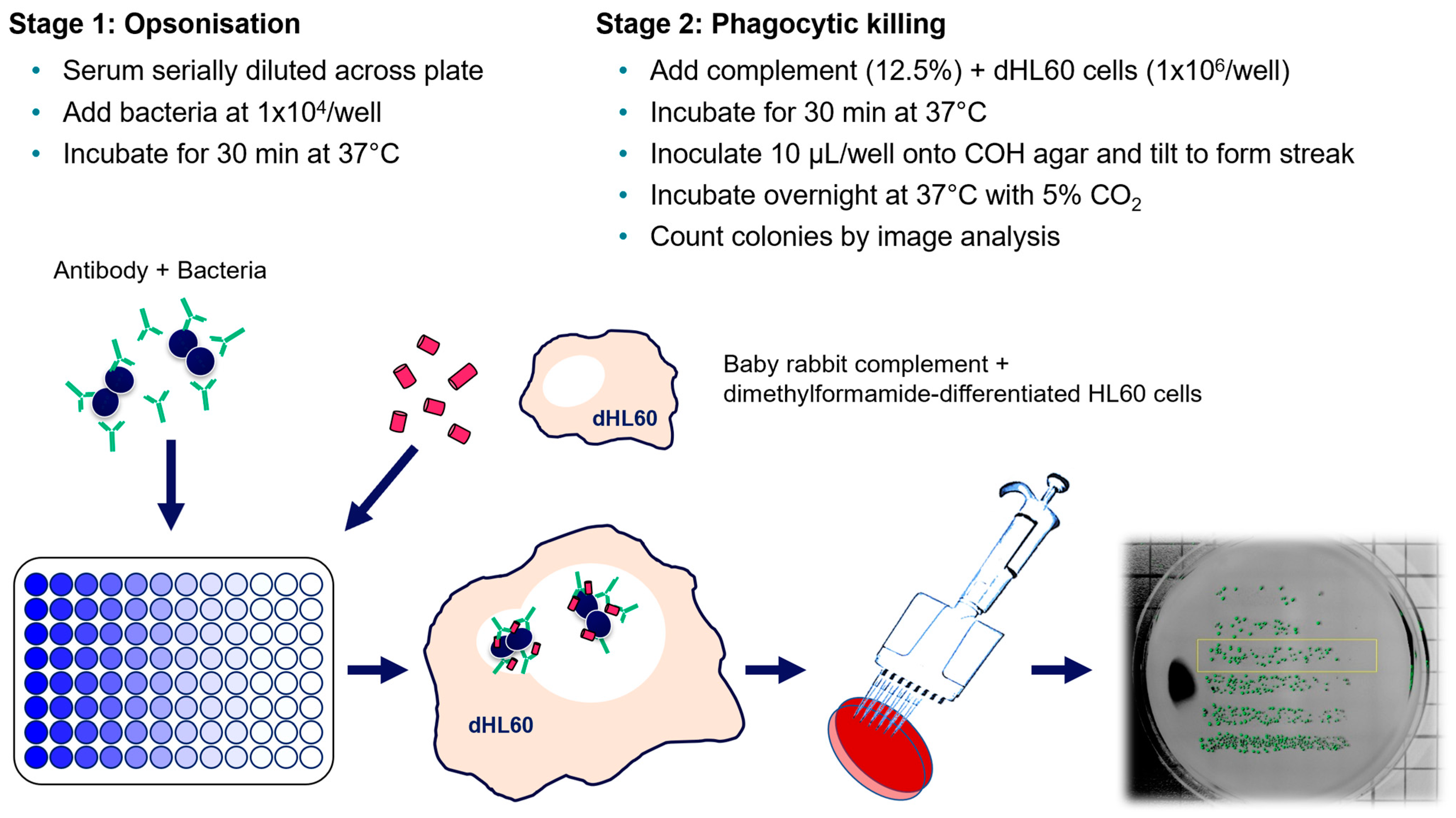
2.6. Opsonophagocytic Killing Assay (OPKA)
2.7. Titer Determination
2.8. Specificity
2.9. Interlaboratory Study
2.10. Statistical Analyses
3. Results
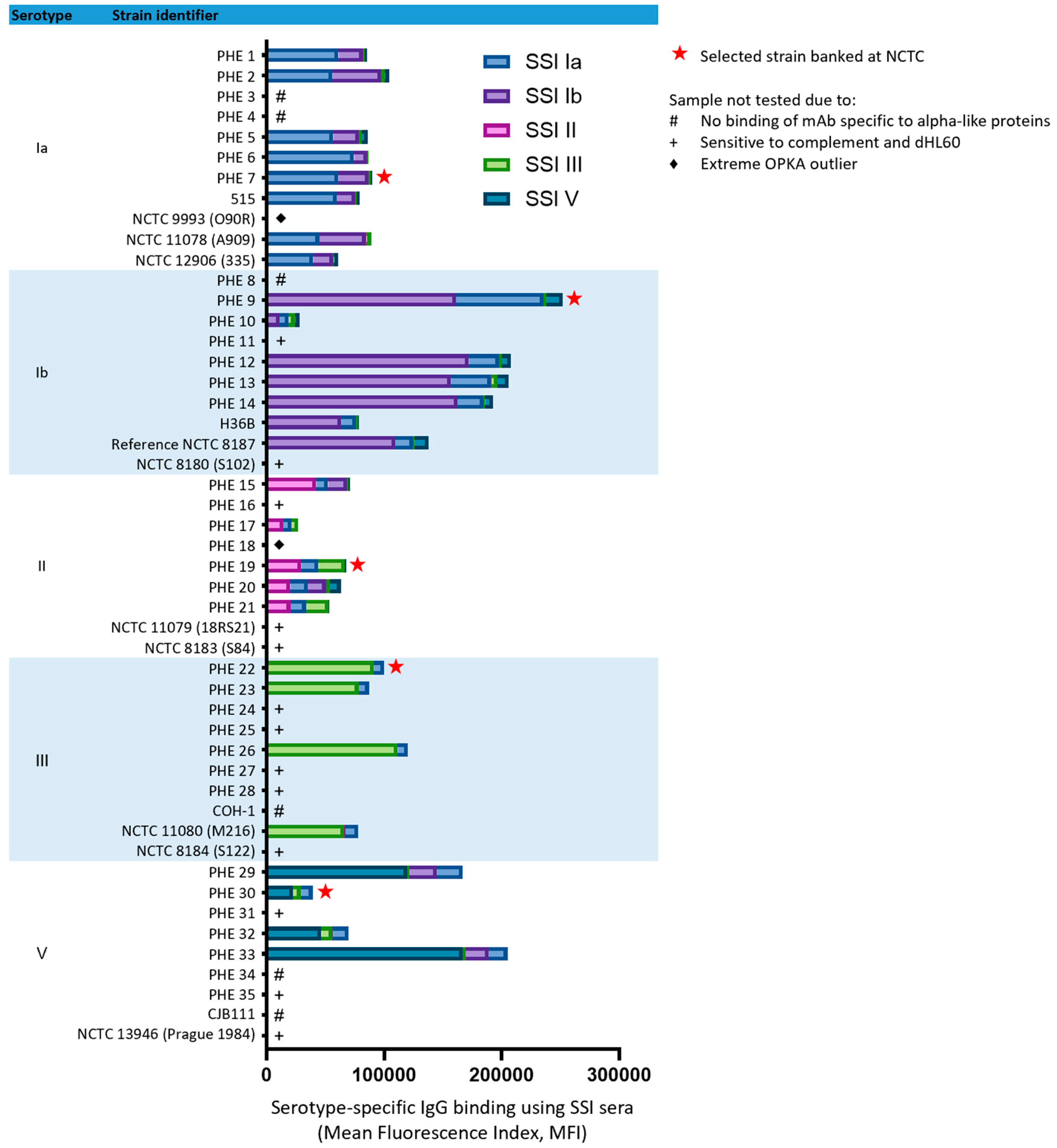
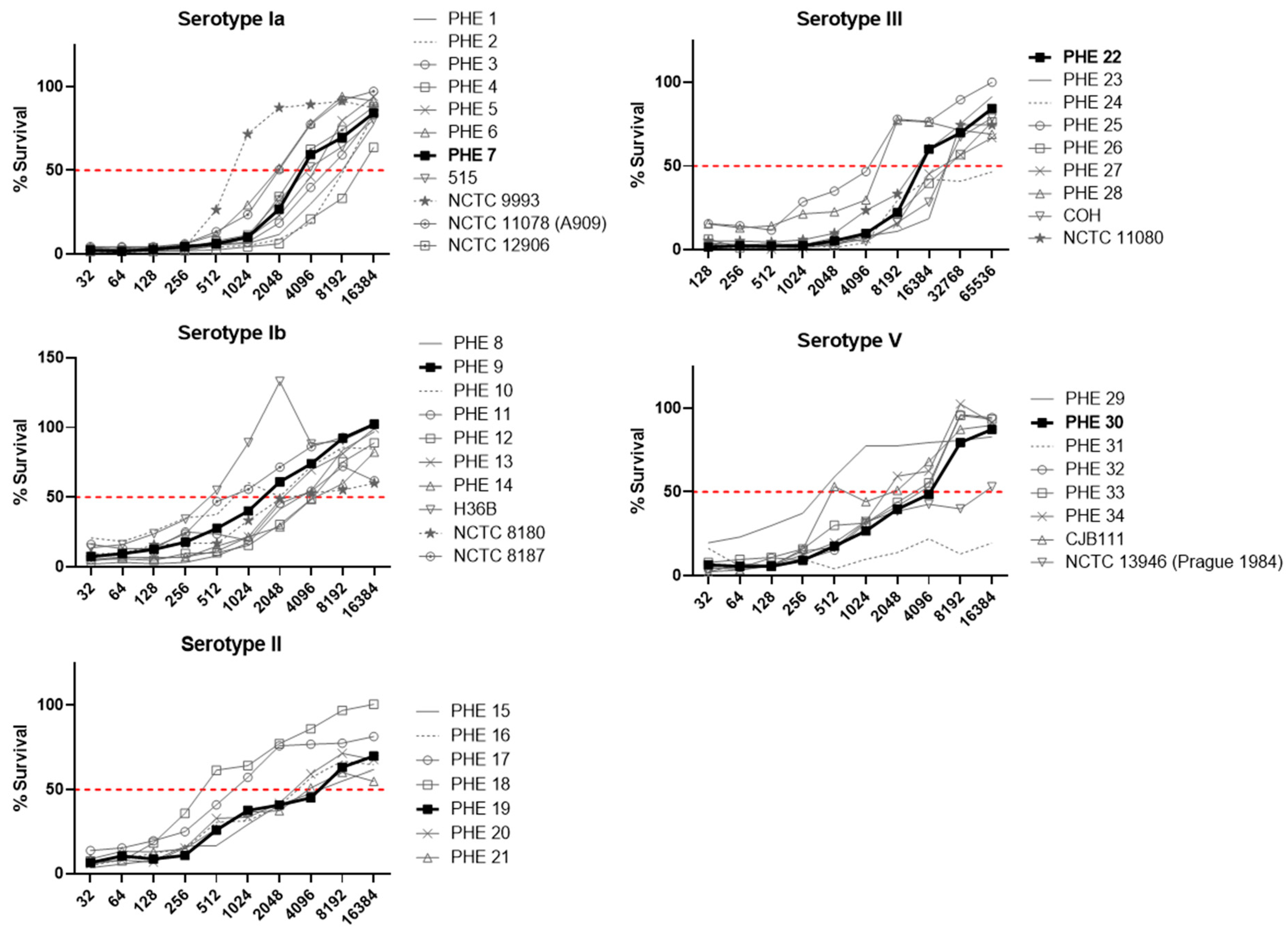
3.1. Strain Selection
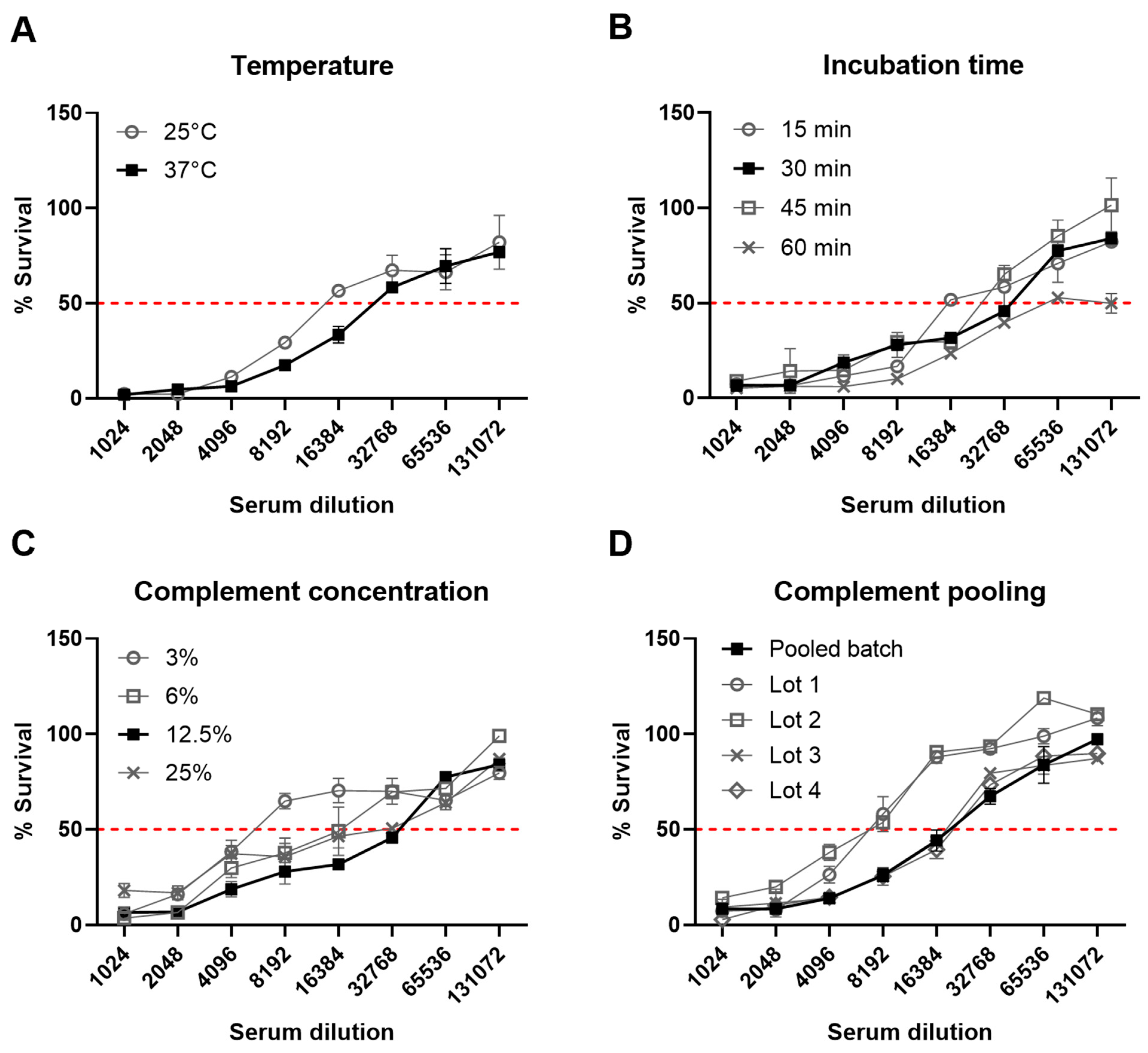
3.2. Assay Development and Optimization
3.3. Assay Specificity
3.4. Assay Sensitivity
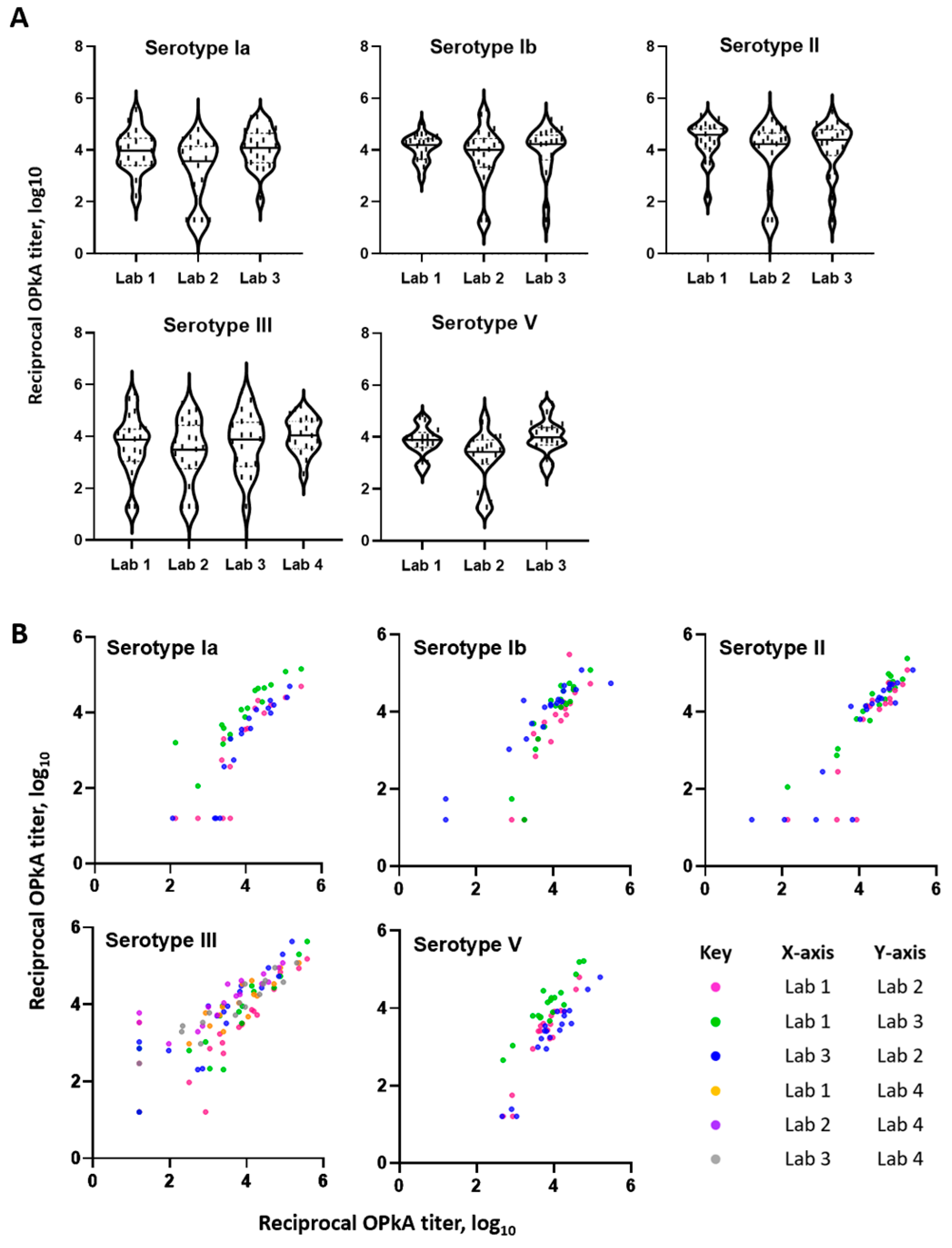
3.5. Interlaboratory Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, P.; Gonçalves, B.P.; Le Doare, K.; Lawn, J.E. 20 Million Pregnant Women with Group B Streptococcus Carriage: Consequences, Challenges, and Opportunities for Prevention. Curr. Opin. Pediatr. 2023, 35, 223. [Google Scholar]
- Vekemans, J.; Crofts, J.; Baker, C.J.; Goldblatt, D.; Heath, P.T.; Madhi, S.A.; Le Doare, K.; Andrews, N.; Pollard, A.J.; Saha, S.K.; et al. The Role of Immune Correlates of Protection on the Pathway to Licensure, Policy Decision and Use of Group B Streptococcus Vaccines for Maternal Immunization: Considerations from World Health Organization Consultations. Vaccine 2019, 37, 3190–3198. [Google Scholar] [CrossRef]
- Davies, H.G.; Carreras-Abad, C.; Le Doare, K.; Heath, P.T. Group B Streptococcus: Trials and Tribulations. Pediatr. Infect. Dis. J. 2019, 38, S72–S76. [Google Scholar] [CrossRef] [PubMed]
- Absalon, J.; Segall, N.; Block, S.L.; Center, K.J.; Scully, I.L.; Giardina, P.C.; Peterson, J.; Watson, W.J.; Gruber, W.C.; Jansen, K.U.; et al. Safety and Immunogenicity of a Novel Hexavalent Group B Streptococcus Conjugate Vaccine in Healthy, Non-Pregnant Adults: A Phase 1/2, Randomised, Placebo-Controlled, Observer-Blinded, Dose-Escalation Trial. Lancet Infect. Dis. 2021, 21, 263–274. [Google Scholar] [CrossRef]
- Gonzalez-Miro, M.; Pawlowski, A.; Lehtonen, J.; Cao, D.; Larsson, S.; Darsley, M.; Kitson, G.; Fischer, P.B.; Johansson-Lindbom, B. Safety and Immunogenicity of the Group B Streptococcus Vaccine AlpN in a Placebo-Controlled Double-Blind Phase 1 Trial. iScience 2023, 26, 106261. [Google Scholar] [CrossRef] [PubMed]
- Absalon, J.; Simon, R.; Radley, D.; Giardina, P.C.; Koury, K.; Jansen, K.U.; Anderson, A.S. Advances towards Licensure of a Maternal Vaccine for the Prevention of Invasive Group B Streptococcus Disease in Infants: A Discussion of Different Approaches. Hum. Vaccin. Immunother. 2022, 18, 2037350. [Google Scholar] [CrossRef]
- Jódar, L.; Butler, J.; Carlone, G.; Dagan, R.; Goldblatt, D.; Käyhty, H.; Klugman, K.; Plikaytis, B.; Siber, G.; Kohberger, R.; et al. Serological Criteria for Evaluation and Licensure of New Pneumococcal Conjugate Vaccine Formulations for Use in Infants. Vaccine 2003, 21, 3265–3272. [Google Scholar] [CrossRef]
- Findlow, J.; Lucidarme, J.; Taha, M.-K.; Burman, C.; Balmer, P. Correlates of Protection for Meningococcal Surface Protein Vaccines: Lessons from the Past. Expert Rev. Vaccines 2022, 21, 739–751. [Google Scholar] [CrossRef]
- Le Doare, K.; Kampmann, B.; Vekemans, J.; Heath, P.T.; Goldblatt, D.; Nahm, M.H.; Baker, C.; Edwards, M.S.; Kwatra, G.; Andrews, N.; et al. Serocorrelates of Protection against Infant Group B Streptococcus Disease. Lancet Infect Dis. 2019, 19, e162–e171. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Schrag, S.J.; Alderson, M.R.; Madhi, S.A.; Baker, C.J.; Sobanjo-ter Meulen, A.; Kaslow, D.C.; Smith, P.G.; Moorthy, V.S.; Vekemans, J. WHO Consultation on Group B Streptococcus Vaccine Development: Report from a Meeting Held on 27–28 April 2016. Vaccine 2019, 37, 7307–7314. [Google Scholar] [CrossRef]
- Sadarangani, M. Protection Against Invasive Infections in Children Caused by Encapsulated Bacteria. Front. Immunol. 2018, 9, 2674. [Google Scholar] [PubMed]
- Edwards, M.S.; Baker, C.J.; Kasper, D.L. Opsonic Specificity of Human Antibody to the Type III Polysaccharide of Group B Streptococcus. J. Infect. Dis. 1979, 140, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Lin, S.-M.; Zhi, Y.; Lim, J.H.; Lim, S.; Seo, H.S.; Song, J.Y. Development of a Multiplexed Opsonophagocytic Killing Assay (MOPA) for Group B Streptococcus. Hum. Vaccin. Immunother. 2018, 14, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, M.; Sammicheli, C.; Margarit, I.; Maione, D.; Grandi, G.; Giuliani, M.M.; Mori, E.; Nuti, S. A New Flow-Cytometry-Based Opsonophagocytosis Assay for the Rapid Measurement of Functional Antibody Levels against Group B Streptococcus. J. Immunol. Methods 2012, 378, 11–19. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.W.; Kim, K.-H. Seroprevalence of Opsonophagocytic Antibodies against Serotype Ia, Ib, II, III, and V Group B Streptococcus among Korean Population. J. Korean Med. Sci. 2018, 33, e127. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Edwards, M.S.; Ewing, K.T.; Lewis, A.L.; Rench, M.A.; Baker, C.J. Group B Streptococcal Conjugate Vaccines Elicit Functional Antibodies Independent of Strain O-Acetylation. Vaccine 2009, 27, 4452–4456. [Google Scholar] [CrossRef]
- Baker, C.J.; Paoletti, L.C.; Wessels, M.R.; Guttormsen, H.-K.; Rench, M.A.; Hickman, M.E.; Kasper, D.L. Safety and Immunogenicity of Capsular Polysaccharide—Tetanus Toxoid Conjugate Vaccines for Group B Streptococcal Types Ia and Ib. J. Infect. Dis. 1999, 179, 142–150. [Google Scholar] [CrossRef]
- Baker, C.J.; Rench, M.A.; Fernandez, M.; Paoletti, L.C.; Kasper, D.L.; Edwards, M.S. Safety and Immunogenicity of a Bivalent Group B Streptococcal Conjugate Vaccine for Serotypes II and III. J. Infect. Dis. 2003, 188, 66–73. [Google Scholar] [CrossRef]
- Baker, C.J.; Paoletti, L.C.; Rench, M.A.; Guttormsen, H.-K.; Carey, V.J.; Hickman, M.E.; Kasper, D.L. Use of Capsular Polysaccharide—Tetanus Toxoid Conjugate Vaccine for Type II Group B Streptococcus in Healthy Women. J. Infect. Dis. 2000, 182, 1129–1138. [Google Scholar] [CrossRef]
- Baker, C.J.; Paoletti, L.C.; Rench, M.A.; Guttormsen, H.-K.; Edwards, M.S.; Kasper, D.L. Immune Response of Healthy Women to 2 Different Group B Streptococcal Type V Capsular Polysaccharide-Protein Conjugate Vaccines. J. Infect. Dis. 2004, 189, 1103–1112. [Google Scholar]
- Paoletti, L.C.; Rench, M.A.; Kasper, D.L.; Molrine, D.; Ambrosino, D.; Baker, C.J. Effects of Alum Adjuvant or a Booster Dose on Immunogenicity during Clinical Trials of Group B Streptococcal Type III Conjugate Vaccines. Infect. Immun. 2001, 69, 6696–6701. [Google Scholar] [CrossRef]
- Baker, C.J.; Rench, M.A.; Paoletti, L.C.; Edwards, M.S. Dose–Response to Type V Group B Streptococcal Polysaccharide–Tetanus Toxoid Conjugate Vaccine in Healthy Adults. Vaccine 2007, 25, 55–63. [Google Scholar] [CrossRef]
- Guttormsen, H.K.; Baker, C.J.; Edwards, M.S.; Paoletti, L.C.; Kasper, D.L. Quantitative Determination of Antibodies to Type III Group B Streptococcal Polysaccharide. J. Infect. Dis. 1996, 173, 142–150. [Google Scholar] [CrossRef]
- Romero-Steiner, S.; Libutti, D.; Pais, L.B.; Dykes, J.; Anderson, P.; Whitin, J.C.; Keyserling, H.L.; Carlone, G.M. Standardization of an Opsonophagocytic Assay for the Measurement of Functional Antibody Activity against Streptococcus pneumoniae Using Differentiated HL-60 Cells. Clin. Diagn. Lab. Immunol. 1997, 4, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Auma, E.; Hall, T.; Chopra, S.; Bilton, S.; Ramkhelawon, L.; Amini, F.; Calvert, A.; Amirthalingam, G.; Jones, C.E.; Andrews, N.; et al. Using Dried Blood Spots for a Sero-Surveillance Study of Maternally Derived Antibody against Group B Streptococcus. Vaccines 2023, 11, 357. [Google Scholar] [CrossRef]
- Nahm, M.H.; Briles, D.E.; Yu, X. Development of a Multi-Specificity Opsonophagocytic Killing Assay. Vaccine 2000, 18, 2768–2771. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.L.; Nahm, M.H. Development of a Fourfold Multiplexed Opsonophagocytosis Assay for Pneumococcal Antibodies against Additional Serotypes and Discovery of Serological Subtypes in Streptococcus pneumoniae Serotype 20. Clin. Vaccine Immunol. 2012, 19, 835–841. [Google Scholar] [CrossRef]
- Guttormsen, H.-K.; Mascuch, S.J.; West, J.C.; Paoletti, L.C. A Fluorescence-Based Opsonophagocytosis Assay to Measure the Functional Activity of Antibody to Group B Streptococcus. Hum. Vaccin. 2009, 5, 461–466. [Google Scholar] [CrossRef]
- Ferrieri, P.; Lynfield, R.; Creti, R.; Flore, A.E. Serotype IV and Invasive Group B Streptococcus Disease in Neonates, Minnesota, USA, 2000–2001. Emerg. Infect. Dis. 2013, 19, 553–558. [Google Scholar] [CrossRef]
- Teatero, S.; Athey, T.B.T.; Van Caeseele, P.; Horsman, G.; Alexander, D.C.; Melano, R.G.; Li, A.; Flores, A.R.; Shelburne, S.A.; McGeer, A.; et al. Emergence of Serotype IV Group B Streptococcus Adult Invasive Disease in Manitoba and Saskatchewan, Canada, Is Driven by Clonal Sequence Type 459 Strains. J. Clin. Microbiol. 2015, 53, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Matsubara, K.; Shibayama, K.; Arakawa, Y. Active Screening of Group B Streptococci with Reduced Penicillin Susceptibility and Altered Serotype Distribution Isolated from Pregnant Women in Kobe, Japan. Jpn. J. Infect. Dis. 2013, 66, 158–160. [Google Scholar] [CrossRef]
- Matsubara, K.; Katayama, K.; Baba, K.; Nigami, H.; Harigaya, H.; Sugiyama, M. Seroepidemiologic Studies of Serotype VIII Group B Streptococcus in Japan. J. Infect. Dis. 2002, 186, 855–858. [Google Scholar] [CrossRef]
- Hoshina, K.; Suzuki, Y.; Nishida, H.; Kaneko, K.; Matsuda, S.; Kobayashi, M.; Kadoi, N. Trend of Neonatal Group B Streptococcal Infection during the Last 15 Years. Pediatr. Int. 2002, 44, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-Specific Effectiveness and Correlates of Protection for the 13-Valent Pneumococcal Conjugate Vaccine: A Postlicensure Indirect Cohort Study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Siber, G.R.; Chang, I.; Baker, S.; Fernsten, P.; O’Brien, K.L.; Santosham, M.; Klugman, K.P.; Madhi, S.A.; Paradiso, P.; Kohberger, R. Estimating the Protective Concentration of Anti-Pneumococcal Capsular Polysaccharide Antibodies. Vaccine 2007, 25, 3816–3826. [Google Scholar] [CrossRef]
- Lee, H.; Nahm, M.H.; Kim, K.-H. The Effect of Age on the Response to the Pneumococcal Polysaccharide Vaccine. BMC Infect. Dis. 2010, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Moseley, M.A.; Burton, R.L.; Nahm, M.H. Pneumococcal Vaccine and Opsonic Pneumococcal Antibody. J. Infect. Chemother. 2013, 19, 412–425. [Google Scholar] [CrossRef]
- Romero-Steiner, S.; Frasch, C.; Concepcion, N.; Goldblatt, D.; Käyhty, H.; Väkeväinen, M.; Laferriere, C.; Wauters, D.; Nahm, M.H.; Schinsky, M.F.; et al. Multilaboratory Evaluation of a Viability Assay for Measurement of Opsonophagocytic Antibodies Specific to the Capsular Polysaccharides of Streptococcus pneumoniae. Clin. Vaccine Immunol. 2003, 10, 1019–1024. [Google Scholar] [CrossRef]
- Rose, C.E.; Romero-Steiner, S.; Burton, R.L.; Carlone, G.M.; Goldblatt, D.; Nahm, M.H.; Ashton, L.; Haston, M.; Ekström, N.; Haikala, R.; et al. Multilaboratory Comparison of Streptococcus pneumoniae Opsonophagocytic Killing Assays and Their Level of Agreement for the Determination of Functional Antibody Activity in Human Reference Sera. Clin. Vaccine Immunol. 2011, 18, 135–142. [Google Scholar] [CrossRef]
- Balloch, A.; Roalfe, L.; Ekstrom, N.; Nguyen, C.D.; Spry, L.; Marimla, R.A.; Licciardi, P.V.; Goldblatt, D.; Mulholland, E.K. Interlaboratory Comparison of the Pneumococcal Multiplex Opsonophagocytic Assays and Their Level of Agreement for Determination of Antibody Function in Pediatric Sera. mSphere 2018, 3, e00070-18. [Google Scholar] [CrossRef]


| NCTC Identification | Serotype | Clonal Complex | Site and Date of Isolate | Surface Protein Expression |
|---|---|---|---|---|
| 14,094 | Ia | 23 | Blood culture, 2014 | Alp1-N |
| 14,092 | Ib | 8 | Blood culture, 2014 | AlpC-N |
| 14,093 | II | 28 | Blood culture, 2014 | Rib-N |
| 14,091 | III | 17 | Blood culture, 2009 | Rib-N |
| 14,095 | V | 1 | Blood culture, 2014 | Alp2-N |
| Laboratory Comparisons | Pearson Correlation (r) | ||||
|---|---|---|---|---|---|
| X, Y | ST Ia | ST Ib | ST II | ST III | ST V |
| Lab 1, Lab 2 | 0.86 | 0.89 | 0.87 | 0.95 | 0.95 |
| Lab 1, Lab 3 | 0.89 | 0.90 | 0.97 | 0.88 | 0.93 |
| Lab 3, Lab 2 | 0.90 | 0.95 | 0.88 | 0.86 | 0.94 |
| Lab 1, Lab 4 | - | - | - | 0.88 | - |
| Lab 2, Lab 4 | - | - | - | 0.87 | - |
| Lab 3, Lab 4 | - | - | - | 0.95 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, S.; Collett, C.F.; Allen, L.; Lim, S.; Maniatis, P.; Bolcen, S.J.; Alston, B.; Patel, P.Y.; Kwatra, G.; Hall, T.; et al. Development of A Standardized Opsonophagocytosis Killing Assay for Group B Streptococcus and Assessment in an Interlaboratory Study. Vaccines 2023, 11, 1703. https://doi.org/10.3390/vaccines11111703
Leung S, Collett CF, Allen L, Lim S, Maniatis P, Bolcen SJ, Alston B, Patel PY, Kwatra G, Hall T, et al. Development of A Standardized Opsonophagocytosis Killing Assay for Group B Streptococcus and Assessment in an Interlaboratory Study. Vaccines. 2023; 11(11):1703. https://doi.org/10.3390/vaccines11111703
Chicago/Turabian StyleLeung, Stephanie, Clare F. Collett, Lauren Allen, Suzanna Lim, Pete Maniatis, Shanna J. Bolcen, Bailey Alston, Palak Y. Patel, Gaurav Kwatra, Tom Hall, and et al. 2023. "Development of A Standardized Opsonophagocytosis Killing Assay for Group B Streptococcus and Assessment in an Interlaboratory Study" Vaccines 11, no. 11: 1703. https://doi.org/10.3390/vaccines11111703
APA StyleLeung, S., Collett, C. F., Allen, L., Lim, S., Maniatis, P., Bolcen, S. J., Alston, B., Patel, P. Y., Kwatra, G., Hall, T., Thomas, S., Taylor, S., Le Doare, K., & Gorringe, A. (2023). Development of A Standardized Opsonophagocytosis Killing Assay for Group B Streptococcus and Assessment in an Interlaboratory Study. Vaccines, 11(11), 1703. https://doi.org/10.3390/vaccines11111703





