False Contraindications for Vaccinations Result in Sub-Optimal Vaccination Coverage in Quito, Ecuador: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data/Measurement Sources
2.3. Control of Sources of Bias
2.4. Study Size
2.5. Statistical Methods
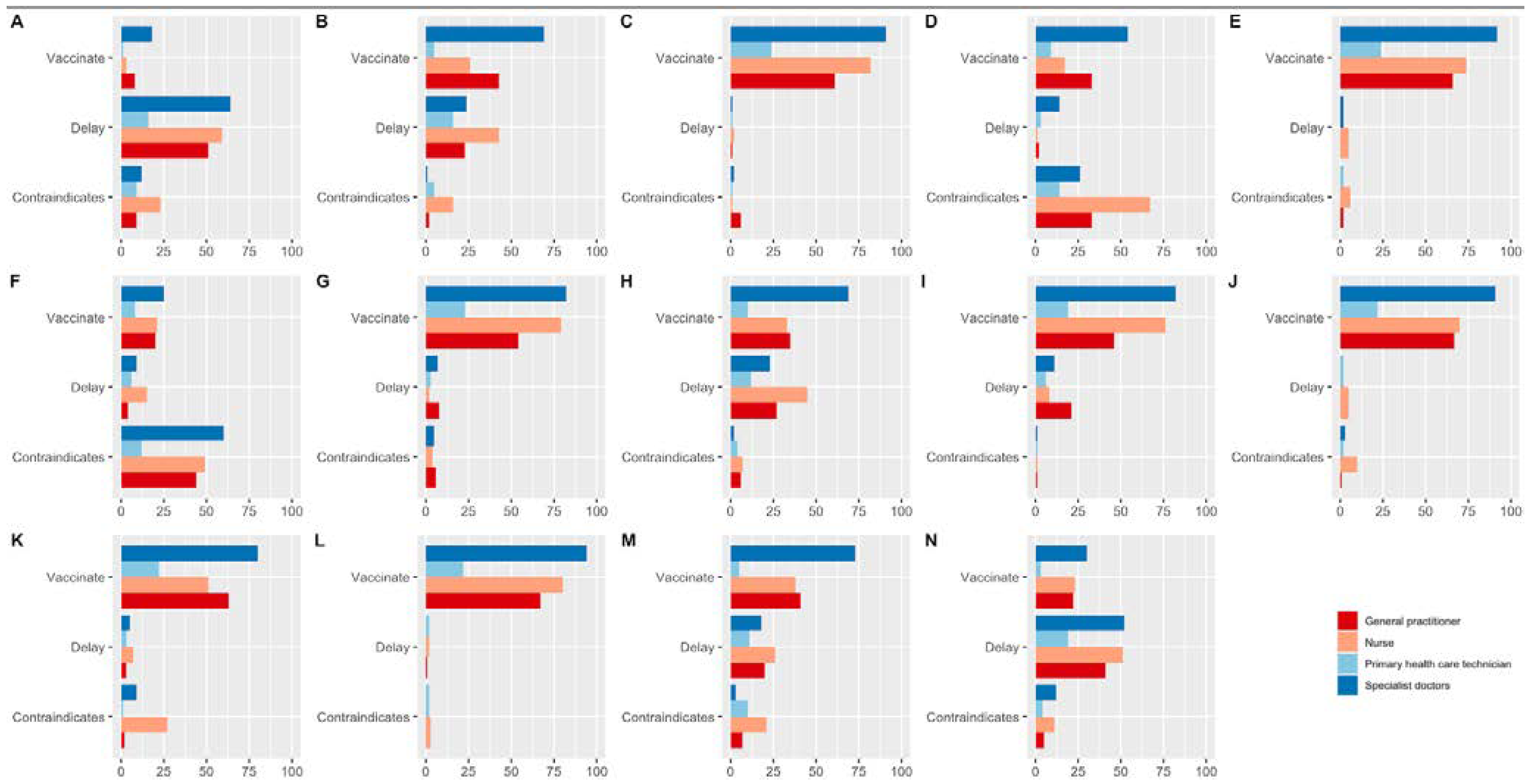
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding Modern-Day Vaccines: What You Need to Know. Ann. Med. 2018, 50, 110–120. [Google Scholar] [CrossRef]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Damitz, B.A. Immunization Practices. In Conn’s Current Therapy; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020; p. 6. [Google Scholar]
- Plotkin, S.A.; Orenstein, W.A.; Offit, P.A. (Eds.) General Aspects of Vaccination. In Vaccines; Elsevier Saunders: Philadelphia, PA, USA, 2013; ISBN 978-1-4557-0090-5. [Google Scholar]
- Comité Asesor de Vacunas de la AEP Coberturas Vacunales En el Mundo. 2019. Available online: https://vacunasaep.org/profesionales/noticias/coberturas-vacunales-en-el-mundo-2019 (accessed on 11 February 2021).
- Organización Mundial de la Salud Cobertura Vacunal. Available online: https://www.who.int/es/news-room/fact-sheets/detail/immunization-coverage (accessed on 5 January 2021).
- Kayser, V.; Ramzan, I. Vaccines and Vaccination: History and Emerging Issues. Hum. Vaccines Immunother. 2021, 17, 5255–5268. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud Pública del Ecuador. OPS Evaluación de la Estrategia Nacional de Inmunizaciones Ecuador 2017; Ministerio de Salud Pública del Ecuador: Quito, Equador, 2017.
- Organización Mundial de la Salud. Plan de Accion Mundial Sobre Vacunas 2011 a 2020; Organización Mundial de la Salud: Geneva, Switzerland, 2013; ISBN 978 92 4 350498 8.
- Organización Panamericana de la Salud Vaccine Coverage. Available online: http://ais.paho.org/imm/IM_JRF_COVERAGE.asp (accessed on 20 October 2020).
- Organización Panamericana de la Salud Coverage DTP3 DTP1 MMr1 for South America. Available online: http://ais.paho.org/imm/IM_ADM2_COVERAGE-MAPS%20-SouthAmerica.asp (accessed on 25 July 2020).
- Jaca, A.; Mathebula, L.; Iweze, A.; Pienaar, E.; Wiysonge, C.S. A Systematic Review of Strategies for Reducing Missed Opportunities for Vaccination. Vaccine 2018, 36, 2921–2927. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud; Organización Mundial de la Salud. Missed Opportunity Vaccination Protocol; Organización Panamericana de la Salud: Washington, DC, USA, 2014; ISBN 978-92-75-31818-8.
- Idris, I.O.; Ayeni, G.O.; Adebisi, Y.A. Why Are Missed Opportunities for Immunisation and Immunisation Defaulting among Children Indistinguishable? Ann. Med. Surgery 2022, 80, 104268. [Google Scholar] [CrossRef]
- Sridhar, S.; Maleq, N.; Guillermet, E.; Colombini, A.; Gessner, B.D. A Systematic Literature Review of Missed Opportunities for Immunization in Low- and Middle-Income Countries. Vaccine 2014, 32, 6870–6879. [Google Scholar] [CrossRef]
- Fatiregun, A.A.; Lochlainn, L.N.; Kaboré, L.; Dosumu, M.; Isere, E.; Olaoye, I.; Akanbiemu, F.A.; Olagbuji, Y.; Onyibe, R.; Boateng, K.; et al. Missed Opportunities for Vaccination among Children Aged 0–23 Months Visiting Health Facilities in a Southwest State of Nigeria, December 2019. PLoS ONE 2021, 16, e0252798. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, L.; Meda, B.; Médah, I.; Shendale, S.; Nic Lochlainn, L.; Sanderson, C.; Ouattara, M.; Kaboré, W.M.F.; Betsem, E.; Ogbuanu, I.U. Assessment of Missed Opportunities for Vaccination (MOV) in Burkina Faso Using the World Health Organization’s Revised MOV Strategy: Findings and Strategic Considerations to Improve Routine Childhood Immunization Coverage. Vaccine 2020, 38, 7603–7611. [Google Scholar] [CrossRef]
- Rivero, I.; Raguindin, P.F.; Buttler, R.; Martinon-Torres, F. False Vaccine Contraindications Among Healthcare Providers in Europe: A Short Survey Among Members of The European Society of Pediatric Infectious Diseases. Pediatric Infect. Dis. J. 2019, 38, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Holst, A.; DeAntonio, R.; Prado-Cohrs, D.; Juliao, P. Barriers to Vaccination in Latin America: A Systematic Literature Review. Vaccine 2020, 38, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Borras-Bermejo, B.; Panunzi, I.; Bachy, C.; Gil-Cuesta, J. Missed Opportunities for Vaccination (MOV) in Children up to 5 Years Old in 19 Médecins Sans Frontières-Supported Health Facilities: A Cross-Sectional Survey in Six Low-Resource Countries. BMJ Open 2022, 12, e059900. [Google Scholar] [CrossRef] [PubMed]
- Tampi, M.; Carrasco-Labra, A.; O’Brien, K.K.; Velandia-González, M.; Brignardello-Petersen, R. Systematic Review on Reducing Missed Opportunities for Vaccinations in Latin America. Rev. Panam. Salud Pública 2022, 46, e65. [Google Scholar] [CrossRef]
- Jimbo Sotomayor, R.; Armijos Acurio, L.; Sánchez Choez, X.; Vilema Ortiz, M.; Ghisays, G.; Moyota Quinzo, D.; Moreta Colcha, F. Oportunidades perdidas de vacunación en centros de atención primaria en Ecuador. Vacunas 2019, 20, 46–52. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Committee on Infectious Diseases. Red Book: 2018 Report of the Committee on Infectious Diseases, 31st ed; Kimberlin, D.W., Brady, M.T., Jackson, M.A., Long, S.S., Eds.; American Academy of Pediatrics: Itasca, IL, USA, 2018. [Google Scholar]
- Opri, R.; Zanoni, G.; Caffarelli, C.; Bottau, P.; Caimmi, S.; Crisafulli, G.; Franceschini, F.; Liotti, L.; Saretta, F.; Vernich, M.; et al. True and False Contraindications to Vaccines. Allergol. Immunopathol. 2018, 46, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Smith, M. Vaccine Safety: Medical Contraindications, Myths, and Risk Communication. Pediatrics Rev. 2015, 36, 227–238. [Google Scholar] [CrossRef] [PubMed]
| Vaccine Period | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccines | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
| HepBB | 5% | 7% | 16% | 69% | 79% | 75% | 47% | 61% | 70% | 71% |
| MMR2 | 91% | 92% | 55% | 83% | 85% | 76% | 64% | 73% | 74% | 76% |
| DTP3 | 100% | 100% | 100% | 87% | 83% | 78% | 83% | 85% | 85% | 85% |
| PCV3 | 17% | 71% | 94% | 90% | 100% | 81% | 84% | 84% | 85% | 83% |
| Polio3 | 100% | 100% | 100% | 87% | 84% | 84% | 79% | 83% | 85% | 85% |
| MMR1 | N/D | N/D | N/D | N/D | N/D | 84% | 86% | 81% | 83% | 83% |
| DTP1 | 100% | 100% | 100% | 87% | 84% | 80% | 82% | 84% | 86% | 86% |
| BCG | 100% | 100% | 100% | 90% | 89% | 88% | 84% | 88% | 90% | 86% |
| Case Scenario | Theme | Vaccinate % (n) | Delay % (n) | Contraindication % (n) |
|---|---|---|---|---|
| Fever (38 °C) with normal physical examination and mild flu symptoms in a 5-year-old child | Fever | 11% (30) | 69.6% (190) | 19.4% (53) |
| Taking antibiotic therapy and going to receive immunization | Antibiotic therapy | 52.4% (143) | 38.8% (106) | 8.8% (24) |
| Breastfeeding a healthy 9-month-old baby | Breastfeeding | 94.5% (258) | 1.8% (5) | 3.7% (10) |
| MMR immunization with egg allergy | Egg allergy | 41.4% (113) | 7.3% (20) | 51.3% (140) |
| Mild, non-anaphylactic allergic reaction prior to previous vaccination | Mild allergic reaction | 93.8% (256) | 2.6% (7) | 3.6% (10) |
| History of autoimmune diseases such as agammaglobulinemia prior to varicella immunization | Autoimmune diseases | 27% (74) | 12.5% (34) | 60.4% (165) |
| History of living with immunosuppressed people for rotavirus immunization | Living with an immunosuppressed person | 87.2% (238) | 7.3% (20) | 5.5% (15) |
| Patient discharged for influenza, however, presents mild symptoms | Convalescence | 53.8% (147) | 39.2% (107) | 7% (19) |
| 2-month-old patient with a history of prematurity | Premature | 81.7% (223) | 16.8% (46) | 1.5% (4) |
| Patient with a family history of epilepsy to receive DPT immunization | Family history of epilepsy | 91.6% (250) | 2.6% (7) | 5.9% (16) |
| Patient with a personal history of epilepsy controlled for immunization of the DPT vaccine | Epilepsy | 79.1% (216) | 6.6% (18) | 14.3% (39) |
| 12-month-old patient with an allergy to penicillin | Allergy to penicillin | 96.3% (263) | 1.8% (5) | 1.8% (5) |
| 4-month-old patient who comes to receive the immunization days before the date according to the vaccination schedule | Advanced immunization | 57.5% (157) | 27.5% (75) | 15% (41) |
| Taking corticosteroid therapy and going to receive chickenpox immunization | Corticosteroid therapy | 28.6% (78) | 59.7% (163) | 11.7% (32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade-Guerrero, F.; Tapia, A.; Andrade, V.; Vásconez-González, J.; Andrade-Guerrero, J.; Noroña-Calvachi, C.; Izquierdo-Condoy, J.S.; Yeager, J.; Ortiz-Prado, E. False Contraindications for Vaccinations Result in Sub-Optimal Vaccination Coverage in Quito, Ecuador: A Cross-Sectional Study. Vaccines 2023, 11, 60. https://doi.org/10.3390/vaccines11010060
Andrade-Guerrero F, Tapia A, Andrade V, Vásconez-González J, Andrade-Guerrero J, Noroña-Calvachi C, Izquierdo-Condoy JS, Yeager J, Ortiz-Prado E. False Contraindications for Vaccinations Result in Sub-Optimal Vaccination Coverage in Quito, Ecuador: A Cross-Sectional Study. Vaccines. 2023; 11(1):60. https://doi.org/10.3390/vaccines11010060
Chicago/Turabian StyleAndrade-Guerrero, Felipe, Adriana Tapia, Vinicio Andrade, Jorge Vásconez-González, José Andrade-Guerrero, Carlos Noroña-Calvachi, Juan S. Izquierdo-Condoy, Justin Yeager, and Esteban Ortiz-Prado. 2023. "False Contraindications for Vaccinations Result in Sub-Optimal Vaccination Coverage in Quito, Ecuador: A Cross-Sectional Study" Vaccines 11, no. 1: 60. https://doi.org/10.3390/vaccines11010060
APA StyleAndrade-Guerrero, F., Tapia, A., Andrade, V., Vásconez-González, J., Andrade-Guerrero, J., Noroña-Calvachi, C., Izquierdo-Condoy, J. S., Yeager, J., & Ortiz-Prado, E. (2023). False Contraindications for Vaccinations Result in Sub-Optimal Vaccination Coverage in Quito, Ecuador: A Cross-Sectional Study. Vaccines, 11(1), 60. https://doi.org/10.3390/vaccines11010060







