Trick-or-Trap: Extracellular Vesicles and Viral Transmission
Abstract
:1. Introduction
2. Extracellular Vesicles
3. Role of Extracellular Vesicles in Viral Infection
3.1. Antiviral Functions of EVs
3.2. Proviral Functions of EVs
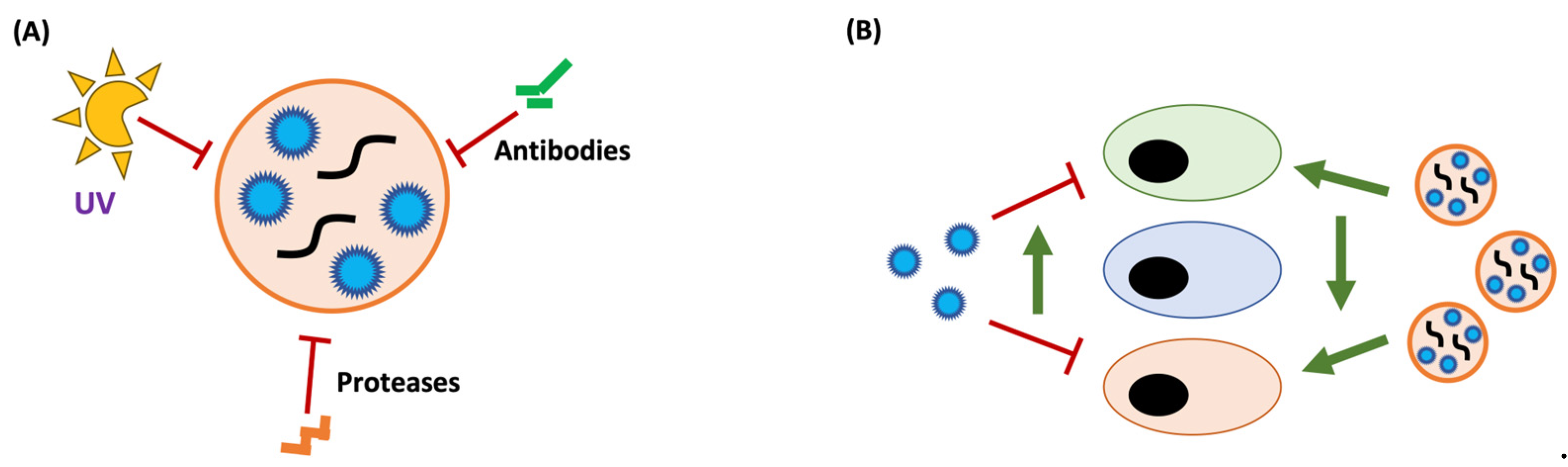
3.3. A New Infectious Particle: EVs as Vehicles for Viral Transmission
4. Pros and Cons of Viral Transmission through Extracellular Vesicles
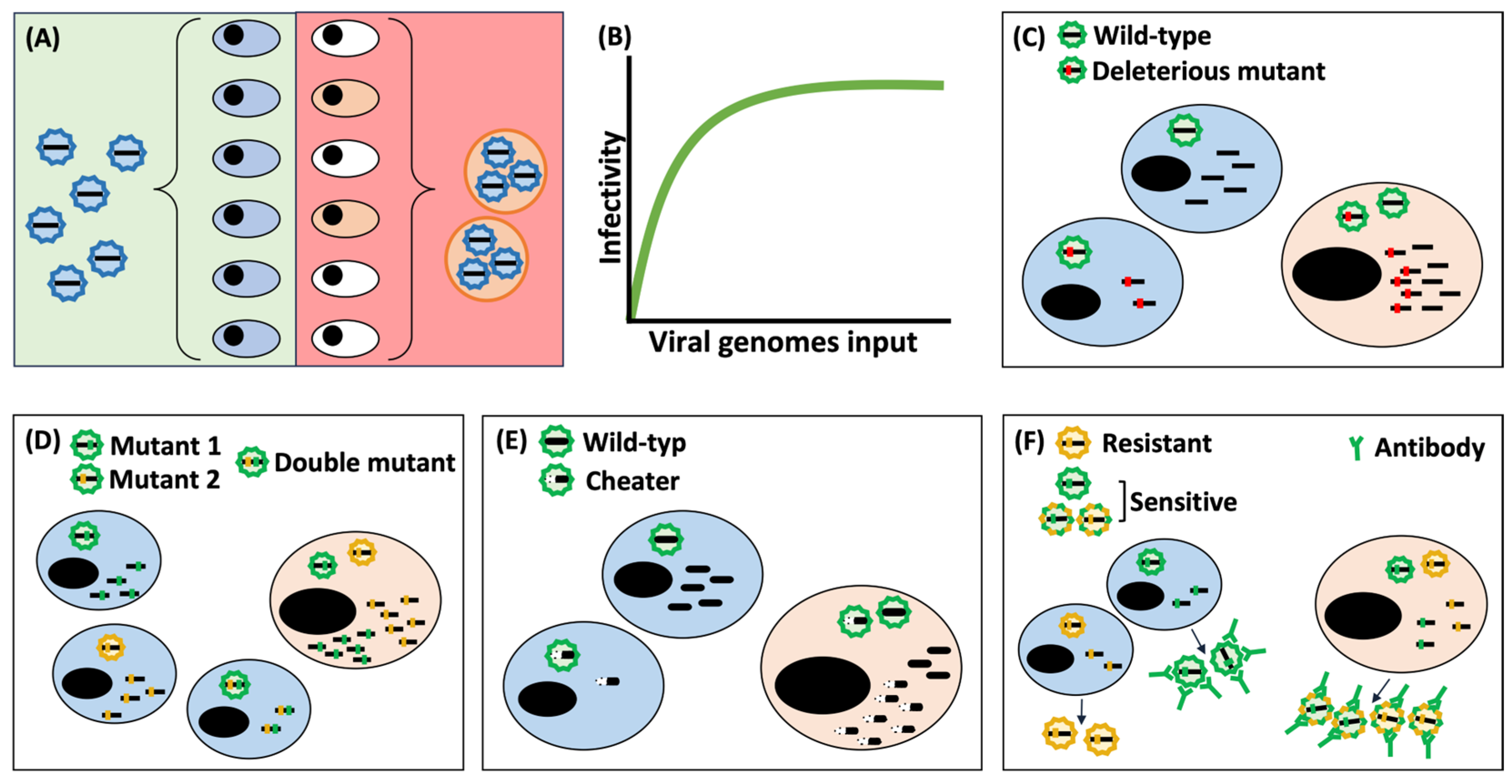
4.1. Effects Derived from an Increase in MOI
4.2. Vehicular Effects of Extracellular Vesicles
5. Clinical Applications and Concluding Insights
| Virus Name | Virus Family | Genome | VP Size | EV Type | EV Size | Infectious Cargo | Markers | References | |
|---|---|---|---|---|---|---|---|---|---|
| Non-enveloped | Human astrovirus | Astroviridae | ss(+)RNA | 30 nm | ES | 100–200 nm | VP | CD63, Alix | [147] |
| Cricket paralysis virus | Dicistroviridae | ss(+)RNA | 30 nm | ES | 30–100 nm | NG & VP | Alix, Flotillin-1, Rab35, Syntaxin-1A | [148] | |
| Hepatitis E virus | Hepeviridae | ss(+)RNA | 30 nm | ES | 50 nm | VP | CD9, CD63, CD81, Alix, Tsg101 | [79,149] | |
| Hepatitis A virus | Picornaviridae | ss(+)RNA | 30 nm | ES | 50–110 nm | VP | CD9, CD63, CD81, Alix, Flotillin-1 | [77,150] | |
| Duck hepatitis A virus | Picornaviridae | ss(+)RNA | 30 nm | ES | 30–150 nm | NG & VP | CD63, Tsg101 | [151] | |
| Poliovirus | Picornaviridae | ss(+)RNA | 30 nm | ALMV | 300–400 nm | VP | LC3, calnexin, PS | [82,152] | |
| ES & MV | 80, 170 nm | NG & VP | CD9, PS | [153] | |||||
| Rhinovirus | Picornaviridae | ss(+)RNA | 30 nm | ALMV | 300–400 nm | VP | LC3, calnexin, PS | [82] | |
| Coxsackievirus B1 | Picornaviridae | ss(+)RNA | 30 nm | NS | 100–300 nm | VP | β-actin | [154] | |
| Coxsackievirus B3 | Picornaviridae | ss(+)RNA | 30 nm | ALMV | 300–400 nm | VP | LC3, calnexin, PS | [82,103] | |
| ES | 100 nm | VP | Alix, CD9 | [155] | |||||
| Enterovirus A71 | Picornaviridae | ss(+)RNA | 30 nm | ES | 100 nm | NG & VP | CD63, Tsg101 | [156] | |
| Enterovirus D68 | Picornaviridae | ss(+)RNA | 30 nm | ALMV ? | 100–300 nm | VP | NS | [157] | |
| Echovirus 16 | Picornaviridae | ss(+)RNA | 30 nm | ES | 70–200 nm | VP | CD9, CD63, CD81 | [158] | |
| Encephalomyocarditis virus (EMCV) | Picornaviridae | ss(+)RNA | 30 nm | ES & MV | 50–350 nm | VP | CD9, Flotillin-1, LC3 | [83] | |
| Foot and mouth disease virus | Picornaviridae | ss(+)RNA | 30 nm | ES | <200 nm | NG | CD9, CD63, Alix | [159] | |
| Norovirus | Caliciviridae | ss(+)RNA | 40 nm | ES | <200 nm | VP | CD9, CD63, CD81 | [84] | |
| Infectious bursal disease virus | Birnaviridae | segmented-dsRNA | 70 nm | ALMV | 500 nm | VP | LC3 | [160] | |
| Rotavirus | Reoviridae | segmented-dsRNA | 80 nm | MV | 300–500 nm | VP | CD98, PS | [84,115] | |
| ES & MV | 110–450 nm | VP | Alix, CD63, GM1, Integrin-α2 | [161] | |||||
| Rice gall dwarf virus | Reoviridae | segmented-dsRNA | 70 nm | ALMV | 200–500 nm | VP | ATG8 | [162] | |
| Avian orthoreoviruses | Reoviridae | segmented-dsRNA | 70–80 nm | ES | 100 nm | VP | Tsg101, Hsp70 | [163] | |
| Bluetongue virus | Reoviridae | segmented-dsRNA | 80 nm | ALMV | 300–1000 nm | VP | Annexin A2, LAMP1, LC3, Tsg101 | [164] | |
| Trichomonasvirus | Totiviridae | linear-dsRNA | 40 nm | ES | 30–150 nm | VP | TvTSP1 | [165] | |
| Torquetenovirus | Anelloviridae | ssDNA | 30 nm | ES | 70 nm | NG & VP | CD63, CD81, Annexin II | [166] | |
| JC polyomavirus | Polyomaviridae | circular dsDNA | 50 nm | ES | 150–200 nm | VP | CD9, CD81, Flotillin-1, Annexin-V, TSG101 | [85,86] | |
| BK polyomavirus | Polyomaviridae | circular dsDNA | 50 nm | ES | 50–100 nm | VP | CD9, CD63, CD81 | [167] | |
| Enveloped | Porcine reproductive and respiratory syndrome virus | Arteriviridae | ss(+)RNA | 45–60 nm | ES | 30–150 nm | NG | CD9, CD63, Alix | [129] |
| Porcine epidemic diarrhea virus | Coronaviridae | ss(+)RNA | 120 nm | ES | 100 nm | NG | CD9, CD63, Alix | [117] | |
| SARS-CoV-2 | Coronaviridae | ss(+)RNA | 120 nm | ES | >120 nm | VP | NS | [168] | |
| AB | 1.6–9.5μm | VP | NS | [169] | |||||
| Hepatitis C virus | Flaviviridae | ss(+)RNA | 50 nm | ES | 50–100 nm | NG | CD9, CD63, Alix, Tsg101 | [69,70] | |
| Hepatitis G virus | Flaviviridae | ss(+)RNA | 50 nm | ES | NS | NG | NS | [170] | |
| Dengue virus | Flaviviridae | ss(+)RNA | 50 nm | ES | 50–150 nm | NG & VP | CD9/AalCD9, CD81/AalCD81 | [71,72] | |
| ALMV ? | 2–5μm | NG & VP | LC3, Rab11, Transferrin receptor | [116,171] | |||||
| West nile virus | Flaviviridae | ss(+)RNA | 50 nm | ES | 30–200 nm | NG | CD9 | [71] | |
| Tick-borne Langat virus | Flaviviridae | ss(+)RNA | 50 nm | ES | 30–200 nm | NG | CD9 | [131] | |
| Zika virus | Flaviviridae | ss(+)RNA | 50 nm | ES | 50–150 nm | NG | CD9, CD63, Alix | [51,132] | |
| ES & MV | 125, 320 nm | NG | CD63, PS | [172] | |||||
| NS | 300–700 nm | VP | NS | [173] | |||||
| Chikungunya virus | Togaviridae | ss(+)RNA | 70 nm | ES | 50–250 nm | NG | CD9, CD63 | [133] | |
| Human immunodeficiency virus | Retroviridae | ss(+)RNA - RT | 80–100 nm | ES | 100 nm | VP | CD1b, CD9, CD63, HLA-DR1 | [67] | |
| Human T-cell lymphotropic virus | Retroviridae | ss(+)RNA - RT | 80–100 nm | ES | NS | NG | CD9, CD63, CD81, LC3, p62 | [134] | |
| Avian leukosis virus J | Retroviridae | ss(+)RNA - RT | 80–100 nm | ES | 50–200 nm | NG | CD63, CD81, Tsg101 | [123] | |
| Severe fever with thrombocytopenia syndrome virus | Phenuiviridae | segmented-ss(-)RNA | 80–120 nm | ES | 50–100 nm | VP | CD63, LC3 | [68] | |
| Rift valley fever virus | Phenuiviridae | segmented-ss(-)RNA | 80–120 nm | ES | 50–150 nm | NG | CD63 | [174] | |
| Spodoptera frugiperda ascovirus | Ascoviridae | circular dsDNA | 200–400 nm | AB | 5–10μm | VP | NS | [175] | |
| Heliothis virescens ascovirus 3h | Ascoviridae | circular dsDNA | 200–400 nm | MV | <1000 nm | VP | NS | [176] | |
| Marseillevirus | Marseilleviridae | circular dsDNA | 250 nm | NS | 0.3–3.5μm | VP | NS | [74] | |
| Hepatitis B virus | Hepadnaviridae | circular dsDNA - RT | 40 nm | ES | 100–150 nm | NG & VP | CD63, CD81, Alix, Tsg101 | [177] | |
| Varicela zoster virus | Herpesviridae | dsDNA | 150–200 nm | ALMV | 300–500 nm | VP | LC3, Rab11 | [136] | |
| Herpes simplex virus 1 (alpha) | Herpesviridae | dsDNA | 150–200 nm | ALMV | 250–1000 nm | VP | CD63, CD81, LC3, Integrin β1, Flotillin-1 | [137] | |
| Swine fever virus | Asfarviridae | dsDNA | 175–215 nm | ALMV | 400–800 nm | VP | BECN1, CD81, LC3 | [128] |
Author Contributions
Funding
Conflicts of Interest
References
- McCormick, W.; Mermel, L.A. The Basic Reproductive Number and Particle-to-Plaque Ratio: Comparison of These Two Parameters of Viral Infectivity. Virol. J. 2021, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral Mutation Rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J. Molecular Determinants of the Ratio of Inert to Infectious Virus Particles. Prog. Mol. Biol. Transl. Sci. 2015, 129, 285–326. [Google Scholar] [CrossRef] [PubMed]
- Fredericksen, B.L.; Wei, B.L.; Yao, J.; Luo, T.; Garcia, J.V. Inhibition of Endosomal/Lysosomal Degradation Increases the Infectivity of Human Immunodeficiency Virus. J. Virol. 2002, 76, 11440–11446. [Google Scholar] [CrossRef]
- Wei, B.L.; Denton, P.W.; O’Neill, E.; Luo, T.; Foster, J.L.; Garcia, J.V. Inhibition of Lysosome and Proteasome Function Enhances Human Immunodeficiency Virus Type 1 Infection. J. Virol. 2005, 79, 5705–5712. [Google Scholar] [CrossRef]
- Stiefel, P.; Schmidt, F.I.; Dörig, P.; Behr, P.; Zambelli, T.; Vorholt, J.A.; Mercer, J. Cooperative Vaccinia Infection Demonstrated at the Single-Cell Level Using FluidFM. Nano Lett. 2012, 12, 4219–4227. [Google Scholar] [CrossRef]
- Andreu-Moreno, I.; Sanjuán, R. Collective Infection of Cells by Viral Aggregates Promotes Early Viral Proliferation and Reveals a Cellular-Level Allee Effect. Curr. Biol. 2018, 28, 3212–3219. [Google Scholar] [CrossRef]
- Sanjuán, R. Collective Properties of Viral Infectivity. Curr. Opin. Virol. 2018, 33, 1–6. [Google Scholar] [CrossRef]
- Sanjuán, R.; Thoulouze, M.-I. Why Viruses Sometimes Disperse in Groups. Virus Evol. 2019, 5, vez014. [Google Scholar] [CrossRef]
- Deatheragea, B.L.; Cooksona, B.T. Membrane Vesicle Release in Bacteria, Eukaryotes, and Archaea: A Conserved yet Underappreciated Aspect of Microbial Life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding Microvesicles: Artefacts No More. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, S.; Wang, X.; Gu, H.; Fan, G.C. Pathologic Function and Therapeutic Potential of Exosomes in Cardiovascular Disease. Biochim. Et Biophys. Acta-Mol. Basis Dis. 2015, 1852, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, C.; Cavallini, L.; Spinelli, C.; Yang, J.; Reis-Sobreiro, M.; Candia, P.D.; Minciacchi, V.R.; Di Vizio, D. Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer. Int. J. Mol. Sci. 2016, 17, 175. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F. Extracellular Vesicles and Neurodegenerative Diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef] [PubMed]
- Bello-Morales, R.; Ripa, I.; López-Guerrero, J.A. Extracellular Vesicles in Viral Spread and Antiviral Response. Viruses 2020, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and Secretion of Exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes). J. Biol. Chem 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Meckes, D.G.; Raab-Traub, N. Microvesicles and Viral Infection. J. Virol. 2011, 85, 12844–12854. [Google Scholar] [CrossRef]
- Ponpuak, M.; Mandell, M.A.; Kimura, T.; Chauhan, S.; Cleyrat, C.; Deretic, V. Secretory Autophagy. Curr. Opin. Cell Biol. 2015, 35, 106–116. [Google Scholar] [CrossRef]
- Ktistakis, N.T.; Tooze, S.A. Digesting the Expanding Mechanisms of Autophagy. Trends Cell Biol. 2016, 26, 624–635. [Google Scholar] [CrossRef]
- Hailey, D.W.; Rambold, A.S.; Satpute-Krishnan, P.; Mitra, K.; Sougrat, R.; Kim, P.K.; Lippincott-Schwartz, J. Mitochondria Supply Membranes for Autophagosome Biogenesis during Starvation. Cell 2010, 141, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Moreau, K.; Jahreiss, L.; Puri, C.; Rubinsztein, D.C. Plasma Membrane Contributes to the Formation of Pre-Autophagosomal Structures. Nat Cell Biol 2010, 12, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M. The Plasma Membrane Brings Autophagosomes to Life. Nat. Cell Biol. 2010, 12, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Escola, J.M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-Lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef]
- Barrès, C.; Blanc, L.; Bette-Bobillo, P.; André, S.; Mamoun, R.; Gabius, H.J.; Vidal, M. Galectin-5 Is Bound onto the Surface of Rat Reticulocyte Exosomes and Modulates Vesicle Uptake by Macrophages. Blood 2010, 115, 696–705. [Google Scholar] [CrossRef]
- Christianson, H.C.; Svensson, K.J.; Van Kuppevelt, T.H.; Li, J.P.; Belting, M. Cancer Cell Exosomes Depend on Cell-Surface Heparan Sulfate Proteoglycans for Their Internalization and Functional Activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Gomzikova, M.; Kletukhina, S.; Kurbangaleeva, S.; Rizvanov, A. Evaluation of Cytochalasin B-Induced Membrane Vesicles Fusion Specificity with Target Cells. Biomed. Res. Int. 2018, 2018, 7053623. [Google Scholar] [CrossRef]
- van Dongen, H.M.; Masoumi, N.; Witwer, K.W.; Pegtel, D.M. Extracellular Vesicles Exploit Viral Entry Routes for Cargo Delivery. Microbiol. Mol. Biol. Rev. MMBR 2016, 80, 369. [Google Scholar] [CrossRef] [PubMed]
- Raab-Traub, N.; Dittmer, D.P. Viral Effects on the Content and Function of Extracellular Vesicles. Nat. Reviews Microbiol. 2017, 15, 559. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Porcellati, S.; Emiliani, C. The Role of Extracellular Vesicles in Viral Infection and Transmission. Vaccines 2019, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- de Toledo Martins, S.; Alves, L.R. Extracellular Vesicles in Viral Infections: Two Sides of the Same Coin? Front. Cell. Infect. Microbiol. 2020, 10, 593170. [Google Scholar] [CrossRef]
- Velandia-Romero, M.L.; Caldern-Pelaez, M.A.; Balbas-Tepedino, A.; Alejandro Marquez-Ortiz, R.; Madroñero, L.J.; Prieto, A.B.; Castellanos, J.E. Extracellular Vesicles of U937 Macrophage Cell Line Infected with DENV-2 Induce Activation in Endothelial Cells EA.Hy926. PLoS ONE 2020, 15, e0227030. [Google Scholar] [CrossRef]
- Gilliet, M.; Cao, W.; Liu, Y.J. Plasmacytoid Dendritic Cells: Sensing Nucleic Acids in Viral Infection and Autoimmune Diseases. Nature Reviews Immunology 2008 8:8 2008, 8, 594–606. [Google Scholar] [CrossRef]
- Bernard, M.A.; Zhao, H.; Yue, S.C.; Anandaiah, A.; Koziel, H.; Tachado, S.D. Novel HIV-1 MiRNAs Stimulate TNFα Release in Human Macrophages via TLR8 Signaling Pathway. PLoS ONE 2014, 9, e106006. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M.; et al. Exosomes Mediate the Cell-to-Cell Transmission of IFN-α-Induced Antiviral Activity. Nat. Immunol. 2013, 14, 793–803. [Google Scholar] [CrossRef]
- Arrode, G.; Boccaccio, C.; Abastado, J.-P.; Davrinche, C. Cross-Presentation of Human Cytomegalovirus Pp65 (UL83) to CD8 + T Cells Is Regulated by Virus-Induced, Soluble-Mediator-Dependent Maturation of Dendritic Cells. J. Virol. 2002, 76, 142–150. [Google Scholar] [CrossRef]
- Montecalvo, A.; Shufesky, W.J.; Beer Stolz, D.; Sullivan, M.G.; Wang, Z.; Divito, S.J.; Papworth, G.D.; Watkins, S.C.; Robbins, P.D.; Larregina, A.T.; et al. Exosomes As a Short-Range Mechanism to Spread Alloantigen between Dendritic Cells during T Cell Allorecognition. J. Immunol. 2008, 180, 3081–3090. [Google Scholar] [CrossRef]
- Utsugi-Kobukai, S.; Fujimaki, H.; Hotta, C.; Nakazawa, M.; Minami, M. MHC Class I-Mediated Exogenous Antigen Presentation by Exosomes Secreted from Immature and Mature Bone Marrow Derived Dendritic Cells. Immunol. Lett. 2003, 89, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Muntasell, A.; Berger, A.C.; Roche, P.A. T Cell-Induced Secretion of MHC Class II-Peptide Complexes on B Cell Exosomes. EMBO J. 2007, 26, 4263–4272. [Google Scholar] [CrossRef] [PubMed]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef]
- Jaszczur, M.; Bertram, J.G.; Pham, P.; Scharff, M.D.; Goodman, M.F. AID and Apobec3G Haphazard Deamination and Mutational Diversity. Cell Mol. Life Sci. 2013, 70, 3089–3108. [Google Scholar] [CrossRef] [PubMed]
- Khatua, A.K.; Taylor, H.E.; Hildreth, J.E.K.; Popik, W. Exosomes Packaging APOBEC3G Confer Human Immunodeficiency Virus Resistance to Recipient Cells. J. Virol. 2009, 83, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Delorme-Axford, E.; Donker, R.B.; Mouillet, J.F.; Chu, T.; Bayer, A.; Ouyang, Y.; Wang, T.; Stolz, D.B.; Sarkar, S.N.; Morelli, A.E.; et al. Human Placental Trophoblasts Confer Viral Resistance to Recipient Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12048–12053. [Google Scholar] [CrossRef]
- Yao, Z.; Qiao, Y.; Li, X.; Chen, J.; Ding, J.; Bai, L.; Shen, F.; Shi, B.; Liu, J.; Peng, L.; et al. Exosomes Exploit the Virus Entry Machinery and Pathway To Transmit Alpha Interferon-Induced Antiviral Activity. J. Virol. 2018, 92, e01578-18. [Google Scholar] [CrossRef]
- De Carvalho, J.V.; De Castro, R.O.; Da Silva, E.Z.M.; Silveira, P.P.; Da Silva-Januário, M.E.; Arruda, E.; Jamur, M.C.; Oliver, C.; Aguiar, R.S.; DaSilva, L.L.P. Nef Neutralizes the Ability of Exosomes from CD4+ T Cells to Act as Decoys during HIV-1 Infection. PLoS ONE 2014, 9, e113691. [Google Scholar] [CrossRef]
- Han, Z.; Liu, X.; Chen, X.; Zhou, X.; Du, T.; Roizman, B.; Zhou, G. MiR-H28 and MiR-H29 Expressed Late in Productive Infection Are Exported and Restrict HSV-1 Replication and Spread in Recipient Cells. Proc. Natl. Acad. Sci. USA 2016, 113, E894–E901. [Google Scholar] [CrossRef]
- Germano, J.F.; Sawaged, S.; Saadaeijahromi, H.; Andres, A.M.; Feuer, R.; Gottlieb, R.A.; Sin, J. Coxsackievirus B Infection Induces the Extracellular Release of MiR-590-5p, a Proviral MicroRNA. Virology 2019, 529, 169–176. [Google Scholar] [CrossRef]
- Zhou, C.; Tan, L.; Sun, Y.; Qiu, X.; Meng, C.; Liao, Y.; Song, C.; Liu, W.; Nair, V.; Ding, C. Exosomes Carry MicroRNAs into Neighboring Cells to Promote Diffusive Infection of Newcastle Disease Virus. Viruses 2019, 11, 527. [Google Scholar] [CrossRef]
- Klibi, J.; Niki, T.; Riedel, A.; Pioche-Durieu, C.; Souquere, S.; Rubinstein, E.; Moulec, S.L.E.; Guigay, J.; Hirashima, M.; Guemira, F.; et al. Blood Diffusion and Th1-Suppressive Effects of Galectin-9-Containing Exosomes Released by Epstein-Barr Virus-Infected Nasopharyngeal Carcinoma Cells. Blood 2009, 113, 1957–1966. [Google Scholar] [CrossRef]
- Dukers, D.F.; Meij, P.; Vervoort, M.B.H.J.; Vos, W.; Scheper, R.J.; Meijer, C.J.L.M.; Bloemena, E.; Middeldorp, J.M. Direct Immunosuppressive Effects of EBV-Encoded Latent Membrane Protein 1. J. Immunol. 2000, 165, 663–670. [Google Scholar] [CrossRef]
- Flanagan, J.; Middeldorp, J.; Sculley, T. Localization of the Epstein-Barr Virus Protein LMP 1 to Exosomes. J. Gen. Virol. 2003, 84, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, L.; Ward, A.; Choi, S.H.; Pushkarsky, T.; Brichacek, B.; Vanpouille, C.; Adzhubei, A.A.; Mukhamedova, N.; Sviridov, D.; Margolis, L.; et al. Inhibition of HIV Replication by Apolipoprotein A-I Binding Protein Targeting the Lipid Rafts. mBio 2020, 11, e02956-19. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Santini, P.A.; Sullivan, J.S.; He, B.; Shan, M.; Ball, S.C.; Dyer, W.B.; Ketas, T.J.; Chadburn, A.; Cohen-Gould, L.; et al. HIV-1 Evades Virus-Specific IgG2 and IgA Responses by Targeting Systemic and Intestinal B Cells via Long-Range Intercellular Conduits. Nat. Immunol. 2009, 10, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Arenaccio, C.; Anticoli, S.; Manfredi, F.; Chiozzini, C.; Olivetta, E.; Federico, M. Latent HIV-1 Is Activated by Exosomes from Cells Infected with Either Replication-Competent or Defective HIV-1. Retrovirology 2015, 12, 87. [Google Scholar] [CrossRef]
- Mack, M.; Kleinschmidt, A.; Brühl, H.; Klier, C.; Nelson, P.J.; Cihak, J.; Plachý, J.; Stangassinger, M.; Erfle, V.; Schlöndorff, D. Transfer of the Chemokine Receptor CCR5 between Cells by Membrane- Derived Microparticles: A Mechanism for Cellular Human Immunodeficiency Virus 1 Infection. Nat. Med. 2000, 6, 769–775. [Google Scholar] [CrossRef]
- Rozmyslowicz, T.; Majka, M.; Kijowski, J.; Murphy, S.L.; Conover, D.O.; Poncz, M.; Ratajczak, J.; Gaulton, G.N.; Ratajczak, M.Z. Platelet- and Megakaryocyte-Derived Microparticles Transfer CXCR4 Receptor to CXCR4-Null Cells and Make Them Susceptible to Infection by X4-HIV. AIDS 2003, 17, 33–42. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The Role of Extracellular Vesicles in COVID-19 Virus Infection. Infect. Genet. Evol. 2020, 85, 104422. [Google Scholar] [CrossRef]
- Tey, S.K.; Lam, H.; Wong, S.W.K.; Zhao, H.; To, K.K.W.; Yam, J.W.P. ACE2-enriched Extracellular Vesicles Enhance Infectivity of Live SARS-CoV-2 Virus. J. Extracell. Vesicles 2022, 11, e12231. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Serada, S.; Naka, T.; Mori, Y. MHC Class I Molecules Are Incorporated into Human Herpesvirus-6 Viral Particles and Released into the Extracellular Environment. Microbiol. Immunol. 2014, 58, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.O.; DeMarino, C.; Pleet, M.L.; Cowen, M.; Branscome, H.; Al Sharif, S.; Jones, J.; Dutartre, H.; Lepene, B.; Liotta, L.A.; et al. HTLV-1 Extracellular Vesicles Promote Cell-to-Cell Contact. Front. Microbiol. 2019, 10, 2147. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Yu, D.; An, D.S.; Baldwin, G.C.; Xie, Y.; Poon, B.; Chow, Y.-H.; Park, N.-H.; Chen, I.S.Y. Human Immunodeficiency Virus Env-Independent Infection of Human CD4− Cells. J. Virol. 2000, 74, 10994. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.H.; Yu, D.; Zhang, J.; Xie, Y.; Wei, O.L.C.; Chiu, C.; Foroohar, M.; Yang, O.O.; Park, N.H.; Chen, I.S.Y.; et al. Gp120-Independent Infection of CD4(-) Epithelial Cells and CD4(+) T-Cells by HIV-1. J. Acquir. Immune Defic. Syndr. 2002, 30, 1–8. [Google Scholar] [CrossRef]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E.K. The Trojan Exosome Hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592. [Google Scholar] [CrossRef]
- Wiley, R.D.; Gummuluru, S. Immature Dendritic Cell-Derived Exosomes Can Mediate HIV-1 Trans Infection. Proc. Natl. Acad. Sci. USA 2006, 103, 738–743. [Google Scholar] [CrossRef]
- Silvas, J.A.; Popov, V.L.; Paulucci-Holthauzen, A.; Aguilar, P.V. Extracellular Vesicles Mediate Receptor-Independent Transmission of Novel Tick-Borne Bunyavirus. J. Virol. 2016, 90, 873–886. [Google Scholar] [CrossRef]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.A.; Stalin Raj, V.; Jenster, G.; et al. Exosome-Mediated Transmission of Hepatitis C Virus between Human Hepatoma Huh7.5 Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef]
- Bukong, T.N.; Momen-Heravi, F.; Kodys, K.; Bala, S.; Szabo, G. Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-MiR122-HSP90. PLoS Pathog. 2014, 10, e1004424. [Google Scholar] [CrossRef]
- Vora, A.; Zhou, W.; Londono-Renteria, B.; Woodson, M.; Sherman, M.B.; Colpitts, T.M.; Neelakanta, G.; Sultana, H. Arthropod EVs Mediate Dengue Virus Transmission through Interaction with a Tetraspanin Domain Containing Glycoprotein Tsp29Fb. Proc. Natl. Acad. Sci. USA 2018, 115, E6604–E6613. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Hurtado-Monzón, A.M.; Farfan-Morales, C.N.; Cervantes-Salazar, M.; Bolaños, J.; Cigarroa-Mayorga, O.E.; Martín-Martínez, E.S.; Medina, F.; et al. Isolation and Characterization of Exosomes Released from Mosquito Cells Infected with Dengue Virus. Virus Res. 2019, 266, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Woodson, M.; Sherman, M.B.; Neelakanta, G.; Sultana, H. Exosomes Mediate Zika Virus Transmission through SMPD3 Neutral Sphingomyelinase in Cortical Neurons. Emerg. Microbes Infect. 2019, 8, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Arantes, T.S.; Rodrigues, R.A.L.; Dos Santos Silva, L.K.; Oliveira, G.P.; de Souza, H.L.; Khalil, J.Y.B.; de Oliveira, D.B.; Torres, A.A.; da Silva, L.L.; Colson, P.; et al. The Large Marseillevirus Explores Different Entry Pathways by Forming Giant Infectious Vesicles. J. Virol. 2016, 90, 5246–5255. [Google Scholar] [CrossRef]
- Le Blanc, I.; Luyet, P.P.; Pons, V.; Ferguson, C.; Emans, N.; Petiot, A.; Mayran, N.; Demaurex, N.; Fauré, J.; Sadoul, R.; et al. Endosome-to-Cytosol Transport of Viral Nucleocapsids. Nature Cell Biol. 2005, 7, 653–664. [Google Scholar] [CrossRef]
- Nour, A.M.; Li, Y.; Wolenski, J.; Modis, Y. Viral Membrane Fusion and Nucleocapsid Delivery into the Cytoplasm Are Distinct Events in Some Flaviviruses. PLOS Pathog. 2013, 9, e1003585. [Google Scholar] [CrossRef]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.-H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A Pathogenic Picornavirus Acquires an Envelope by Hijacking Cellular Membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef]
- Nagashima, S.; Jirintai, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Kouki, T.; Yashiro, T.; Okamoto, H. Hepatitis E Virus Egress Depends on the Exosomal Pathway, with Secretory Exosomes Derived from Multivesicular Bodies. J. Gen. Virol. 2014, 95, 2166–2175. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Nishiyama, T.; Primadharsini, P.P.; Okamoto, H. Characterization of the Quasi-Enveloped Hepatitis E Virus Particles Released by the Cellular Exosomal Pathway. J. Virol. 2017, 91, 822–839. [Google Scholar] [CrossRef]
- Bird, S.W.; Maynard, N.D.; Covert, M.W.; Kirkegaard, K. Nonlytic Viral Spread Enhanced by Autophagy Components. Proc. Natl. Acad. Sci. USA 2014, 111, 13081–13086. [Google Scholar] [CrossRef]
- Robinson, S.M.; Tsueng, G.; Sin, J.; Mangale, V.; Rahawi, S.; McIntyre, L.L.; Williams, W.; Kha, N.; Cruz, C.; Hancock, B.M.; et al. Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers. PLoS Pathog. 2014, 10, e1004045. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Du, W.; Hagemeijer, M.C.; Takvorian, P.M.; Pau, C.; Cali, A.; Brantner, C.A.; Stempinski, E.S.; Connelly, P.S.; Ma, H.-C.; et al. Phosphatidylserine Vesicles Enable Efficient En Bloc Transmission of Enteroviruses. Cell 2015, 160, 619–630. [Google Scholar] [CrossRef] [PubMed]
- van der Grein, S.G.; Defourny, K.A.Y.; Rabouw, H.H.; Galiveti, C.R.; Langereis, M.A.; Wauben, M.H.M.; Arkesteijn, G.J.A.; van Kuppeveld, F.J.M.; Nolte-’t Hoen, E.N.M. Picornavirus Infection Induces Temporal Release of Multiple Extracellular Vesicle Subsets That Differ in Molecular Composition and Infectious Potential. PLoS Pathog. 2019, 15, e1007594. [Google Scholar] [CrossRef] [PubMed]
- Santiana, M.; Ghosh, S.; Ho, B.A.; Rajasekaran, V.; Du, W.-L.; Mutsafi, Y.; De Jésus-Diaz, D.A.; Sosnovtsev, S.V.; Levenson, E.A.; Parra, G.I.; et al. Vesicle-Cloaked Virus Clusters Are Optimal Units for Inter-Organismal Viral Transmission. Cell Host Microbe 2018, 24, 208–220.e8. [Google Scholar] [CrossRef]
- Morris-Love, J.; Gee, G.V.; O’Hara, B.A.; Assetta, B.; Atkinson, A.L.; Dugan, A.S.; Haley, S.A.; Atwood, W.J. JC Polyomavirus Uses Extracellular Vesicles to Infect Target Cells. mBio 2019, 10, e00379-19. [Google Scholar] [CrossRef]
- O’Hara, B.A.; Morris-Love, J.; Gee, G.V.; Haley, S.A.; Atwood, W.J. JC Virus Infected Choroid Plexus Epithelial Cells Produce Extracellular Vesicles That Infect Glial Cells Independently of the Virus Attachment Receptor. PLoS Pathog. 2020, 16, e1008371. [Google Scholar] [CrossRef]
- Díaz-Muñoz, S.L.; Sanjuán, R.; West, S. Sociovirology: Conflict, Cooperation, and Communication among Viruses. Cell Host Microbe 2017, 22, 437–441. [Google Scholar] [CrossRef]
- Altan-Bonnet, N.; Perales, C.; Domingo, E. Extracellular Vesicles: Vehicles of En Bloc Viral Transmission. Virus Res. 2019, 265, 143–149. [Google Scholar] [CrossRef]
- Stephens, P.A.; Sutherland, W.J.; Freckleton, R.P. What Is the Allee Effect? Oikos 1999, 87, 185. [Google Scholar] [CrossRef]
- Andreu-Moreno, I.; Bou, J.V.; Sanjuán, R. Cooperative Nature of Viral Replication. Sci. Adv. 2020, 6, eabd4942. [Google Scholar] [CrossRef]
- Sigal, A.; Kim, J.T.; Balazs, A.B.; Dekel, E.; Mayo, A.; Milo, R.; Baltimore, D. Cell-to-Cell Spread of HIV Permits Ongoing Replication despite Antiretroviral Therapy. Nature 2011, 477, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Agosto, L.M.; Ilinskaya, A.; Dorjbal, B.; Truong, R.; Derse, D.; Uchil, P.D.; Heidecker, G.; Mothes, W. Cell-to-Cell Transmission Can Overcome Multiple Donor and Target Cell Barriers Imposed on Cell-Free HIV. PLoS ONE 2013, 8, e53138. [Google Scholar] [CrossRef] [PubMed]
- Boullé, M.; Müller, T.G.; Dähling, S.; Ganga, Y.; Jackson, L.; Mahamed, D.; Oom, L.; Lustig, G.; Neher, R.A.; Sigal, A. HIV Cell-to-Cell Spread Results in Earlier Onset of Viral Gene Expression by Multiple Infections per Cell. PLOS Pathog. 2016, 12, e1005964. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.L.; Zhang, J.Y.; Rollins, M.F.; Osuna, B.A.; Wiedenheft, B.; Bondy-Denomy, J. Bacteriophage Cooperation Suppresses CRISPR-Cas3 and Cas9 Immunity. Cell 2018, 174, 917–925.e10. [Google Scholar] [CrossRef] [PubMed]
- Andino, R.; Domingo, E. Viral Quasispecies. Virology 2015, 479–480, 46–51. [Google Scholar] [CrossRef]
- Vignuzzi, M.; Stone, J.K.; Arnold, J.J.; Cameron, C.E.; Andino, R. Quasispecies Diversity Determines Pathogenesis through Cooperative Interactions in a Viral Population. Nature 2006, 439, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Bordería, A.V.; Isakov, O.; Moratorio, G.; Henningsson, R.; Agüera-González, S.; Organtini, L.; Gnädig, N.F.; Blanc, H.; Alcover, A.; Hafenstein, S.; et al. Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype. PLoS Pathog. 2015, 11, e1004838. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.S.; Hooper, K.A.; Ollodart, A.R.; Dingens, A.S.; Bloom, J.D. Cooperation between Distinct Viral Variants Promotes Growth of H3N2 Influenza in Cell Culture. eLife 2016, 5, e13974. [Google Scholar] [CrossRef]
- Tanner, E.J.; Liu, H.; Oberste, M.S.; Pallansch, M.; Collett, M.S.; Kirkegaard, K. Dominant Drug Targets Suppress the Emergence of Antiviral Resistance. eLife 2014, 3, e03830. [Google Scholar] [CrossRef]
- Turner, P.E.; Chao, L. Prisoner’s Dilemma in an RNA Virus. Nature 1999, 398, 441–443. [Google Scholar] [CrossRef]
- Marriott, A.C.; Dimmock, N.J. Defective Interfering Viruses and Their Potential as Antiviral Agents. Rev. Med. Virol. 2010, 20, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Moreno, I.; Sanjuán, R. Collective Viral Spread Mediated by Virion Aggregates Promotes the Evolution of Defective Interfering Particles. mBio 2020, 11, e02156-19. [Google Scholar] [CrossRef] [PubMed]
- Bou, J.-V.; Geller, R.; Sanjuán, R. Membrane-Associated Enteroviruses Undergo Intercellular Transmission as Pools of Sibling Viral Genomes. Cell Rep. 2019, 29, 714–723.e4. [Google Scholar] [CrossRef] [PubMed]
- Hsu, N.-Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.-H.; Takvorian, P.M.; Pau, C.; van der Schaar, H.; Kaushik-Basu, N.; Balla, T.; et al. Viral Reorganization of the Secretory Pathway Generates Distinct Organelles for RNA Replication. Cell 2010, 141, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Belov, G.A. Dynamic Lipid Landscape of Picornavirus Replication Organelles. Curr. Opin. Virol. 2016, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Castro, I.; Tenorio, R.; Risco, C. Virus Assembly Factories in a Lipid World. Curr. Opin. Virol. 2016, 18, 20–26. [Google Scholar] [CrossRef]
- Ravindran, M.S.; Bagchi, P.; Cunningham, C.N.; Tsai, B. Opportunistic Intruders: How Viruses Orchestrate ER Functions to Infect Cells. Nat. Rev. Microbiol. 2016, 14, 407–420. [Google Scholar] [CrossRef]
- Romero-Brey, I.; Bartenschlager, R. Endoplasmic Reticulum: The Favorite Intracellular Niche for Viral Replication and Assembly. Viruses 2016, 8, 160. [Google Scholar] [CrossRef]
- Shulla, A.; Randall, G. (+) RNA Virus Replication Compartments: A Safe Home for (Most) Viral Replication. Curr. Opin. Microbiol. 2016, 32, 82–88. [Google Scholar] [CrossRef]
- Altan-Bonnet, N. Lipid Tales of Viral Replication and Transmission. Trends Cell Biol. 2017, 27, 201–213. [Google Scholar] [CrossRef]
- Bou, J.-V.; Sanjuán, R. Experimental Evolution Reveals a Genetic Basis for Membrane-Associated Virus Release. Mol. Biol. Evol. 2020, 38, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Kerviel, A.; Zhang, M.; Altan-Bonnet, N. A New Infectious Unit: Extracellular Vesicles Carrying Virus Populations. Annu. Rev. Cell Dev. Biol. 2021, 37, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Llorente, A.; Skotland, T.; Sylvänne, T.; Kauhanen, D.; Róg, T.; Orłowski, A.; Vattulainen, I.; Ekroos, K.; Sandvig, K. Molecular Lipidomics of Exosomes Released by PC-3 Prostate Cancer Cells. Biochim. Biophys. Acta 2013, 1831, 1302–1309. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-Resolution Proteomic and Lipidomic Analysis of Exosomes and Microvesicles from Different Cell Sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ghosh, S.; Li, M.; Altan-Bonnet, N.; Shuai, D. Vesicle-Cloaked Rotavirus Clusters Are Environmentally Persistent and Resistant to Free Chlorine Disinfection. Environ. Sci. Technol. 2022, 56, 8475–8484. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wu, J.; Shen, L.; Yang, J.; Chen, J.; Xu, H. Enterovirus 71 Transmission by Exosomes Establishes a Productive Infection in Human Neuroblastoma Cells. Virus Genes 2016, 52, 189–194. [Google Scholar] [CrossRef]
- Ding, T.; Cheng, T.; Zhu, X.; Xiao, W.; Xia, S.; Fang, L.; Fang, P.; Xiao, S. Exosomes Mediate the Antibody-Resistant Intercellular Transmission of Porcine Epidemic Diarrhea Virus. Vet. Microbiol. 2023, 284, 109834. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; McKnight, K.L.; Hensley, L.; Lanford, R.E.; Walker, C.M.; Lemon, S.M. Human PDCs Preferentially Sense Enveloped Hepatitis A Virions. J. Clin. Investig. 2015, 125, 169. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, Y.; Liu, N.; Lv, D.; Chen, Y.; Liu, Z.; Jin, X.; Xiao, M.; Lavillette, D.; Zhong, J.; et al. Extracellular Vesicles from Zika Virus-Infected Cells Display Viral E Protein That Binds ZIKV-Neutralizing Antibodies to Prevent Infection Enhancement. EMBO J. 2023, 42, e112096. [Google Scholar] [CrossRef]
- Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef]
- Morizono, K.; Chen, I.S.Y. Receptors and Tropisms of Envelope Viruses. Curr. Opin. Virol. 2011, 1, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; de Jesús-González, L.A.; Palacios-Rápalo, S.N.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Hurtado-Monzón, A.M.; Gallardo-Flores, C.E.; Alcaraz-Estrada, S.L.; Salas-Benito, J.S.; et al. The Regulation of Flavivirus Infection by Hijacking Exosome-Mediated Cell–Cell Communication: New Insights on Virus–Host Interactions. Viruses 2020, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Chen, W.; Zhang, X.; Zhang, H.; Li, A.; Yan, Y.; Xie, Z.; Li, H.; Lin, W.; Ma, J.; et al. Semen Extracellular Vesicles Mediate Vertical Transmission of Subgroup J Avian Leukosis Virus. Virol. Sin. 2022, 37, 284–294. [Google Scholar] [CrossRef]
- Ravichandran, K.S.; Lorenz, U. Engulfment of Apoptotic Cells: Signals for a Good Meal. Nat. Rev. Immunol. 2007, 7, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.; Mercer, J. Viral Apoptotic Mimicry. Nat. Reviews. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Altan-Bonnet, N. Extracellular Vesicles Are the Trojan Horses of Viral Infection. Curr. Opin. Microbiol. 2016, 32, 77–81. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, C.; Hui, L.; Song, Y.; Fu, Y.; Li, M.; Yang, H.; Wu, J.; Sun, J.; Xu, W.; et al. Cathelicidins Target HSP60 To Restrict CVB3 Transmission via Disrupting the Exosome and Reducing Cardiomyocyte Apoptosis. J. Virol. 2023, 97, e0143322. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Liang, W.; Liu, S.; Deng, W.; Liu, Y.; Liu, Y.; Song, M.; Guo, K.; Zhang, Y. Extracellular Vesicles Originating from Autophagy Mediate an Antibody-Resistant Spread of Classical Swine Fever Virus in Cell Culture. Autophagy 2022, 18, 1433–1449. [Google Scholar] [CrossRef]
- Wang, T.; Fang, L.; Zhao, F.; Wang, D.; Xiao, S. Exosomes Mediate Intercellular Transmission of Porcine Reproductive and Respiratory Syndrome Virus. J. Virol. 2018, 92, e01734-17. [Google Scholar] [CrossRef]
- Wu, Y.W.; Mettling, C.; Wu, S.R.; Yu, C.Y.; Perng, G.C.; Lin, Y.S.; Lin, Y.L. Autophagy-Associated Dengue Vesicles Promote Viral Transmission Avoiding Antibody Neutralization. Sci. Rep. 2016, 6, 32243. [Google Scholar] [CrossRef]
- Zhou, W.; Woodson, M.; Neupane, B.; Bai, F.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes Serve as Novel Modes of Tick-Borne Flavivirus Transmission from Arthropod to Human Cells and Facilitates Dissemination of Viral RNA and Proteins to the Vertebrate Neuronal Cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef]
- York, S.B.; Sun, L.; Cone, A.S.; Duke, L.C.; Cheerathodi, M.R.; Meckes, D.G., Jr. Zika Virus Hijacks Extracellular Vesicle Tetraspanin Pathways for Cell-to-Cell Transmission. mSphere 2021, 6, e0019221. [Google Scholar] [CrossRef] [PubMed]
- Le, B.C.T.; Burassakarn, A.; Tongchai, P.; Ekalaksananan, T.; Aromseree, S.; Phanthanawiboon, S.; Polsan, Y.; Alexander, N.; Overgaard, H.J.; Pientong, C. Characterization and Involvement of Exosomes Originating from Chikungunya Virus-Infected Epithelial Cells in the Transmission of Infectious Viral Elements. Int. J. Mol. Sci. 2022, 23, 12117. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Mensah, G.A.; Sharif, S.A.; Pinto, D.O.; Branscome, H.; Yelamanchili, S.V.; Cowen, M.; Erickson, J.; Khatkar, P.; Mahieux, R.; et al. Extracellular Vesicles from Infected Cells Are Released Prior to Virion Release. Cells 2021, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Glitscher, M.; Tonnemacher, S.; Schollmeier, A.; Raupach, J.; Zahn, T.; Eberle, R.; Krijnse-Locker, J.; Basic, M.; Hildt, E. Presence of Intact Hepatitis B Virions in Exosomes. Cell. Mol. Gastroenterol. Hepatol. 2022, 15, 237–259. [Google Scholar] [CrossRef]
- Buckingham, E.M.; Jarosinski, K.W.; Jackson, W.; Carpenter, J.E.; Grose, C. Exocytosis of Varicella-Zoster Virus Virions Involves a Convergence of Endosomal and Autophagy Pathways. J. Virol. 2016, 90, 8673–8685. [Google Scholar] [CrossRef] [PubMed]
- Bello-Morales, R.; Praena, B.; de la Nuez, C.; Rejas, M.T.; Guerra, M.; Galán-Ganga, M.; Izquierdo, M.; Calvo, V.; Krummenacher, C.; López-Guerrero, J.A. Role of Microvesicles in the Spread of Herpes Simplex Virus 1 in Oligodendrocytic Cells. J. Virol. 2018, 92, 88–106. [Google Scholar] [CrossRef]
- Kuate, S.; Cinatl, J.; Doerr, H.W.; Überla, K. Exosomal Vaccines Containing the S Protein of the SARS Coronavirus Induce High Levels of Neutralizing Antibodies. Virology 2007, 362, 26–37. [Google Scholar] [CrossRef]
- Rolls, M.M.; Webster, P.; Balba, N.H.; Rose, J.K. Novel Infectious Particles Generated by Expression of the Vesicular Stomatitis Virus Glycoprotein from a Self-Replicating RNA. Cell 1994, 79, 497–506. [Google Scholar] [CrossRef]
- Reynolds, T.D.; Buonocore, L.; Rose, N.F.; Rose, J.K.; Robek, M.D. Virus-Like Vesicle-Based Therapeutic Vaccine Vectors for Chronic Hepatitis B Virus Infection. J. Virol. 2015, 89, 10407–10415. [Google Scholar] [CrossRef]
- Schell, J.B.; Rose, N.F.; Bahl, K.; Diller, K.; Buonocore, L.; Hunter, M.; Marx, P.A.; Gambhira, R.; Tang, H.; Montefiori, D.C.; et al. Significant Protection against High-Dose Simian Immunodeficiency Virus Challenge Conferred by a New Prime-Boost Vaccine Regimen. J. Virol. 2011, 85, 5764–5772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tian, Y.; Chen, C.; Wang, Z.; Pei, J.; Lin, C.; Zhou, M.; Fu, Z.F.; Zhao, L. Virus-Like Vesicles Based on Semliki Forest Virus-Containing Rabies Virus Glycoprotein Make a Safe and Efficacious Rabies Vaccine Candidate in a Mouse Model. J. Virol. 2021, 95, 790–811. [Google Scholar] [CrossRef]
- Choi, S.; Yang, Z.; Wang, Q.; Qiao, Z.; Sun, M.; Wiggins, J.; Xiang, S.-H.; Lu, Q. Displaying and Delivering Viral Membrane Antigens via WW Domain–Activated Extracellular Vesicles. Sci. Adv. 2023, 9, eade2708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Li, Y.; Lu, J.; Xiong, S.; Yue, Y. Exosome-Based Delivery of VP1 Protein Conferred Enhanced Resistance of Mice to CVB3-Induced Viral Myocarditis. Virology 2023, 579, 46–53. [Google Scholar] [CrossRef]
- Syromiatnikova, V.; Prokopeva, A.; Gomzikova, M. Methods of the Large-Scale Production of Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 10522. [Google Scholar] [CrossRef] [PubMed]
- Taheri, B.; Zarei-Behjani, Z.; Babaei, A.; Moradkhan, F.M. Extracellular Vesicles: A Trojan Horse Delivery Method for Systemic Administration of Oncolytic Viruses. Regen. Eng. Transl. Med. 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Baez-Navarro, C.; Quevedo, I.R.; López, S.; Arias, C.F.; Iša, P. The Association of Human Astrovirus with Extracellular Vesicles Facilitates Cell Infection and Protects the Virus from Neutralizing Antibodies. J. Virol. 2022, 96, e0084822. [Google Scholar] [CrossRef]
- Kerr, C.H.; Dalwadi, U.; Scott, N.E.; Yip, C.K.; Foster, L.J.; Jan, E. Transmission of Cricket Paralysis Virus via Exosome-like Vesicles during Infection of Drosophila Cells. Sci. Rep. 2018, 8, 17353. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Dubois, M.; Plisson-Chastang, C.; Bonnefois, T.; Lhomme, S.; Bertrand-Michel, J.; You, B.; Simoneau, S.; Gleizes, P.E.; Flan, B.; et al. Characterization of the Lipid Envelope of Exosome Encapsulated HEV Particles Protected from the Immune Response. Biochimie 2017, 141, 70–79. [Google Scholar] [CrossRef]
- McKnight, K.L.; Xie, L.; González-López, O.; Rivera-Serrano, E.E.; Chen, X.; Lemon, S.M. Protein Composition of the Hepatitis A Virus Quasi-Envelope. Proc. Natl. Acad. Sci. USA 2017, 114, 6587–6592. [Google Scholar] [CrossRef]
- Xu, G.; Yan, H.; Zhu, Y.; Xie, Z.; Zhang, R.; Jiang, S. Duck Hepatitis A Virus Type 1 Transmission by Exosomes Establishes a Productive Infection in Vivo and in Vitro. Vet. Microbiol. 2023, 277, 109621. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.T.; Giddings, T.H.; Taylor, M.P.; Mulinyawe, S.; Rabinovitch, M.; Kopito, R.R.; Kirkegaard, K. Subversion of Cellular Autophagosomal Machinery by RNA Viruses. PLoS Biol. 2005, 3, e156. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.E.; Rossignol, E.D.; Chang, D.; Zaia, J.; Forrester, I.; Raja, K.; Winbigler, H.; Nicastro, D.; Jackson, W.T.; Bullitt, E. Complexity and Ultrastructure of Infectious Extracellular Vesicles from Cells Infected by Non-Enveloped Virus. Sci. Rep. 2020, 10, 7939. [Google Scholar] [CrossRef]
- Jorfi, S.; Ansa-Addo, E.A.; Mariniello, K.; Warde, P.; Bin Senian, A.A.; Stratton, D.; Bax, B.E.; Levene, M.; Lange, S.; Inal, J.M. A Coxsackievirus B1-Mediated Nonlytic Extracellular Vesicle-to-Cell Mechanism of Virus Transmission and Its Possible Control through Modulation of EV Release. J. Gen. Virol. 2023, 104, 001884. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xiongid, S. Exosomes Mediate Coxsackievirus B3 Transmission and Expand the Viral Tropism. PLOS Pathog. 2023, 19, e1011090. [Google Scholar] [CrossRef]
- Gu, J.; Zhao, Y.; Wu, J.; Chen, Y.; Yin, Y.; Jia, X.; Mao, L. Enterovirus-71 Utilizes Small Extracellular Vesicles to Cross the Blood–Brain Barrier for Infecting the Central Nervous System via Transcytosis. J. Med. Virol. 2023, 95. [Google Scholar] [CrossRef]
- Corona, A.K.; Saulsbery, H.M.; Corona Velazquez, A.F.; Jackson, W.T. Enteroviruses Remodel Autophagic Trafficking through Regulation of Host SNARE Proteins to Promote Virus Replication and Cell Exit. Cell Rep. 2018, 22, 3304–3314. [Google Scholar] [CrossRef]
- Netanyah, E.; Calafatti, M.; Arvastsson, J.; Cabrera-Rode, E.; Cilio, C.M.; Sarmiento, L. Extracellular Vesicles Released by Enterovirus-Infected EndoC-ΒH1 Cells Mediate Non-Lytic Viral Spread. Microorganisms 2020, 8, 1753. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, S.; Shi, X.; Xu, G.; Shen, C.; Liu, X.; Zheng, H. Exosomes-Mediated Transmission of Foot-and-Mouth Disease Virus in Vivo and in Vitro. Vet. Microbiol. 2019, 233, 164–173. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.; Han, C.; Yao, S.; Qi, X.; Gao, Y.; Maier, H.J.; Britton, P.; Chen, L.; Zhang, L.; et al. Infectious Bursal Disease Virus Subverts Autophagic Vacuoles To Promote Viral Maturation and Release. J. Virol. 2017, 91, e01883-16. [Google Scholar] [CrossRef]
- Iša, P.; Pérez-Delgado, A.; Quevedo, I.R.; López, S.; Arias, C.F. Rotaviruses Associate with Distinct Types of Extracellular Vesicles. Viruses 2020, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Q.; Li, M.; Mao, Q.; Chen, H.; Wu, W.; Jia, D.; Wei, T. Autophagy Pathway Induced by a Plant Virus Facilitates Viral Spread and Transmission by Its Insect Vector. PLOS Pathog. 2017, 13, e1006727. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, M.; He, H.; Kilby, K.; de Antueno, R.; Castle, E.; McMullen, N.; Qian, Z.; Zeev-Ben-Mordehai, T.; Duncan, R.; et al. Nonenveloped Avian Reoviruses Released with Small Extracellular Vesicles Are Highly Infectious. Viruses 2023, 15, 1610. [Google Scholar] [CrossRef] [PubMed]
- Labadie, T.; Roy, P. A Non-Enveloped Arbovirus Released in Lysosome-Derived Extracellular Vesicles Induces Super-Infection Exclusion. PLoS Pathog. 2020, 16, e1009015. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.C.; Cheng, W.H.; Ku, F.M.; Tsai, C.Y.; Huang, P.J.; Lee, C.C.; Yeh, Y.M.; Rada, P.; Hrdý, I.; Narayanasamy, R.K.; et al. Identification of Endosymbiotic Virus in Small Extracellular Vesicles Derived from Trichomonas Vaginalis. Genes 2022, 13, 531. [Google Scholar] [CrossRef]
- Martelli, F.; Macera, L.; Spezia, P.G.; Medici, C.; Pistello, M.; Guasti, D.; Romagnoli, P.; Maggi, F.; Giannecchini, S. Torquetenovirus Detection in Exosomes Enriched Vesicles Circulating in Human Plasma Samples. Virol. J. 2018, 15, 145. [Google Scholar] [CrossRef]
- Handala, L.; Blanchard, E.; Raynal, P.-I.; Roingeard, P.; Morel, V.; Descamps, V.; Castelain, S.; Francois, C.; Duverlie, G.; Brochot, E.; et al. BK Polyomavirus Hijacks Extracellular Vesicles for En Bloc Transmission. J. Virol. 2020, 94, e01834-19. [Google Scholar] [CrossRef]
- Eymieux, S.; Uzbekov, R.; Rouillé, Y.; Blanchard, E.; Hourioux, C.; Dubuisson, J.; Belouzard, S.; Roingeard, P. Secretory Vesicles Are the Principal Means of SARS-CoV-2 Egress. Cells 2021, 10, 2047. [Google Scholar] [CrossRef]
- Xia, B.; Pan, X.; Luo, R.H.; Shen, X.; Li, S.; Wang, Y.; Zuo, X.; Wu, Y.; Guo, Y.; Xiao, G.; et al. Extracellular Vesicles Mediate Antibody-Resistant Transmission of SARS-CoV-2. Cell Discov. 2023, 9, 1–15. [Google Scholar] [CrossRef]
- Chivero, E.T.; Bhattarai, N.; Rydze, R.T.; Winters, M.A.; Holodniy, M.; Stapleton, J.T. Human Pegivirus RNA Is Found in Multiple Blood Mononuclear Cells in Vivo and Serum-Derived Viral RNA-Containing Particles Are Infectious in Vitro. J. Gen. Virol. 2014, 95, 1307. [Google Scholar] [CrossRef]
- Li, M.Y.; Naik, T.S.; Siu, L.Y.L.; Acuto, O.; Spooner, E.; Wang, P.; Yang, X.; Lin, Y.; Bruzzone, R.; Ashour, J.; et al. Lyn Kinase Regulates Egress of Flaviviruses in Autophagosome-Derived Organelles. Nat. Commun. 2020, 11, 5189. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rojas, P.P.; Quiroz-García, E.; Monroy-Martínez, V.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Ruiz-Ordaz, B.H. Participation of Extracellular Vesicles from Zika-Virus-Infected Mosquito Cells in the Modification of Naïve Cells’ Behavior by Mediating Cell-to-Cell Transmission of Viral Elements. Cells 2020, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Sexton, N.R.; Bellis, E.D.; Murrieta, R.A.; Spangler, M.C.; Cline, P.J.; Weger-Lucarelli, J.; Ebel, G.D. Genome Number and Size Polymorphism in Zika Virus Infectious Units. J. Virol. 2021, 95, e00787-20. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.A.; Sampey, G.C.; Lepene, B.; Akpamagbo, Y.; Barclay, R.A.; Iordanskiy, S.; Hakami, R.M.; Kashanchi, F. Presence of Viral RNA and Proteins in Exosomes from Cellular Clones Resistant to Rift Valley Fever Virus Infection. Front. Microbiol. 2016, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Bideshi, D.K.; Tan, Y.; Bigot, Y.; Federici, B.A. A Viral Caspase Contributes to Modified Apoptosis for Virus Transmission. Genes Dev. 2005, 19, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, H.-Y.; Li, N.; Yang, C.-J.; Huang, G.-H. An Ascovirus Utilizes Different Types of Host Larval Regulated Cell Death Mechanisms To Produce and Release Vesicles. J. Virol. 2022, 97, e0156622. [Google Scholar] [CrossRef]
- Sanada, T.; Hirata, Y.; Naito, Y.; Yamamoto, N.; Kikkawa, Y.; Ishida, Y.; Yamasaki, C.; Tateno, C.; Ochiya, T.; Kohara, M. Transmission of HBV DNA Mediated by Ceramide-Triggered Extracellular Vesicles. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 272–283. [Google Scholar] [CrossRef]
| Function | Mechanism | |
|---|---|---|
| Antiviral | Stimulate immune system | Transport cytokines (such as IFN or IL-6) |
| Deliver PAMPs to PRRs (such as TLR) | ||
| Damage viral RNA | Transport antiviral protein APOBEC3G | |
| Prevent infection | Transference of viral resistance miRNAs | |
| Reduce viral load | Viral receptor–viral particle binding on EV surface | |
| Antigen presentation | Deliver viral proteins to APCs | |
| Direct CD4+ and CD8+ activation | Carrying MHC–antigen complexes on EV surface | |
| Proviral | Suppress immune system | Transport viral miRNAs/proteins, Galectin-9… |
| Increase cell susceptibility | Transfer viral receptors to recipient cell’s membrane | |
| Promoting fusion of viral particles | ||
| Stimulating cell–cell contacts | ||
| Block antigen presentation | Packaging MHC complexes inside EVs | |
| Viral transmission | Transport infectious cargo (viral genomes and/or viral particles) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou, J.-V.; Taguwa, S.; Matsuura, Y. Trick-or-Trap: Extracellular Vesicles and Viral Transmission. Vaccines 2023, 11, 1532. https://doi.org/10.3390/vaccines11101532
Bou J-V, Taguwa S, Matsuura Y. Trick-or-Trap: Extracellular Vesicles and Viral Transmission. Vaccines. 2023; 11(10):1532. https://doi.org/10.3390/vaccines11101532
Chicago/Turabian StyleBou, Juan-Vicente, Shuhei Taguwa, and Yoshiharu Matsuura. 2023. "Trick-or-Trap: Extracellular Vesicles and Viral Transmission" Vaccines 11, no. 10: 1532. https://doi.org/10.3390/vaccines11101532
APA StyleBou, J.-V., Taguwa, S., & Matsuura, Y. (2023). Trick-or-Trap: Extracellular Vesicles and Viral Transmission. Vaccines, 11(10), 1532. https://doi.org/10.3390/vaccines11101532






