Web-Based Reporting of Post-Vaccination Symptoms for Inactivated COVID-19 Vaccines in Jordan: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Survey Development
2.3. Survey Implementation
2.4. Sample Size
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 1 August 2021).
- Ndwandwe, D.; Wiysonge, C.S. COVID-19 vaccines. Curr. Opin. Immunol. 2021, 71, 111–116. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 1 August 2021).
- Kashte, S.; Gulbake, A.; El-Amin, S.F.; Gupta, A. COVID-19 vaccines: Rapid development, implications, challenges and future prospects. Hum. Cell 2021, 34, 711–733. [Google Scholar] [CrossRef] [PubMed]
- Tavilani, A.; Abbasi, E.; Kian Ara, F.; Darini, A.; Asefy, Z. COVID-19 vaccines: Current evidence and considerations. Metab. Open 2021, 12, 100124. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.; Mantovani, A.; on behalf of the COVID-19 Commission of Accademia Nazionale dei Lincei. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Pang, Y.; Lyu, Z.; Wang, R.; Wu, X.; You, C.; Zhao, H.; Manickam, S.; Lester, E.; Wu, T.; et al. The COVID-19 vaccines: Recent development, challenges and prospects. Vaccines 2021, 9, 349. [Google Scholar] [CrossRef]
- Holder, J. Tracking Coronavirus Vaccinations around the World. 2022. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 1 October 2022).
- MacDonald, N.E.; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Wagner, A.L.; Masters, N.B.; Domek, G.J.; Mathew, J.L.; Sun, X.; Asturias, E.J.; Ren, J.; Huang, Z.; Contreras-Roldan, I.L.; Gebremeskel, B.; et al. Comparisons of vaccine hesitancy across five low- and middle-income countries. Vaccines 2019, 7, 155. [Google Scholar] [CrossRef]
- Lane, S.; MacDonald, N.E.; Marti, M.; Dumolard, L. Vaccine hesitancy around the globe: Analysis of three years of WHO/UNICEF Joint Reporting Form data—2015–2017. Vaccine 2018, 36, 3861–3867. [Google Scholar] [CrossRef]
- Thomson, A.; Robinson, K.; Vallée-Tourangeau, G. The 5As: A practical taxonomy for the determinants of vaccine uptake. Vaccine 2016, 34, 1018–1024. [Google Scholar] [CrossRef]
- Karafillakis, E.; Larson, H.J. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine 2017, 35, 4840–4850. [Google Scholar] [CrossRef]
- Yaqub, O.; Castle-Clarke, S.; Sevdalis, N.; Chataway, J. Attitudes to vaccination: A critical review. Soc. Sci. Med. 2014, 112, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.C.; Soveri, A.; Lewandowsky, S.; Karlsson, L.; Karlsson, H.; Nolvi, S.; Karukivi, M.; Lindfelt, M.; Antfolk, J. Fearing the disease or the vaccine: The case of COVID-19. Pers. Individ. Differ. 2021, 172, 110590. [Google Scholar] [CrossRef] [PubMed]
- Nassar, R.I.; Alnatour, D.; Thiab, S.; Nassar, A.; El-Hajji, F.; Basheti, I.A. Short-term side effects of COVID-19 vaccines: A cross-sectional study in Jordan. Hum. Vaccines Immunother. 2022, 18, 2082792. [Google Scholar] [CrossRef] [PubMed]
- Jordan, U. COVID-19 Vaccine. Available online: https://help.unhcr.org/jordan/en/frequently-asked-questions-unhcr/covid-19-vaccine/#:~:text=TheNationalVaccinationCampaignto,havetopayanyfees (accessed on 1 September 2022).
- Abu Farha, R.K.; Alzoubi, K.H.; Khabour, O.F.; Alfaqih, M.A. Exploring perception and hesitancy toward COVID-19 vaccine: A study from Jordan. Hum. Vaccines Immunother. 2021, 17, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.; Dababseh, D.; Eid, H.; Al-Mahzoum, K.; Al-Haidar, A.; Taim, D.; Yaseen, A.; Ababneh, N.A.; Bakri, F.G.; Mahafzah, A. High Rates of COVID-19 Vaccine Hesitancy and Its Association with Conspiracy Beliefs: A Study in Jordan and Kuwait among Other Arab Countries. Vaccines 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.; Dababseh, D.; Yaseen, A.; Al-Haidar, A.; Taim, D.; Eid, H.; Ababneh, N.A.; Bakri, F.G.; Mahafzah, A. COVID-19 misinformation: Mere harmless delusions or much more? A knowledge and attitude cross-sectional study among the general public residing in Jordan. PLoS ONE 2020, 15, e0243264. [Google Scholar] [CrossRef]
- Lataifeh, L.; Al-Ani, A.; Lataifeh, I.; Ammar, K.; Alomary, A.; Al-Hammouri, F.; Al-Hussaini, M. Knowledge, Attitudes, and Practices of Healthcare Workers in Jordan towards the COVID-19 Vaccination. Vaccines 2022, 10, 263. [Google Scholar] [CrossRef]
- Jordanian Ministry of Health. Available online: https://www.moh.gov.jo/Default/En (accessed on 1 August 2021).
- Eng, J. Sample size estimation: How many individuals should be studied? Radiology 2003, 227, 309–313. [Google Scholar] [CrossRef]
- Vogel, W.H. Infusion reactions: Diagnosis, assessment, and management. Clin. J. Oncol. Nurs. 2010, 14, E10. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. What to expect at your appointment to get vaccinated for COVID-19. 2020. Available online: https://stacks.cdc.gov/view/cdc/99715 (accessed on 1 September 2022).
- Lounis, M.; Rais, M.A.; Bencherit, D.; Aouissi, H.A.; Oudjedi, A.; Klugarová, J.; Pokorná, A.; Klugar, M.; Riad, A. Side Effects of COVID-19 Inactivated Virus vs. Adenoviral Vector Vaccines: Experience of Algerian Healthcare Workers. Front. Public Health 2022, 10, 896343. [Google Scholar] [CrossRef]
- Zahid, M.N. Unfolding the mild to moderate short-term side effects of four COVID-19 vaccines used in bahrain: A cross-sectional study. Vaccines 2021, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Attash, H.M.; Al-Obaidy, L.M.; Al-Qazaz, H.K. Which Type of the Promising COVID-19 Vaccines Produces Minimal Adverse Effects? A Retrospective Cross-Sectional Study. Vaccines 2022, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Omeish, H.; Najadat, A.; Al-Azzam, S.; Tarabin, N.; Abu Hameed, A.; Al-Gallab, N.; Abbas, H.; Rababah, L.; Rabadi, M.; Karasneh, R.; et al. Reported COVID-19 vaccines side effects among Jordanian population: A cross sectional study. Hum. Vaccines Immunother. 2021, 18, 1981086. [Google Scholar] [CrossRef] [PubMed]
- Saeed, B.Q.; Al-Shahrabi, R.; Alhaj, S.S.; Alkokhardi, Z.M.; Adrees, A.O. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int. J. Infect. Dis. 2021, 111, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Almufty, H.B.; Mohammed, S.A.; Abdullah, A.M.; Merza, M.A. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102207. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Hatmal, M.; Alhaj-Qasem, D.M.; Olaimat, T.M.; Mohamud, R. Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects. Vaccines 2021, 9, 556. [Google Scholar] [CrossRef]
- The World Health Organization. Side Effects of COVID-19 Vaccines. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines#:~:text=Typicalsideeffectsincludepain,accordingtothespecificvaccine (accessed on 1 October 2022).
- Dadras, O.; Mehraeen, E.; Karimi, A.; Tantuoyir, M.M.; Afzalian, A.; Nazarian, N.; Mojdeganlou, H.; Mirzapour, P.; Shamsabadi, A.; Dashti, M.; et al. Safety and Adverse Events Related to Inactivated COVID-19 Vaccines and Novavax; a Systematic Review. Arch. Acad. Emerg. Med. 2022, 10, e54. [Google Scholar]
- Rossi, A.; Magri, F.; Michelini, S.; Caro, G.; Di Fraia, M.; Fortuna, M.C.; Pellacani, G.; Carlesimo, M. Recurrence of alopecia areata after COVID-19 vaccination: A report of three cases in Italy. J. Cosmet. Dermatol. 2021, 20, 3753–3757. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, Y.Y.; Lan, C.C.E. Intractable alopecia areata following the second dose of COVID-19 vaccination: Report of two cases. Dermatol. Ther. 2022, 35, e15689. [Google Scholar] [CrossRef]
- Di Resta, C.; Ferrari, D.; Viganò, M.; Moro, M.; Sabetta, E.; Minerva, M.; Ambrosio, A.; Locatelli, M.; Tomaiuolo, R. The Gender Impact Assessment among Healthcare Workers in the SARS-CoV-2 Vaccination—An Analysis of Serological Response and Side Effects. Vaccines 2021, 9, 522. [Google Scholar] [CrossRef]
- Elnaem, M.H.; Taufek, N.H.M.; Ab Rahman, N.S.; Mohd Nazar, N.I.; Zin, C.S.; Nuffer, W.; Turner, C.J. COVID-19 Vaccination Attitudes, Perceptions, and Side Effect Experiences in Malaysia: Do Age, Gender, and Vaccine Type Matter? Vaccines 2021, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Gianfredi, V.; Tomaselli, V.; Polosa, R. The Effect of Smoking on Humoral Response to COVID-19 Vaccines: A Systematic Review of Epidemiological Studies. Vaccines 2022, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Mahmud, S.; Uddin Mian, A.; Hasan, P.; Muyeed, A.; Taif Ali, M.; Ahmed, F.F.; Islam, A.; Rahman, M.M.; Islam, M.; et al. Side effects of COVID-19 vaccines and perceptions about COVID-19 and its vaccines in Bangladesh: A Cross-sectional study. Vaccine X 2022, 12, 100207. [Google Scholar] [CrossRef]
- Dar-Odeh, N.; Abu-Hammad, O.; Qasem, F.; Jambi, S.; Alhodhodi, A.; Othman, A.; Abu-Hammad, A.; Al-Shorman, H.; Ryalat, S.; Abu-Hammad, S. Long-term adverse events of three COVID-19 vaccines as reported by vaccinated physicians and dentists, a study from Jordan and Saudi Arabia. Hum. Vaccines Immunother. 2022, 18, 2039017. [Google Scholar] [CrossRef]
- Adedeji-Adenola, H.; Olugbake, O.A.; Adeosun, S.A. Factors influencing COVID-19 vaccine uptake among adults in Nigeria. PLoS ONE 2022, 17, e0264371. [Google Scholar] [CrossRef]
- Batarseh, Y.S.; ElHajji, F.W.D.; Shammas, S.; Darwish, R.M.; Fakhoury, R.; Al Haj Ahmad, M.; Al Rusasi, A.; Jarrar, L. Perception and attitude of the public on vaccine practices and pharmacists as immunisers in Jordan. J. Pharm. Health Serv. Res. 2021, 12, 114–121. [Google Scholar] [CrossRef]

| Parameter | n (%) |
|---|---|
Sex
| 108 (28.1) 278 (71.9) |
Age
| 149 (38.6) 155 (40.2) 63 (16.3) 15 (3.9) 4 (1.0) |
Marital Status
| 229 (59.3) 141 (36.5) 14 (3.7) 2 (0.5) |
Living place
| 293 (75.9) 93 (24.1) |
Nationality
| 366 (94.8) 20 (5.2) |
Educational Level
| 2 (0.5) 22 (5.8) 28 (7.3) 235 (60.6) 99 (25.7) |
Employment
| 250 (64.8) 125 (32.3) 11 (2.9) |
Smoking Status
| 124 (32.0) 250 (64.8) 12 (3.1) |
| Parameter | Side Effects [0: No, 1: Yes] | |||
|---|---|---|---|---|
| OR | p-Value # | OR | p-Value $ | |
Sex
| Reference 0.438 | 0.003 ^ | 0.382 | 0.027 * |
Age
| Reference 0.897 | 0.606 | --- | --- |
Smoking status
| Reference 1.121 | 0.603 | ---- | ---- |
Infected before vaccination
| Reference 0.697 | 0.081 ^ | 0.700 | 0.085 |
| Reported Side Effects | Sex (0: Male; 1: Female) | Age (0: <30 Years Old; 1: ≥30 Years Old) | Smoking Status (0: Non-Smoker; 1: Smoker) | Infected before the Vaccine (0: Yes; 1: No) |
|---|---|---|---|---|
| p-Value | ||||
| Sore arm at the site of injection | 0.684 | 0.801 | 0.450 | 0.588 |
| Injection site swelling | 0.908 | 0.870 | 0.546 | 0.896 |
| Redness at the injection site | 0.514 | 0.435 | 0.588 | 0.898 |
| Discomfort feeling | 0.840 | 0.402 | 0.275 | 0.250 |
| Fatigue | 0.105 | 0.704 | 0.591 | 0.621 |
| Flu-like symptoms | 0.481 | 0.711 | 0.889 | 0.167 |
| High temperature | 0.439 | 0.327 | 0.290 | 0.633 |
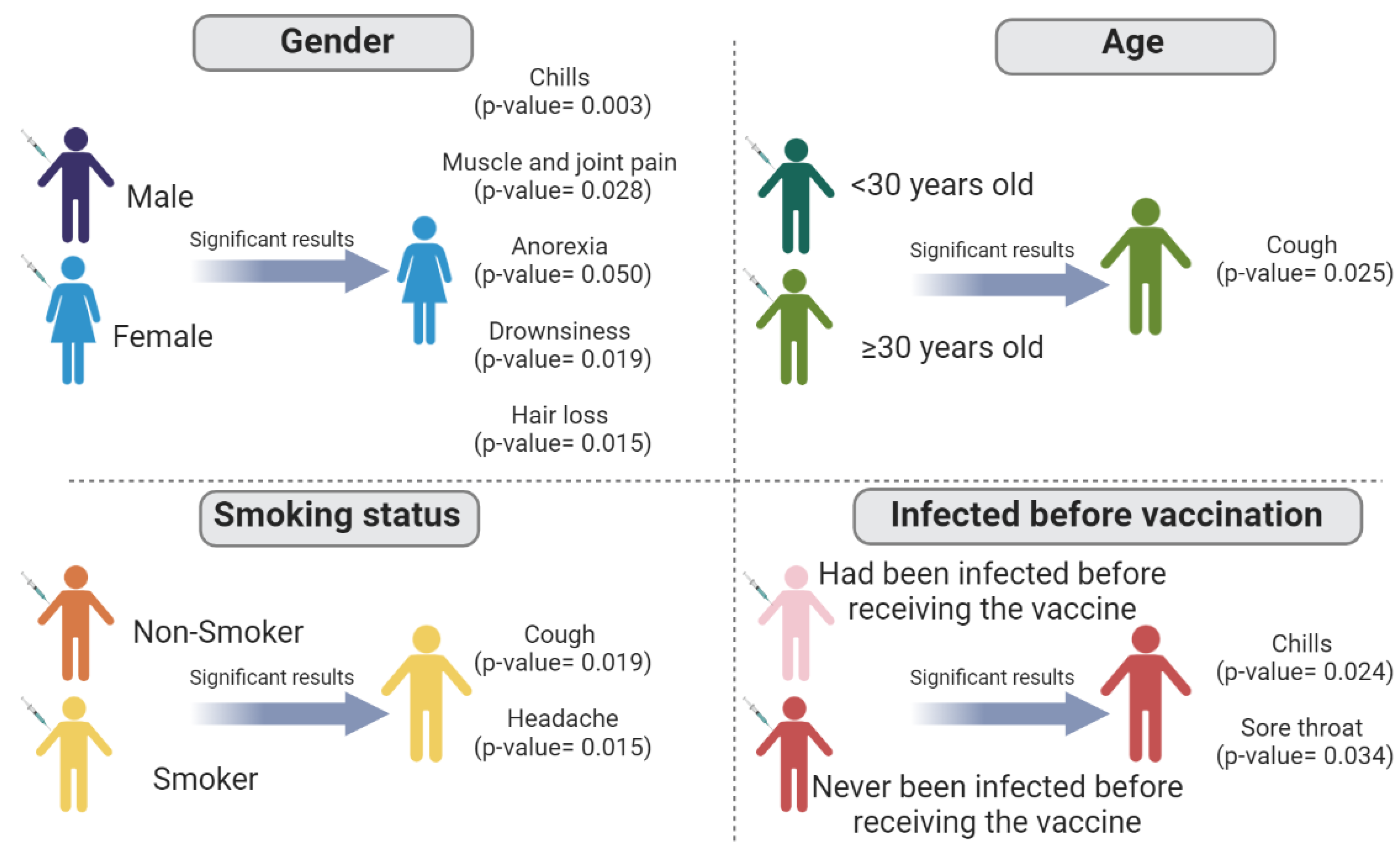
| Chills | 0.003 * | 0.643 | 0.212 | 0.024 * |
| Headache | 0.536 | 0.485 | 0.015 * | 0.888 |
| Shortness of breath | 0.209 | 0.801 | 0.450 | 0.125 |
| Cough | 0.712 | 0.025 * | 0.019 * | 0183 |
| Muscles/joint pain | 0.028 * | 0.610 | 0.589 | 0.686 |
| Gastrointestinal symptoms such as nausea, vomiting, and diarrhea | 0.166 | 0.543 | 0.442 | 0.605 |
| Sore throat | 0.820 | 0.780 | 0.656 | 0.034 * |
| Eye pain | 0.157 | 0.354 | 0.439 | 0.976 |
| Runny nose | 0.806 | 0.216 | 0.749 | 0.268 |
| Loss or change in the sense of taste or smell | 0.282 | 0.987 | 0.828 | 0.697 |
| Anorexia | 0.050 * | 0.550 | 0.372 | 0.502 |
| Chest pressure/pain | 0.545 | 0.747 | 0.618 | 0.392 |
| Drowsiness | 0.019 * | 0.060 | 0.367 | 0.760 |
| Hair loss | 0.015 * | 0.573 | 0.081 | 0.153 |
| Tachycardia or heart palpitations | 0.902 | 0.590 | 0.055 | 0.442 |
| Lymphadenopathy | 0.157 | 0.934 | 0.811 | 0.976 |
| Reported Side Effect | n (%) |
|---|---|
| Fatigue and tiredness | 144 (80.9) |
| Sore arm at the site of injection | 137 (77.0) |
| Discomfort | 112 (62.9) |
| Muscles/joint pain | 105 (59.0) |
| Headache | 99 (55.6) |
| Drowsiness | 90 (50.6) |
| High temperature | 69 (38.8) |
| Chills | 67 (37.6) |
| Flu-like symptoms | 54 (30.3) |
| Injection site swelling | 50 (28.1) |
| Redness at the injection site | 39 (21.9) |
| Gastrointestinal symptoms such as nausea, vomiting, and diarrhea | 34 (19.1) |
| Hair loss | 32 (18.0) |
| Tachycardia or heart palpitations | 30 (16.8) |
| Chest pressure/pain | 29 (16.3) |
| Shortness of breath | 28 (15.7) |
| Runny nose | 23 (12.9) |
| Anorexia | 20 (11.2) |
| Sore throat | 19 (10.8) |
| Cough | 16 (9.0) |
| Eye pain | 15 (8.4) |
| Lymphadenopathy | 13 (7.3) |
| Loss or change in the sense of taste or smell | 10 (5.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, R.I.; Barakat, M.; Thiab, S.; El-Hajji, F.; Barqawi, H.; El-Huneidi, W.; Basheti, I.A.; Abu-Gharbieh, E. Web-Based Reporting of Post-Vaccination Symptoms for Inactivated COVID-19 Vaccines in Jordan: A Cross-Sectional Study. Vaccines 2023, 11, 44. https://doi.org/10.3390/vaccines11010044
Nassar RI, Barakat M, Thiab S, El-Hajji F, Barqawi H, El-Huneidi W, Basheti IA, Abu-Gharbieh E. Web-Based Reporting of Post-Vaccination Symptoms for Inactivated COVID-19 Vaccines in Jordan: A Cross-Sectional Study. Vaccines. 2023; 11(1):44. https://doi.org/10.3390/vaccines11010044
Chicago/Turabian StyleNassar, Razan I., Muna Barakat, Samar Thiab, Feras El-Hajji, Hiba Barqawi, Waseem El-Huneidi, Iman A. Basheti, and Eman Abu-Gharbieh. 2023. "Web-Based Reporting of Post-Vaccination Symptoms for Inactivated COVID-19 Vaccines in Jordan: A Cross-Sectional Study" Vaccines 11, no. 1: 44. https://doi.org/10.3390/vaccines11010044
APA StyleNassar, R. I., Barakat, M., Thiab, S., El-Hajji, F., Barqawi, H., El-Huneidi, W., Basheti, I. A., & Abu-Gharbieh, E. (2023). Web-Based Reporting of Post-Vaccination Symptoms for Inactivated COVID-19 Vaccines in Jordan: A Cross-Sectional Study. Vaccines, 11(1), 44. https://doi.org/10.3390/vaccines11010044








