Abstract
Following an extremely low incidence of influenza during the first waves of the ongoing COVID-19 pandemic, the 2021/22 Northern Hemisphere winter season saw a resurgence of influenza virus circulation. The aim of this study was to describe epidemiology of severe acute respiratory infections (SARIs) among Italian adults and estimate the 2021/22 season influenza vaccine effectiveness. For this purpose, a test-negative case-control study was conducted in a geographically representative sample of Italian hospitals. Of 753 SARI patients analyzed, 2.5% (N = 19) tested positive for influenza, most of which belonged to the A(H3N2) subtype. Phylogenetic analysis showed that these belonged to the subclade 3C.2a1b.2a.2, which was antigenically different from the 2021/22 A(H3N2) vaccine component. Most (89.5%) cases were registered among non-vaccinated individuals, suggesting a protective effect of influenza vaccination. Due to a limited number of cases, vaccine effectiveness estimated through the Firth’s penalized logistic regression was highly imprecise, being 83.4% (95% CI: 25.8–97.4%) and 83.1% (95% CI: 22.2–97.3%) against any influenza type A and A(H3N2), respectively. Exclusion of SARS-CoV-2-positive controls from the model did not significantly change the base-case estimates. Within the study limitations, influenza vaccination appeared to be effective against laboratory-confirmed SARI.
1. Introduction
Seasonal influenza carries a significant socioeconomic burden, and each year causes up to 650,000 respiratory deaths worldwide [1]. Despite vaccine effectiveness (VE) still being suboptimal [2], seasonal influenza vaccination (SIV) remains the most powerful public health means able to reduce the burden of disease and is currently recommended for several population groups, including pregnant women, older adults, young children and subjects with underlying morbidities [3].
Non-specific measures adopted during the first waves of the ongoing COVID-19 pandemic had a dramatic impact on influenza virus circulation. For instance, the 2020/21 winter season in Europe was associated with little influenza activity; indeed, a 99.8% reduction in positive detections was observed [4]. In the following 2021/22 Northern Hemisphere (NH) season influenza returned (although by a comparably small amount compared to pre-pandemic seasons) and was mostly represented by the A(H3N2) virus subtype [5]. However, the last 2022 Southern Hemisphere season (April–October 2022) was particularly severe, with influenza activity exceeding the 5-year average [6].
Monitoring of SIV VE has recently become well-established in North America, Europe and Australasia to complement existing epidemiological and virological surveillance systems [7]. This monitoring is usually performed through conducting test-negative case–control studies [7], which have an advantage of reducing differences in healthcare-seeking behavior between cases and controls [8]. Evaluation of the SIV VE may be even more important during the current COVID-19 pandemic stage. Indeed, while the initial non-specific public health measures (e.g., mask wearing and lockdowns) were able to drastically reduce influenza attack rates, these interventions have also determined an up to 60% increase in the population fraction susceptible to influenza virus [9]. These indirect effects may be substantiated by a recently report sharp increase in influenza virus circulation [6].
First available studies for the 2021/22 NH season have been conducted in an outpatient setting and suggested [10,11] a relatively low VE against A(H3N2). This was probably a consequence of predominance of the subclade 3C.2a1b.2a.2, which is antigenically different from the A(H3N2) SIV component (subclade 3C.2a1b.2a.1) [5]. In particular, the interim analysis from the United States (US) has shown that, in outpatient settings, the unadjusted and adjusted VE was 32% (95% CI: 10–50%) and 14% (95% CI: −17–37%), respectively [10]. In the Spanish region of Navarre, the observed VE was 37% (95% CI: 16–52%) against any influenza and 39% (95% CI: 16–55%) against A(H3N2) [11].
In line with other countries, the 2021/22 season in Italy was characterized by substantial influenza activity: a total of 14.5% (N = 13,063) of samples tested positive between November 2021 and April 2022 [12]. Considering that outpatients differ from inpatients in terms of several baseline characteristics (e.g., age and risk factors), which may affect SIV VE [13], the main goal of this hospital-based study was to analyze the epidemiology of severe acute respiratory infections (SARIs) among Italian adults and establish the 2021/22 SIV VE. Indeed, according to the World Health Organization (WHO) guidelines [8], among other influenza-related outcomes, laboratory-confirmed SARI is particularly suitable for test-negative case–control studies, as it is highly specific and may be easily integrated into the available influenza surveillance systems.
2. Materials and Methods
2.1. Overall Study Design
This study was conducted between October 2021 and May 2022 and was part of the DRIVE (Development of Robust and Innovative Vaccine Effectiveness) project, which is a multi-country network aiming to assess the brand-specific SIV VE in Europe [14]. Objectives and methods of the DRIVE project are comprehensively described at https://www.drive-eu.org/ (accessed on 14 November 2022). In the 2021/22 season, the Italian network IT-BIVE-HOSP performed a test-negative case–control study and was composed of five hospitals located in northern (regions of Liguria and Lombardy), central (regions of Tuscany and Lazio) and southern (region of Apulia) Italy.
For the 2021/22 season, SIV was recommended and fully reimbursed for older adults aged ≥60 years, pregnant women, individuals of any age with underlying medical conditions that increase their risk of influenza complications, workers operating in sectors of primary public interest (e.g., healthcare professionals, police) and some other categories [15].
2.2. Study Population and Procedures
All adult subjects aged ≥18 years and presenting at emergency department with SARI were potentially eligible. The per-protocol SARI definition was as follows: ≥1 systemic symptom (fever or feverishness, malaise, headache, or myalgia) or deterioration of general conditions, and ≥1 respiratory symptom (cough, sore throat, or shortness of breath) at admission or within 48 h following admission. Subjects were excluded from the analysis for the following reasons: (i) unknown or uncertain 2021/22 SIV status or SIV administered ≤14 days before SARI onset; (ii) any contraindication for SIV receipt; (iii) previous hospitalization within 48 h prior to onset of the current SARI episode or SARI onset ≥48 h following hospitalization; (iv) documented positivity to influenza before the onset of symptoms leading to the current hospital encounter; (v) institutionalized subjects.
Following the informed consent signature, samples were taken with a naso-/oropharyngeal swab that was tested for influenza types, subtypes and lineages and SARS-CoV-2 by means of reverse-transcription real-time polymerase chain reaction (RT-PCR). Cases were defined as SARI patients who tested positive for influenza, while controls were those who tested negative. At enrollment, a trained physician collected (through both interview of patient/legal representative and if possible patient’s general practitioner) demographic (sex, age, region of residence) and relevant clinical (presence of co-morbidities, current and previous SIV, and COVID-19 vaccination status) data.
2.3. Virus Characterization and Phylogenetic Analysis
A subset of virus detections was characterized molecularly using the Sanger method [16]. Briefly, following the RNA extraction from clinical specimens, the hemagglutinin (HA) gene of A(H3N2) viruses was amplified using the SuperScript IV One-Step RT-PCR kit (Invitrogen; Carlsbad, CA, USA) and specific primers. A nested PCR of the amplified cDNA as template was then performed. Amplification was performed using the Platinum II Taq Hot-Start DNA Polymerase (Invitrogen; Carlsbad, CA, USA). PCR products were then purified with the ExoSAP-IT PCR Product Cleanup Reagent (Applied Biosystems; Waltham, MA, USA). Finally, sequencing reactions were performed on the SeqStudio Genetic Analyzer System (Applied Biosystems; Waltham, MA, USA) for Sanger sequencing. The obtained sequences were assembled with SeqScape Software v4.0 (Applied Biosystems; Waltham, MA, USA). The nucleotide sequences generated in this study were submitted to the GISAID database (EPI_ISL_14699640, EPI_ISL_14699961, EPI_ISL_14699962, EPI_ISL_14810878, EPI_ISL_14810879, EPI_ISL_14810881).
The sequence alignment to relevant reference strains and phylogenetic analysis were performed using the “One click” workflow in the NGPhylogeny.fr software [17], which is freely accessible at https://ngphylogeny.fr (accessed on 14 November 2022).
2.4. Statistical Analysis
Categorical data were expressed as proportions with binomial 95% confidence intervals (CIs), while the skewed variable of age was expressed as medians with interquartile ranges (IQRs). The Fisher’s exact and Mann–Whitney–Wilcoxon’s tests were used to compare categorical and continuous variables, respectively. VE was expressed as [1 − adjusted odds ratio (aOR)] × 100% [18], and the aOR was estimated through Firth’s penalized logistic regression, which is particularly useful for rare events [19,20]. Two types of model specification were considered, namely the fully adjusted (age, sex, area of residence, month of symptom onset, time delay between the onset of symptoms and swabbing, presence of comorbidities, previous season SIV and COVID-19 vaccination pattern) and a more parsimonious specification selected by minimizing the corrected Akaike’s information criterion (AICc) [21]. We planned a priori to estimate VE by virus (sub)type, age-class and type of SIV.
Two kinds of sensitivity analyses were conducted. First, considering that SIV and COVID-19 vaccination behaviors may be correlated, we performed an additional de-confounding by excluding SARS-CoV-2-positive controls [22]. Second, E-values for both VE point estimates and 95% CIs were calculated. These latter were interpreted as the minimum strength of association that an unmeasured confounder would need to have with both the SIV receipt and positivity to influenza to fully explain away the observed association, which is conditional on the measured covariates [23].
All analyses were performed in R (v. 4.1.0) environment (R Core Team; Vienna, Austria).
3. Results
A total of 755 adult individuals were enrolled. Two (0.3%) subjects had an unknown 2021/22 SIV status and were excluded. In summary, a total of 753 subjects were analyzed, and their main demographic and clinical characteristics are reported in Table 1. Briefly, their median age was 73 years, and both sexes were approximately equally distributed. On median, the swab was taken 2 days after the onset of symptoms. Most (55.5%) subjects were enrolled in the southern region of Apulia, between February and March 2022 (51.5%), had no underlying health conditions (73.7%), received both the primary cycle and a booster COVID-19 vaccine dose (56.4%) and did not receive the 2020/21 SIV (59.4%). In the overall cohort, the 2021/22 SIV coverage was 42.8%. Most (62.7%; 202/322) subjects received the adjuvanted quadrivalent SIV.

Table 1.
Characteristics of the study participants (N = 753).
During the study period, a total of 19 influenza virus detections occurred with an overall prevalence of 2.5% (95% CI: 1.5–3.9%). Eighteen (94.7%) detections were caused by A(H3N2), while the remaining case (5.3%) was a non-subtyped A virus. The HA gene of 6 out of 18 (33.3%) A(H3N2) viruses was sequenced and all belonged to the 3C.2a1b.2a.2 subclade (Figure 1. SARS-CoV-2 was detected in 283 (37.6%; 95% CI: 34.1–41.2%) samples. Moreover, there were three (0.4%; 95% CI: 0.1–1.2%) influenza and SARS-CoV-2 co-detections.
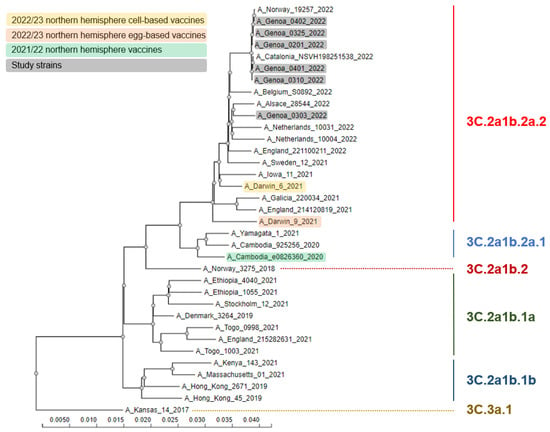
Figure 1.
Phylogenetic comparison of influenza A(H3N2) hemagglutinin genes identified in the study.
As shown in Table 2, compared with controls, cases were significantly younger and more frequently enrolled during March 2022 and in the Italian north and center. The 2021/22 SIV coverage rate was significantly (p = 0.004) higher in controls than in cases (43.6% vs. 10.5%), with an absolute risk reduction of 3.3% (95% CI: 1.3–5.4%).

Table 2.
Comparison of cases and controls.
As shown in Figure 2, when adjusted for potential confounders, the 2020/21 SIV was effective against influenza type A and A(H3N2) with point estimates of 83–84%. The corresponding E-values were sufficiently large, suggesting that the unmeasured confounding was unlikely to explain away the observed protective effect of SIV (Figure 2). Owing to the paucity of cases, no subgroup analysis by age-class and SIV type could be conducted.
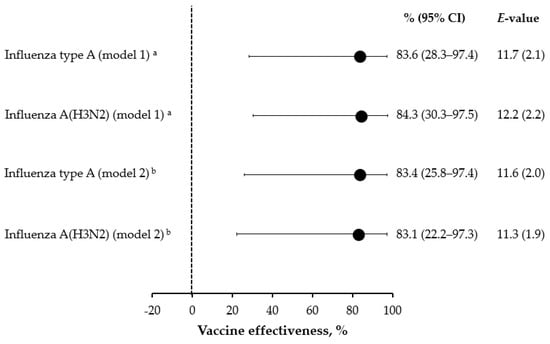
Figure 2.
Effectiveness of the 2021/22 seasonal influenza vaccination against severe acute respiratory infection. a Covariates (age, area, month of symptom onset, COVID-19 vaccination pattern and previous season influenza vaccination) selected on the basis of minimization of the corrected Akaike’s information criterion; b Fully adjusted (age, sex, area, month of symptom onset, swab delay, presence of chronic conditions, COVID-19 vaccination pattern, and previous season influenza vaccination) model.
In the sensitivity analysis, when 280 SARS-CoV-2-positive controls were excluded, no substantial changes occurred (Supplementary Material, Figure S1).
4. Discussion
This study showed the “return” of influenza to the Italian epidemiological scene during the 2021/22 winter season. Although most SARI cases were caused by SARS-CoV-2, a still considerable number of SARI were caused by the influenza virus. Similarly to other countries, this season in Italy was clearly dominated by the 3C.2a1b.2a.2 subclade of the A(H3N2) virus subtype, which is antigenically different from the 2021/22 NH SIV A(H3N2) component A/Cambodia/e0826360/2020 (subclade 3C.2a1b.2a.1) [5,24]. Despite this apparent mismatch, in our study, most (89.5%; 17/19) SARI cases were not vaccinated, suggesting a significant protection provided by SIV. In this regard, a systematic review and meta-analysis of adult randomized controlled trials by Tricco et al. [25] has reported a similar vaccine efficacy against both matched (61%; 95% CI: 46–73%) and mismatched (64%; 95% CI: 23–82%) influenza A virus.
As compared with our findings and despite similarities in virus circulation, the available US [10] and Spanish [11] estimates for the 2021/22 VE are substantially lower (14–39%). This apparent inconsistency may be explained by a number of concurrent reasons. First, both US [10] and Spanish [11] studies were conducted in the outpatient setting, and therefore cases were not severe. In this regard, Tendorfe et al. [13] have documented that hospital-based and community-based SIV VE studies are composed of two distinct populations, and in some instances, the VE computed in an inpatient setting may be higher than among outpatients. Analogously, the pooled analysis performed within the DRIVE project showed that the VE among working-aged adults was 0% (95% CI: −96–49%) and 85% (95% CI: 12–97%) in primary-care-based (data from four networks located in Austria, Iceland, Italy and United Kingdom) and hospital-based (data from nine networks located in France, Iceland, Italy, Romania and Spain) test-negative studies, respectively [26]. In summary, it is highly plausible that SIV VE may be higher against more severe clinical outcomes, as it may reduce the severity of disease in cases where it did not prevent infection per se [27]. Second, in our study, two-thirds of vaccinated subjects received the adjuvanted SIV. Bolton et al. [24] have recently shown that antibodies elicited by the 2021/22 NH non-adjuvanted standard-dose egg-based SIV poorly neutralized strains belonging to the 3C.2a1b.2a.2 A(H3N2) subclade. By contrast, the MF59 adjuvant may broaden the immune response beyond the strains included in SIV [28]. Indeed, the adjuvanted formulation has been extensively shown to be superior to non-adjuvanted SIVs in inducing heterologous and cross-clade immune responses, particularly within the A(H3N2) subtype [29,30,31,32]. Analogously, our recent study [33] demonstrated that during the 2018/19 and 2019/20 seasons, which were characterized by a significant proportion of drifted A(H3N2) strains, the adjuvanted SIV was 59.2% (95% CI: 14.6–80.5%), more effective than non-adjuvanted comparators. Finally, it should be kept in mind that our effect sizes were imprecise, and the previously reported VE point estimates [10,11] were usually within the 95% CIs reported in the present report.
Interestingly, SARI patients positive for influenza were significantly younger than those positive for SARS-CoV-2. Similar observations have been reported from other studies carried out in the United Kingdom [34] and Brazil [35]. In Italy, older age is among the most important predictors of both SIV [36] and COVID-19 [37] vaccination uptake. On considering this positive relationship between SIV and COVID-19 vaccination, in test-negative case–control studies, a bias driven by the conditional probability of vaccination may be introduced [22]. It seems that this bias may be particularly important for older adults. Although in our sensitivity analysis (in which SARS-CoV-2-positive SARI patients were excluded), no major changes in the SIV VE took place, the limited number of cases did not allow us to assess age-specific estimates. Further studies on this purpose are warranted.
As we mentioned earlier, the main drawback of the present study is a limited number of cases imposed by a lower than average circulation of influenza. This limitation determined both the imprecise VE estimates with large 95% CIs and the impossibility of performing further subgroup analyses. We tried to limit the impact of this limitation at the phase of data analysis by applying Firth’s penalized estimation methods able to provide bias-reduced regression coefficients in datasets with rare events [38]. Moreover, although the calculated E-values were sufficiently large and suggested that a relatively strong unmeasured confounding was needed to negate our estimates, we cannot definitively rule out the issue of residual confounding.
In conclusion, the 2021/22 winter season in Italy saw a resurgence of influenza virus circulation, and a substantial proportion of SARI cases were caused by influenza A(H3N2). These latter were part of the 3C.2a1b.2a.2 subclade, which was antigenically different from the A(H3N2) component of the 2021/22 NH SIV. Despite vaccine mismatch, most laboratory-confirmed SARI cases were recorded among non-vaccinated inpatients, suggesting a protective effect of the 2021/22 NH SIV. Effective public health interventions to increase SIV uptake (with the updated 2022/23 NH SIV formulation containing a 3C.2a1b.2a.2-like virus) in all principal target groups [3] should be pursued.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11010083/s1, Figure S1: Effectiveness of the 2021/22 seasonal influenza vaccination against severe acute respiratory infection: Sensitivity analysis by excluding SARS-CoV-2-positive controls.
Author Contributions
Conceptualization: G.I., A.O. and D.P.; data collection: D.L., A.T., I.M., E.P., S.C. and A.O.; laboratory analyses: A.D., C.N., M.C., D.L., E.P. and I.M.; interpretation of the data: A.D. and D.P.; writing—original draft preparation: A.D.; critical revision of manuscript: D.P., A.O., E.M., M.C., S.C., E.P. and G.I.; hospital coordinators: G.I., M.C., C.N., I.M. and S.C.; supervision: G.I. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 777363. This joint undertaking received support from the European Union’s Horizon 2020 Research and Innovation program and EFPIA.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Liguria Region (coordinator center) (protocol # 566/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Further details and results from the DRIVE studies are available at the DRIVE website via the following link: https://www.drive-eu.org/ (accessed on 14 November 2022).
Acknowledgments
IT-BIVE-HOSP Network Study Group (Piero Luigi Lai, Stefano Mosca, Martina Porretto, Giovanna Iudica, Allegra Ferrari, Carola Minet, Bianca Bruzzone, Laura Pellegrinelli, Cristina Galli, Francesca Centrone, Lavinia Bianco, Giovanna Milano, Andrea Camarri, Giovanni Bova, Pier Leopoldo Capecchi). The authors thank Irene Giberti, Carlo-Simone Trombetta, Paola Spatera and Nicola Calcavecchia for their support with patient enrollment and Giada Garzillo and Valentina Ricucci for their support with laboratory analyses.
Conflicts of Interest
A.D. was previously a permanent employee of Seqirus, a pharmaceutical company who manufacture and commercialize influenza vaccines. Other authors declare no conflict of interest.
References
- Bresee, J.; Fitzner, J.; Campbell, H.; Cohen, C.; Cozza, V.; Jara, J.; Krishnan, A.; Lee, V.; WHO Working Group on the Burden of Influenza Disease. Progress and remaining gaps in estimating the global disease burden of influenza. Emerg. Infect. Dis. 2018, 24, 1173–1177. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Past Seasons Vaccine Effectiveness Estimates. Available online: https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html (accessed on 6 August 2022).
- World Health Organization (WHO). Vaccines against influenza WHO position paper–November 2012. Wkly. Epidemiol. Rec. 2012, 87, 461–476. [Google Scholar]
- Adlhoch, C.; Mook, P.; Lamb, F.; Ferland, L.; Melidou, A.; Amato-Gauci, A.J.; Pebody, R.; European Influenza Surveillance Network. Very little influenza in the WHO European Region during the 2020/21 season, weeks 40 2020 to 8 2021. Euro Surveill. 2021, 26, 2100221. [Google Scholar] [CrossRef] [PubMed]
- Melidou, A.; Ködmön, C.; Nahapetyan, K.; Kraus, A.; Alm, E.; Adlhoch, C.; Mooks, P.; Dave, N.; Carvalho, C.; Meslé, M.M.; et al. Influenza returns with a season dominated by clade 3C.2a1b.2a.2 A(H3N2) viruses, WHO European Region, 2021/22. Euro Surveill. 2022, 27, 2200255. [Google Scholar] [CrossRef]
- Australian Government. Department of Health and Aged Care. Australian Influenza Surveillance Report-2022 Influenza Season in Australia. Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-ozflu-flucurr.htm/$File/flu-10-2022.pdf (accessed on 6 August 2022).
- Pebody, R.G.; Mølbak, K. Importance of timely monitoring of seasonal influenza vaccine effectiveness. Euro Surveill. 2016, 21, 30209. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Evaluation of Influenza Vaccine Effectiveness. A Guide to the Design and Interpretation of Observational Studies. Available online: https://apps.who.int/iris/bitstream/handle/10665/255203/9789241512121-eng.pdf (accessed on 25 November 2022).
- Ali, S.T.; Lau, Y.C.; Shan, S.; Ryu, S.; Du, Z.; Wang, L.; Xu, X.K.; Chen, D.; Xiong, J.; Tae, J.; et al. Prediction of upcoming global infection burden of influenza seasons after relaxation of public health and social measures during the COVID-19 pandemic: A modelling study. Lancet Glob. Health 2022, 10, e1612–e1622. [Google Scholar] [CrossRef]
- Chung, J.R.; Kim, S.S.; Kondor, R.J.; Smith, C.; Budd, A.P.; Tartof, S.Y.; Florea, A.; Talbot, H.K.; Grijalva, C.G.; Wernli, K.J.; et al. Interim estimates of 2021-22 seasonal influenza vaccine effectiveness-United States, February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 365–370. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Casado, I.; Miqueleiz, A.; Navascués, A.; Pozo, F.; Trobajo-Sanmartín, C.; Albéniz, E.; Elía, F.; Burgui, C.; Fernández-Huerta, M.; et al. Effectiveness of influenza vaccination in preventing influenza in primary care, Navarre, Spain, 2021/22. Euro Surveill. 2022, 27, 2200488. [Google Scholar] [CrossRef]
- Italian National Institute of Health. Virological Surveillance of Influenza. Available online: https://w3.iss.it/site/rmi/influnet/pagine/rapportoinflunet.aspx (accessed on 6 August 2022).
- Tenforde, M.W.; Chung, J.; Smith, E.R.; Talbot, H.K.; Trabue, C.H.; Zimmerman, R.K.; Silveira, F.P.; Gaglani, M.; Murthy, K.; Monto, A.S.; et al. Influenza vaccine effectiveness in inpatient and outpatient settings in the United States, 2015–2018. Clin. Infect. Dis. 2021, 73, 386–392. [Google Scholar] [CrossRef]
- Carmona, A.; Muñoz-Quiles, C.; Stuurman, A.; Descamps, A.; Mira-Iglesias, A.; Torcel-Pagnon, L.; Díez-Domingo, J. Challenges and adaptation of a European influenza vaccine effectiveness study platform in response to the COVID-19 emergence: Experience from the DRIVE project. Int. J. Environ. Res. Public Health 2021, 18, 1058. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Prevention and Control of Influenza: Recommendations for the 2021–2022 Season. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=79647&parte=1%20&serie=null (accessed on 25 November 2022).
- Pariani, E.; Amendola, A.; Zappa, A.; Bianchi, S.; Colzani, D.; Anselmi, G.; Zanetti, A.; Tanzi, E. Molecular characterization of influenza viruses circulating in Northern Italy during two seasons (2005/2006 and 2006/2007) of low influenza activity. J. Med. Virol. 2008, 80, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.G.; Cowling, B.J. “Crude vaccine effectiveness” is a misleading term in test-negative studies of influenza vaccine effectiveness. Epidemiology 2015, 26, e60. [Google Scholar] [CrossRef]
- Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Leir, S.; Sabaiduc, S.; Chambers, C.; Zou, M.; Rose, C.; Olsha, R.; Dickinson, J.A.; Winter, A.L.; Jassem, A.; et al. Influenza vaccine effectiveness by A(H3N2) phylogenetic sub-cluster and prior vaccination history: 2016-17 and 2017-18 epidemics in Canada. J. Infect. Dis. 2020, 225, 1387–1398. [Google Scholar] [CrossRef]
- Calcagno, V.; de Mazancourt, C. glmulti: An R package for easy automated model selection with (generalized) linear models. J. Stat. Soft. 2010, 34, 1–29. [Google Scholar] [CrossRef]
- Doll, M.K.; Pettigrew, S.M.; Ma, J.; Verma, A. Effects of confounding bias in coronavirus disease 2019 (COVID-19) and influenza vaccine effectiveness test-negative designs due to correlated influenza and COVID-19 vaccination behaviors. Clin. Infect. Dis. 2022, 75, e564–e571. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity analysis in observational research: Introducing the E-value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef]
- Bolton, M.J.; Ort, J.T.; McBride, R.; Swanson, N.J.; Wilson, J.; Awofolaju, M.; Furey, C.; Greenplate, A.R.; Drapeau, E.M.; Pekosz, A.; et al. Antigenic and virological properties of an H3N2 variant that continues to dominate the 2021-22 Northern Hemisphere influenza season. Cell Rep. 2022, 39, 110897. [Google Scholar] [CrossRef]
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef]
- DRIVE (Development of Robust and Innovative Vaccine Effectiveness) Project. Brand-Specific Influenza Vaccine Effectiveness in Europe Season 2021/22. Available online: https://www.drive-eu.org/wp-content/uploads/2022/07/DRIVE_D7.9-IVE-Results-Report_Season-2021-22_FINAL.pdf (accessed on 6 August 2022).
- Godoy, P.; Romero, A.; Soldevila, N.; Torner, N.; Jané, M.; Martínez, A.; Caylà, J.A.; Rius, C.; Domínguez, A.; The Working Group on Surveillance of Severe Influenza Hospitalized Cases In Catalonia. Influenza vaccine effectiveness in reducing severe outcomes over six influenza seasons, a case-case analysis, Spain, 2010/11 to 2015/16. Euro Surveill. 2018, 23, 1700732. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Rappuoli, R.; De Gregorio, E.; Tsai, T.; Del Giudice, G. MF59 adjuvant: The best insurance against influenza strain diversity. Expert Rev. Vaccines 2011, 10, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Ansaldi, F.; Bacilieri, S.; Durando, P.; Sticchi, L.; Valle, L.; Montomoli, E.; Icardi, G.; Gasparini, R.; Crovari, P. Cross-protection by MF59-adjuvanted influenza vaccine: Neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine 2008, 26, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Ansaldi, F.; Zancolli, M.; Durando, P.; Montomoli, E.; Sticchi, L.; Del Giudice, G.; Icardi, G. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 2010, 28, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Nicolay, U.; Heijnen, E.; Nacci, P.; Patriarca, P.A.; Leav, B. Immunogenicity of aIIV3, MF59-adjuvanted seasonal trivalent influenza vaccine, in older adults ≥65 years of age: Meta-analysis of cumulative clinical experience. Int. J. Infect. Dis. 2019, 85S, S1–S9. [Google Scholar] [CrossRef]
- Kavian, N.; Hachim, A.; Li, A.P.; Cohen, C.A.; Chin, A.W.; Poon, L.L.; Fang, V.J.; Leung, N.H.; Cowling, B.J.; Valkenburg, S.A. Assessment of enhanced influenza vaccination finds that FluAd conveys an advantage in mice and older adults. Clin. Transl. Immunol. 2020, 9, e1107. [Google Scholar] [CrossRef]
- Domnich, A.; Panatto, D.; Pariani, E.; Napoli, C.; Chironna, M.; Manini, I.; Rizzo, C.; Orsi, A.; Icardi, G.; IT-BIVE-HOSP Network Study Group. Relative effectiveness of the adjuvanted vs non-adjuvanted seasonal influenza vaccines against severe laboratory-confirmed influenza among hospitalized Italian older adults. Int. J. Infect. Dis. 2022, 125, 164–169. [Google Scholar] [CrossRef]
- Woodcock, T.; Greenfield, G.; Lalvani, A.; Majeed, A.; Aylin, P. Patient outcomes following emergency admission to hospital for COVID-19 compared with influenza: Retrospective cohort study. Thorax 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Niquini, R.P.; Lana, R.M.; Pacheco, A.G.; Cruz, O.G.; Coelho, F.C.; Carvalho, L.M.; Villela, D.A.M.; Gomes, M.F.D.C.; Bastos, L.S. Description and comparison of demographic characteristics and comorbidities in SARI from COVID-19, SARI from influenza, and the Brazilian general population. Cad. Saude Publica. 2020, 36, e00149420. [Google Scholar] [CrossRef]
- Domnich, A.; Grassi, R.; Fallani, E.; Spurio, A.; Bruzzone, B.; Panatto, D.; Marozzi, B.; Cambiaggi, M.; Vasco, A.; Orsi, A.; et al. Changes in attitudes and beliefs concerning vaccination and influenza vaccines between the first and second COVID-19 pandemic waves: A longitudinal study. Vaccines 2021, 9, 1016. [Google Scholar] [CrossRef]
- Steinert, J.I.; Sternberg, H.; Prince, H.; Fasolo, B.; Galizzi, M.M.; Büthe, T.; Veltri, G.A. COVID-19 vaccine hesitancy in eight European countries: Prevalence, determinants, and heterogeneity. Sci. Adv. 2022, 8, eabm9825. [Google Scholar] [CrossRef] [PubMed]
- Heinze, G.; Schemper, M. A solution to the problem of separation in logistic regression. Stat. Med. 2002, 21, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).