NEURO-COVAX: An Italian Population-Based Study of Neurological Complications after COVID-19 Vaccinations
Abstract
:1. Introduction
2. Methods
2.1. Design, Setting and Participants
2.2. NEURO-COVAX Questionnaire
2.3. Statistical Analysis
3. Results
3.1. Study Population
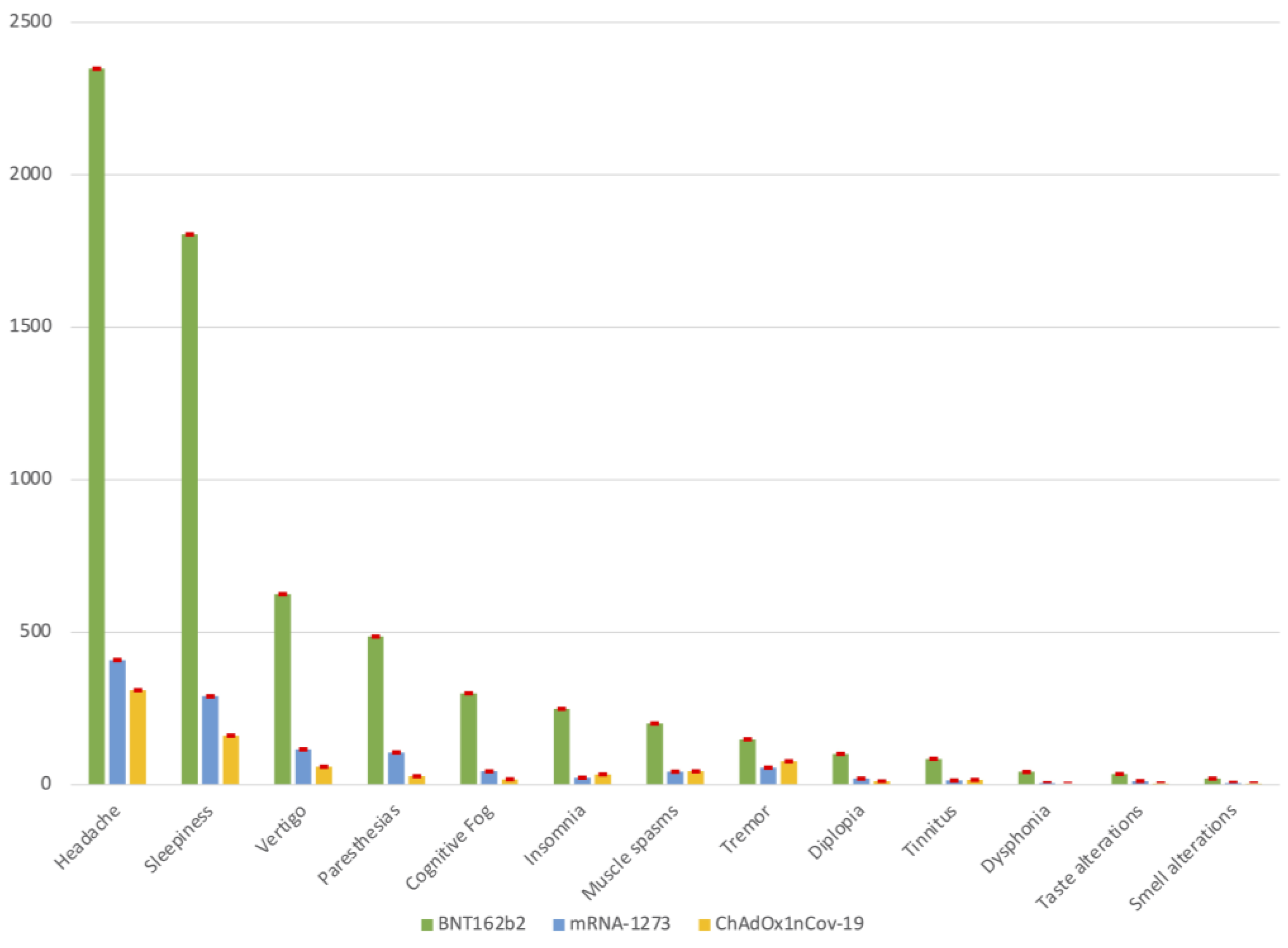
3.2. Neurological Complications Post-Vaccination
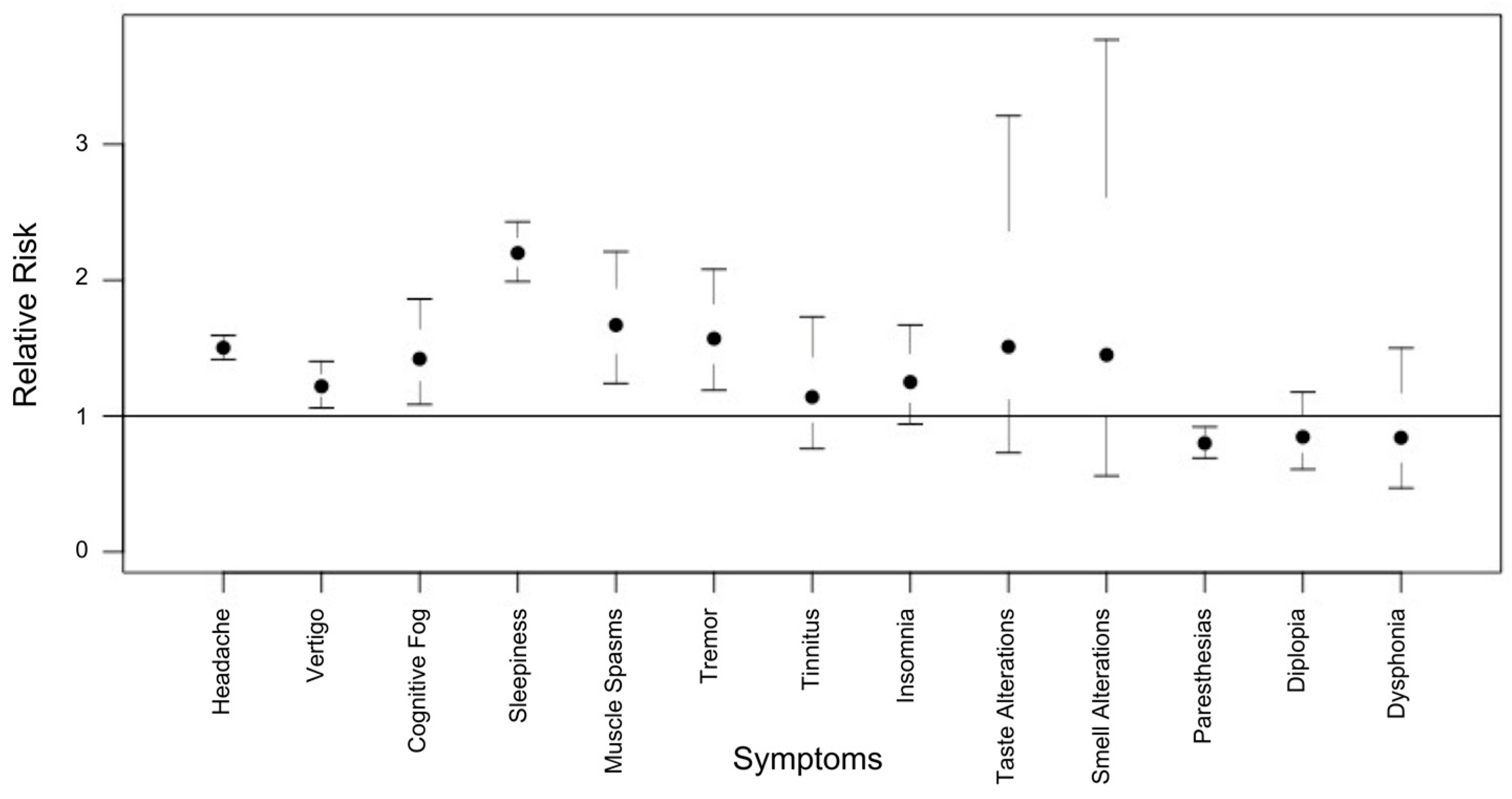
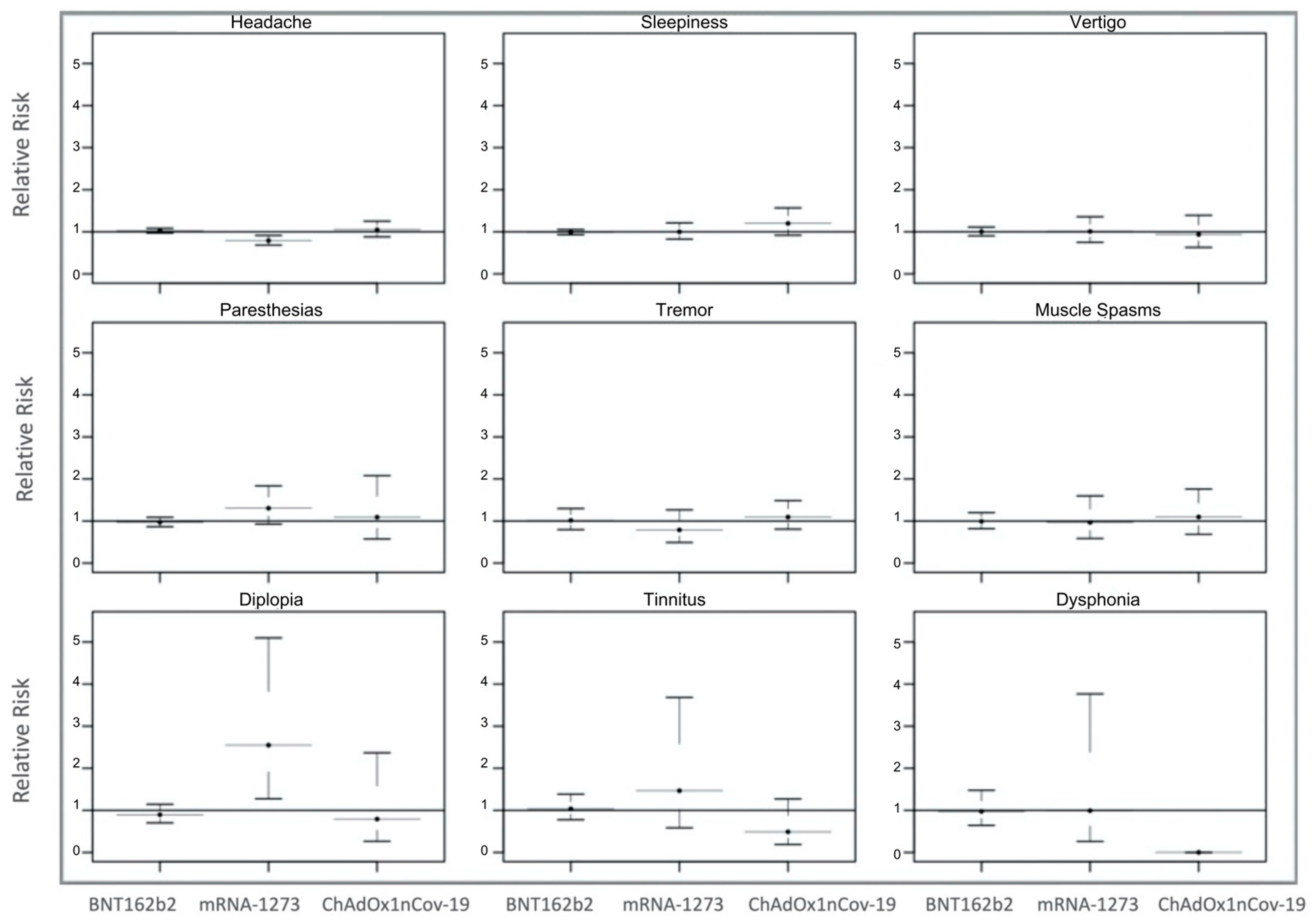
3.3. Neurological Risk Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francis, A.I.; Ghany, S.; Gilkes, T.; Umakathan, S. Review of COVID-19 vaccine subtypes, efficacy and geographical. Postgrad. Med. J. 2022, 98, 389–394. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Allen, A.; Szabo, L. Kaiser Health News. NIH Very Concerned about Serious Side Effect in Coronavirus Vaccine Trial. Scientific American 2020. Available online: https://www.scientificamerican.com/article/nih-very-concerned-about-serious-side-effect-in-coronavirus-vaccine-trial/ (accessed on 27 February 2021).
- Goss, A.L.; Samudralwar, R.D.; Das, R.R.; Nath, A. ANA Investigates: Neurological Complications of COVID-19 Vaccines. Ann. Neurol. 2021, 89, 856–857. [Google Scholar] [CrossRef]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2022, 43, 3–40. [Google Scholar] [CrossRef]
- Tondo, G.; Virgilio, E.; Naldi, A.; Bianchi, A.; Comi, C. Safety of COVID-19 Vaccines: Spotlight on Neurological Complications. Life 2022, 12, 1338. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Bentivegna, E.; Cho, S.J.; Harriott, A.M.; García-Azorín, D.; Labastida-Ramirez, A.; Ornello, R.; Raffaelli, B.; Beltrán, E.R.; Ruscheweyh, R.; et al. Long COVID headache. J. Headache Pain 2022, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- Straburzyński, M.; Kuca-Warnawin, E.; Waliszewska-Prosół, M. COVID-19-related headache and innate immune response. Neurol. Neurochir. Pol. 2023, 57, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; Odone, A.; Gianfredi, V.; Capraro, M.; Kacerik, E.; Chiecca, G.; Scardoni, A.; Minerva, M.; Mantecca, R.; Musarò, P.; et al. Application of the “immunization islands” model to improve quality, efficiency and safety of a COVID-19 mass vaccination site. Ann. Ig. 2021, 33, 499–512. [Google Scholar] [PubMed]
- Castaldo, M.; Waliszewska-Prosół, M.; Koutsokera, M.; Robotti, M.; Straburzyński, M.; Apostolakopoulou, L.; Capizzi, M.; Çibuku, O.; Ambat, F.D.F.; Frattale, I.; et al. Headache onset after vaccination against SARS-CoV-2: A systematic literature review and meta-analysis. J. Headache Pain 2022, 23, 41. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Caronna, E.; Pozo-Rosich, P. Headache as a Symptom of COVID-19: Narrative Review of 1-Year Research. Curr. Pain Headache Rep. 2021, 25, 73. [Google Scholar] [CrossRef]
- Caronna, E.; Ballvé, A.; Llauradó, A.; Gallardo, V.J.; Ariton, D.M.; Lallana, S.; López Maza, S.; Olivé Gadea, M.; Quibus, L.; Restrepo, J.L.; et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia 2020, 40, 1410–1421. [Google Scholar] [CrossRef]
- Magdy, R.; Hussein, M.; Ragaie, C.; Abdel-Hamid, H.M.; Khallaf, A.; Rizk, H.I.; Dahshan, A. Characteristics of headache attributed to COVID-19 infection and predictors of its frequency and intensity: A cross sectional study. Cephalalgia 2020, 40, 1422–1431. [Google Scholar] [CrossRef]
- Rocha-Filho, P.A.S.; Magalhães, J.E. Headache associated with COVID-19: Frequency, characteristics and association with anosmia and ageusia. Cephalalgia 2020, 40, 1443–1451. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Harris, S.R. Psychogenic movement disorders in children and adolescents: An update. Eur. J. Pediatr. 2019, 178, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Brandão, R.P.P.; Grippe, T.C.; Pereira, A.D.; Munhoz, R.P.; Cardoso, F. New-Onset Movement Disorders Associated with COVID-19. Tremor Other Hyperkinet. Mov. 2021, 11, 26. [Google Scholar] [CrossRef]
- Wallbridge Bourmistrova, N.; Solomon, T.; Braude, P.; Strawbridge, R. Long-term effects of COVID-19 on mental health: A systematic review. J. Affect. Disord. 2022, 299, 118–125. [Google Scholar] [CrossRef] [PubMed]
- De Mello, M.T.; Silva, A.; Guerreiro, R.C.; da-Silva, F.R.; Esteves, A.M.; Poyares, D.; Piovezan, R.; Treptow, E.; Starling, M.; Rosa, D.S.; et al. Sleep and COVID-19: Considerations about immunity, pathophysiology, and treatment. Sleep Sci. 2020, 13, 199–209. [Google Scholar]
- Spiegel, K.; Sheridan, J.F.; Van Cauter, E. Effect of sleep deprivation on response to immunization. JAMA 2002, 288, 1471–1472. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Waseem, S.; Shaikh, T.G.; Qadir, N.A.; Siddiqui, S.A.; Ullah, I.; Waris, A.; Yousaf, Z. SARS-CoV-2 Vaccine-Associated Tinnitus: A Review. Ann. Med. Surg. 2022, 75, 103293. [Google Scholar] [CrossRef]
- Tseng, P.T.; Chen, T.Y.; Sun, Y.S.; Chen, Y.W.; Chen, J.J. The reversible tinnitus and cochleopathy followed first-dose AstraZeneca COVID-19 vaccination. QJM 2021, 114, 663–664. [Google Scholar] [CrossRef]
- Feltelius, N.; Persson, I.; Ahlqvist-Rastad, J.; Andersson, M.; Arnheim-Dahlström, L.; Bergman, P.; Granath, F.; Adori, C.; Hökfelt, T.; Kühlmann-Berenzon, S.; et al. A coordinated cross-disciplinary research initiative to address an increased incidence of narcolepsy following the 2009–2010 Pandemrix vaccination programme in Sweden. J. Intern. Med. 2015, 278, 335–353. [Google Scholar] [CrossRef]
- Wu, M.; Li, S.X.; Pei Xue, P.; Zhou, J.; Tang, X. COVID-19 Vaccine Could Trigger the Relapse of Secondary Hypersomnia. Nat. Sci. Sleep 2021, 13, 2267–2271. [Google Scholar] [CrossRef]
- Liguori, C.; Pierantozzi, M.; Spanetta, M.; Sarmati, L.; Cesta, N.; Iannetta, M.; Ora, J.; Mina, G.G.; Puxeddu, E.; Balbi, O.; et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV-2 infection. Brain Behav. Immun. 2020, 88, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy—Clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Lippert, J.; Young, P.; Gross, C.; Meuth, S.G.; Dräger, B.; Schirmacher, A.; Heidbreder, A. Specific T-cell activation in peripheral blood and cerebrospinal fluid in central disorders of hypersomnolence. Sleep 2019, 42, zsy223. [Google Scholar] [CrossRef]
- Di Mauro, P.; La Mantia, I.; Cocuzza, S.; Sciancalepore, P.I.; Rasà, D.; Maniaci, A.; Ferlito, S.; Tundo, I.; Anzivino, R. Acute Vertigo After COVID-19 Vaccination: Case Series and Literature Review. Front. Med. 2022, 8, 790931. [Google Scholar] [CrossRef]
- Ciorba, A.; Corazzi, V.; Bianchini, C.; Aimoni, C.; Pelucchi, S.; Skarżyński, P.H.; Hatzopoulos, S. Autoimmune inner ear disease (AIED): A diagnostic challenge. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418808680. [Google Scholar] [CrossRef]
- García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Michel-Chávez, A.; García-Alanis, M.; Cadena-Fernández, A.; Galnares-Olalde, J.A.; Carbajal-Sandoval, G.; Carrillo-García, D.A.; Hernández-Valdivia, N.; Hernández-Vanegas, L.E.; et al. Transient sensory symptoms among first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine: A case-control study. Vaccine 2021, 39, 6975–6979. [Google Scholar] [CrossRef] [PubMed]
- Khayat-Khoei, M.; Bhattacharyya, S.; Katz, J.; Harrison, D.; Tauhid, S.; Bruso, P.; Houtchens, M.K.; Edwards, K.R.; Bakshi, R. COVID-19 mRNA vaccination leading to CNS inflammation: A case series. J. Neurol. 2022, 269, 1006–1093. [Google Scholar] [CrossRef]
- Etemadifar, M.; Sigari, A.A.; Sedaghat, N.; Salari, M.; Nouri, H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum. Vaccin. Immunother. 2021, 17, 348–383. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.J.; Sahiba Khurana, S.; Murthy, G.; Dawson, E.T.; Jazebi, N.; Haas, C.J. A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 597–600. [Google Scholar] [CrossRef]
- Pappaterra, M.C.; Rivera, E.J.; Oliver, A.L. Transient Oculomotor Palsy Following the Administration of the Messenger RNA-1273 Vaccine for SARS-CoV-2 Diplopia Following the COVID-19 Vaccine. J. Neurophthalmol. 2021. ahead of print. [Google Scholar] [CrossRef]
- de Medeiros, A.L.; Martins, T.; Kattah, M.; Soares, A.K.A.; Ventura, L.O.; Ventura, C.V.; Barros, E. Isolated abducens nerve palsy associated with coronavirus disease: An 8-month follow-up. Arq. Bras. Oftalmol. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Avcı, H.; Karabulut, B.; Eken, H.D.; Faraşoğlu, A.; Çakil, T.; Çoruk, S.; Özel, H.; Kaya, N.K.; Özbalta, S. Otolaryngology-Specific Symptoms May Be Highly Observed in Patients with a History of COVID-19 Infection After Inactivated Coronavirus Vaccination. Ear Nose Throat J. 2021. ahead of print. [Google Scholar] [CrossRef]
- Shimohata, T. Neuro-COVID-19. Clin. Exp. Neuroimmunol. 2021, 13, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee for the COMEBAC Study Group; Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.-L.; Meyrignac, O.; et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar]
- García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Hernández-Vanegas, L.E.; Núñez, I.; Hernández-Valdivia, N.; Carrillo-García, D.A.; Michel-Chávez, A.; Galnares-Olalde, J.A.; Carbajal-Sandoval, G.; Del Mar Saniger-Alba, M.; et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: A nationwide descriptive study. Clin. Immunol. 2021, 229, 108786. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Schultze, A.; Tazare, J.; Tamborska, A.; Singh, B.; Donegan, K.; Stowe, J.; Morton, C.E.; Hulme, W.J.; Curtis, H.J.; et al. Safety of COVID-19 vaccination and acute neurological events: A self-controlled case series in England using the OpenSAFELY platform. Vaccine 2022, 40, 4479–4487. [Google Scholar] [CrossRef]
- Botton, J.; Jabagi, M.J.; Bertrand, M.; Baricault, B.; Drouin, J.; Le Vu, S.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Risk for Myocardial Infarction, Stroke, and Pulmonary Embolism Following COVID-19 Vaccines in Adults Younger Than 75 Years in France. Ann. Intern. Med. 2022, 175, 1250–1257. [Google Scholar] [CrossRef]
- Kelly, J.D.; Leonard, S.; Hoggatt, K.J.; Boscardin, W.J.; Lum, E.N.; Moss-Vazquez, T.A.; Andino, R.; Wong, J.K.; Byers, A.; Bravata, D.M.; et al. Incidence of Severe COVID-19 Illness Following Vaccination and Booster With BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines. JAMA 2022, 328, 1427–1437. [Google Scholar] [CrossRef]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef]
- Aksoyalp, Z.S.; Nemutlu-Samur, D. Sex-related susceptibility in coronavirus disease 2019 (COVID-19): Proposed mechanisms. Eur. J. Pharmacol. 2021, 912, 174548. [Google Scholar] [CrossRef]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, I.; Lleo, A.; Gershwin, M.E.; Invernizzi, P. The X chromosome and immune associated genes. J. Autoimmun. 2012, 38, J187–J192. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Faidah, H.; Al-Maiahy, T.J.; Cruz-Martins, N.; Batiha, G.E.-S. The looming effects of estrogen in COVID-19: A rocky rollout. Front. Nutr. 2021, 8, 649128. [Google Scholar] [CrossRef]
| BNT162b2 n(%) | Vaccine Lines | ChAdOx1nCov-19 n(%) | |
|---|---|---|---|
| mRNA-1273 n(%) | |||
| Total number of people stratified for vaccines | 15.368 (80.4) | 2077 (10.8) | 1651 (8.6) |
| Sex | |||
| Female | 7497 (48.7) | 988 (47.5) | 867 (52.5) |
| Male | 7869 (51.2) | 1089 (52.4) | 784 (47.4) |
| Not Determined | 2 (0.01) | - | - |
| Age Groups (years) | |||
| Mean (s.d.) | 45.9 ± 11.1 | 43.7 ± 10.8 | 66.1 ± 5.49 |
| 18–29 | 747 (4.86) | 133 (6.40) | 4 (0.24) |
| 30–39 | 3834 (24.94) | 764 (36.8) | 12 (0.72) |
| 40–49 | 4007 (26.07) | 335 (16.1) | 9 (0.55) |
| 50–59 | 5736 (37.32) | 799 (38.5) | 23 (1.39) |
| 60–69 | 684 (4.45) | 31 (1.49) | 1352 (81.89) |
| 70–79 | 213 (1.39) | 10 (0.48) | 242 (14.66) |
| ≥80 | 56 (0.37) | 2 (0.09) | 8 (0.49) |
| Not Determined | 91 (0.6) | 3 (0.14) | 1 (0.06) |
| Non-Neurological complicationspost-vaccination | |||
| Total number stratified for vaccines | 553 (3.6) | 131 (6.3) | 171 (10.3) |
| Neurological complications post-vaccination | |||
| Total number stratified for vaccines | 4650 (30.2) | 729 (35.1) | 583 (35.3) |
| BNT162b2 (N = 4650) | Vaccine Lines | ChAdOx1nCov-19 (N = 583) | |
|---|---|---|---|
| mRNA-1273 (N = 729) | |||
| Sex n, (%) | |||
| Female | 2788 (60) | 439 (60) | 380 (65) |
| Male | 1862 (40) | 290 (40) | 203 (35) |
| Age Groups (years) | |||
| Mean (s.d.) | 46.0 ± 11.2 | 39.0 ± 11.2 | 66.0 ± 5.88 |
| 18–29 | 282 (6.06) | 56 (7.68) | 1 (0.17) |
| 30–39 | 1340 (28.82) | 315 (43.2) | 4 (0.68) |
| 40–49 | 1252 (26.92) | 107 (14.6) | 4 (0.68) |
| 50–59 | 1495 (32.1) | 232 (31.8) | 9 (1.54) |
| 60–69 | 192 (4.12) | 16 (2.19) | 487 (83.5) |
| 70–79 | 44 (0.94) | 2 (0.27) | 78 (13.3) |
| ≥80 | 15 (0.32) | 1 (0.13) | - |
| Not Determined | 30 (0.64) | - | - |
| Positive SARS-CoV2 test (before vaccination) § | 34 (0.73) | 22 (3.0) | 8 (1.37) |
| Comorbidities or concomitant conditions | |||
| Total Number n, (%) | 1933 (41.5) | 283 (38.8) | 278 (47.6) |
| Allergies x | 1310 (67.7) | 184 (65.0) | 140 (50.4) |
| Seasonal (pollen) | 406 (31.0) | 60 (32.6) | 30 (21.4) |
| Food | 389 (29.7) | 67 (36.4) | 38 (27.1) |
| Materials (latex) | 55 (4.2) | 6 (3.26) | 4 (2.90) |
| Drugs | 668 (51) | 82 (44.5) | 82 (58.6) |
| Cardiovascular diseases, lung diseases, kidney diseases, diabetes and blood diseases | 600 (31.0) | 98 (34.6) | 138 (49.6) |
| Immunodeficiency disorders (leukemia, lymphoma, HIV and transplantation) | 130 (6.72) | 13 (4.59) | 14 (5.03) |
| -Antitumoral Drugs | 106 (5.48) | 16 (5.65) | 19 (6.83) |
| Neurological comorbidities (central and peripheral nervous system disorders) | 114 (5.89) | 17 (6.00) | 9 (3.23) |
| Transfusions for hematological disorders | 35 (1.81) | 6 (2.12) | 4 (1.43) |
| Pregnancy | 36 (1.86) | 7 (2.47) | - |
| Breastfeeding | 33 (1.7) | 5 (1.77) | |
| History of anticoagulant drugs | 19 (0.98) | 2 (0.70) | 18 (6.47) |
| History of adverse reactions to previous vaccination | 13 (0.67) | 1 (0.35) | 5 (1.79) |
| Neurological Complications | Symptomatic Group x (N = 5962) | BNT162b2 (N = 4650) | Vaccine Lines x | ChAdOx1nCov-19 (N = 583) |
|---|---|---|---|---|
| mRNA-1273 (N = 729) | ||||
| Headache | 3067 (51.4) | 2348 (50.5) | 409 (56.1) | 310 (53.2) |
| Sleepiness | 2256 (37.8) | 1805 (38.8) | 290 (39.7) | 161 (27.6) |
| Vertigo | 800 (13.4) | 625 (13.4) | 116 (15.9) | 59 (10.1) |
| Paresthesia | 620 (10.4) | 486 (10.4) | 106 (14.5) | 28 (4.8) |
| Cognitive fog (difficulty concentrating) | 362 (6.1) | 300 (6.4) | 44 (6.0) | 18 (3.1) |
| Insomnia | 306 (5.1) | 249 (5.3) | 23 (3.2) | 34 (5.8) |
| Muscle spasms | 288 (4.8) | 201 (4.3) | 43 (5.9) | 44 (7.5) |
| Tremor | 282 (4.7) | 149 (3.2) | 56 (7.7) | 77 (13.2) |
| Diplopia | 132 (2.2) | 101 (2.2) | 20 (2.7) | 11 (1.9) |
| Tinnitus | 115 (1.9) | 85 (1.8) | 14 (1.9) | 16 (2.7) |
| Taste alterations | 51 (0.9) | 35 (0.7) | 12 (1.6) | 4 (0.7) |
| Dysphonia | 50 (0.8) | 42 (0.9) | 5 (0.7) | 3 (0.5) |
| Smell alterations | 30 (0.5) | 20 (0.4) | 6 (0.8) | 4 (0.7) |
| Neurological Complication | First Dose | Second Dose | ||
|---|---|---|---|---|
| Clinical Onset (Minutes/Days) | Duration (Day/Week) | Clinical Onset (Minutes/Days) | Duration | |
| Headache | Within first 15 min | 1 day | Within first 3 days | 1 day |
| Sleepiness | From 15 to 30 min | Up to a week | Within first 3 days | Up a week |
| Vertigo | From15 to 30 min | 1 day | Within 30 min | 1 day |
| Paresthesia | From 15 to 30 min | 1 day | From 15 to 30 min | 1 day |
| Cognitive fog | Within first 3 days | Up to a week | Within first 3 days | Up a week |
| Insomnia | Within first 3 days | Up to a week | Within first 3 days | Up a week |
| Tremor | Within first 15 min | 1 day | Within first 3 days | 1 day |
| Muscle spasms | Within first 15 min | <1 day | Within first 3 days | <1 day |
| Diplopia | From 15 to 30 min | 1 day | Within first 15 min | 1 day |
| Tinnitus | From 15 to 30 min | 1 day | From 15 within 30 min | 1 day |
| Taste alterations | Within 3 days | Up to a week | Within 3 days | Up a week |
| Dysphonia | Within first 3 days | <1 day | Within first 3 days | <1 day |
| Smell alterations | Within 3 days | >a week | Within 3 days | >a week |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salsone, M.; Signorelli, C.; Oldani, A.; Alberti, V.F.; Castronovo, V.; Mazzitelli, S.; Minerva, M.; Ferini-Strambi, L. NEURO-COVAX: An Italian Population-Based Study of Neurological Complications after COVID-19 Vaccinations. Vaccines 2023, 11, 1621. https://doi.org/10.3390/vaccines11101621
Salsone M, Signorelli C, Oldani A, Alberti VF, Castronovo V, Mazzitelli S, Minerva M, Ferini-Strambi L. NEURO-COVAX: An Italian Population-Based Study of Neurological Complications after COVID-19 Vaccinations. Vaccines. 2023; 11(10):1621. https://doi.org/10.3390/vaccines11101621
Chicago/Turabian StyleSalsone, Maria, Carlo Signorelli, Alessandro Oldani, Valerio Fabio Alberti, Vincenza Castronovo, Salvatore Mazzitelli, Massimo Minerva, and Luigi Ferini-Strambi. 2023. "NEURO-COVAX: An Italian Population-Based Study of Neurological Complications after COVID-19 Vaccinations" Vaccines 11, no. 10: 1621. https://doi.org/10.3390/vaccines11101621
APA StyleSalsone, M., Signorelli, C., Oldani, A., Alberti, V. F., Castronovo, V., Mazzitelli, S., Minerva, M., & Ferini-Strambi, L. (2023). NEURO-COVAX: An Italian Population-Based Study of Neurological Complications after COVID-19 Vaccinations. Vaccines, 11(10), 1621. https://doi.org/10.3390/vaccines11101621





