Peri-oral Monkeypox Virus Infection: A Clinical Report with Confirmatory Polymerase Chain Reaction Findings
Abstract
1. Introduction
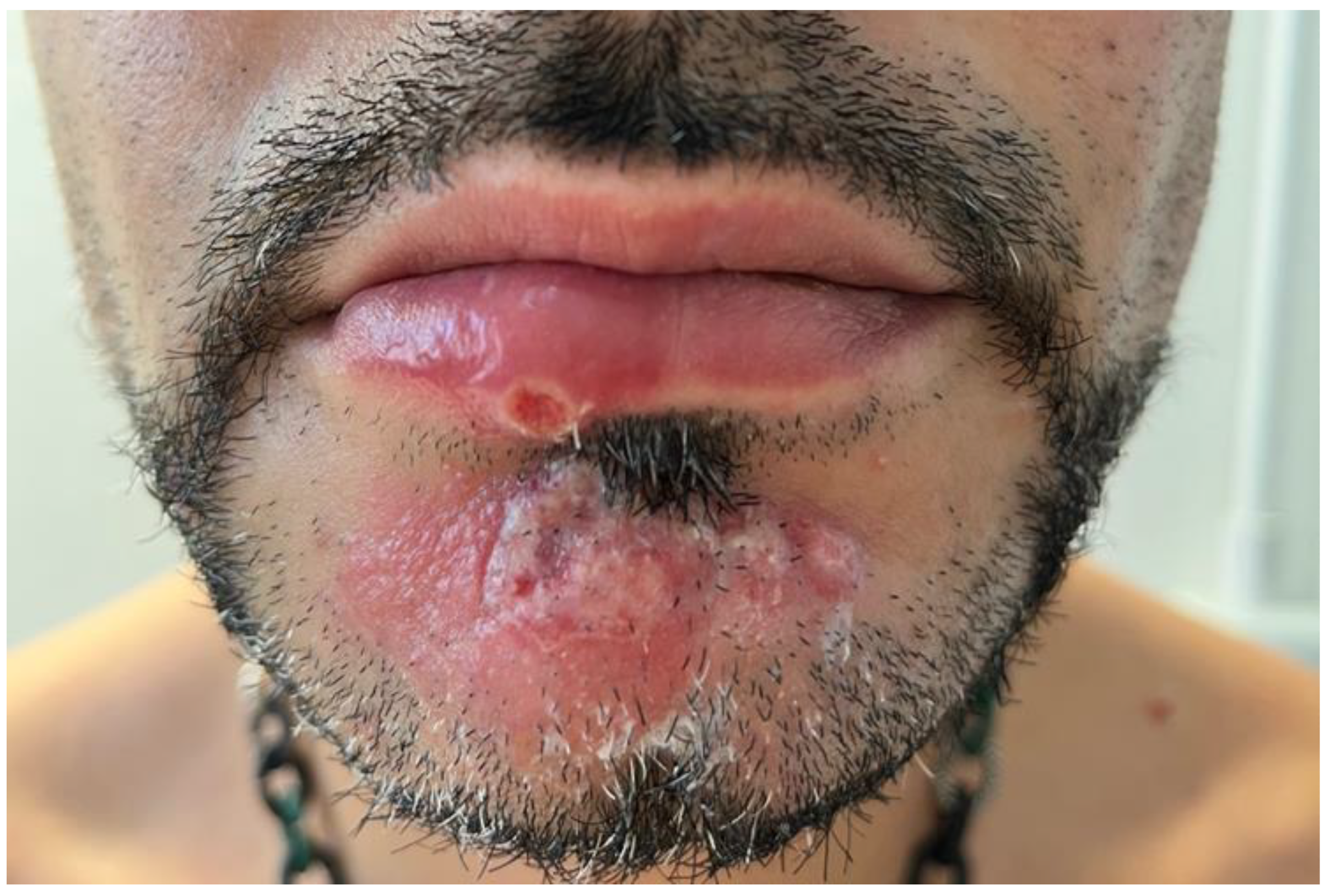
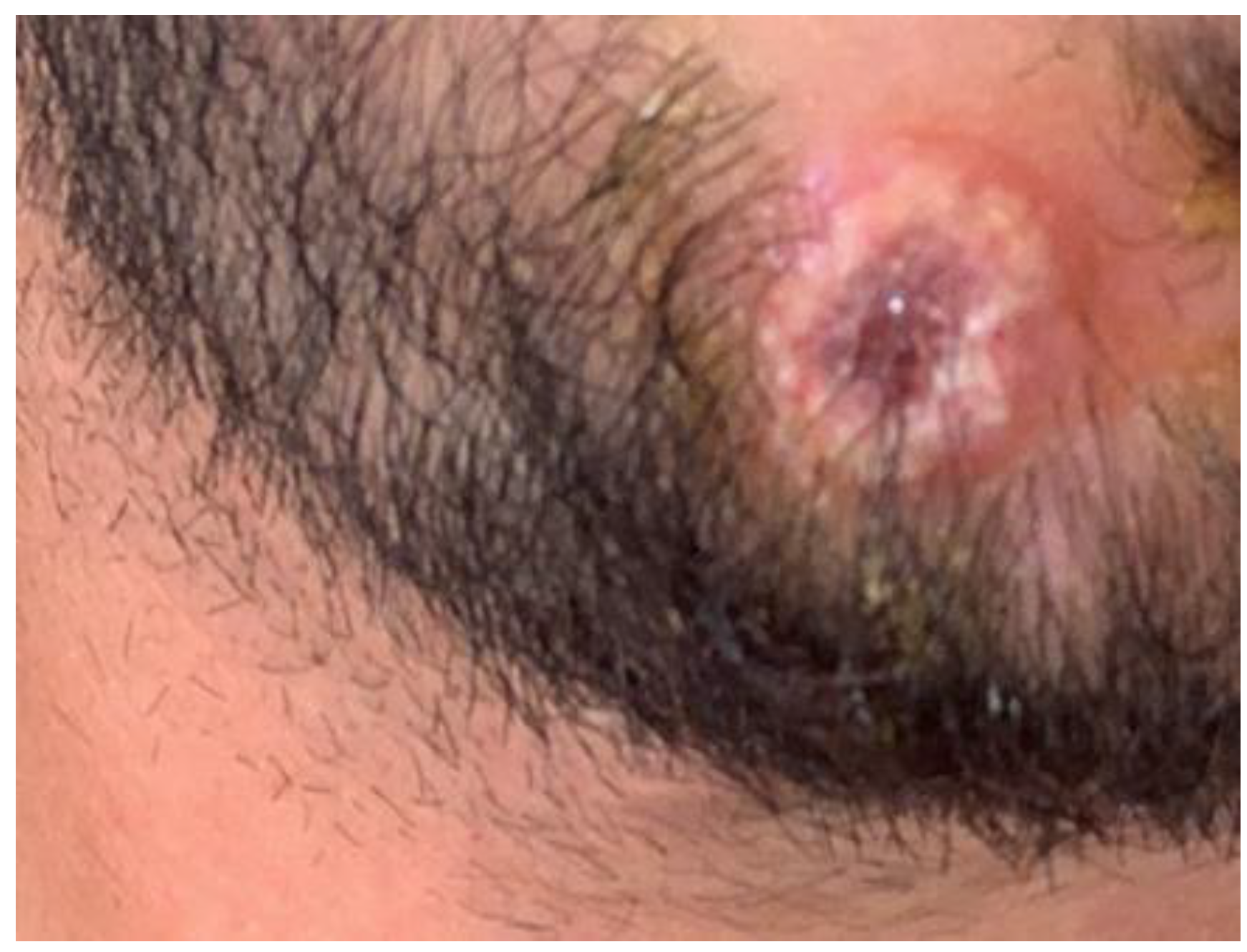
2. Case presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Safronov, P.F.; Ryazankina, O.I.; Petrov, N.A.; Gutorov, V.V.; Uvarova, E.A.; Mikheev, M.V.; Sisler, J.R.; et al. Human monkeypox and smallpox viruses: Genomic comparison. FEBS Lett. 2001, 509, 66–70. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Multi-Country Monkeypox Outbreak in Non-Endemic Countries. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390 (accessed on 7 June 2022).
- Sah, R.; Hada, V.; Mohanty, A.; Alshahrani, N.Z.; Chakraborty, S.; Bhattacharya, M.; Chakraborty, C.; Dhama, K. Recent first report of human-to-dog transmission of Monkeypox virus emphasizes an urgent need of enhancing surveillance and strengthen further explorative research to reveal its real magnitude of reverse zoonosis from other animals including pets as like that happened with SARS-CoV-2/COVID-19 pandemic—Correspondence. Int. J. Surg. 2022, 106, 106949. [Google Scholar] [CrossRef]
- Alshahrani, N.Z.; Alzahrani, F.; Alarifi, A.M.; Algethami, M.R.; Alhumam, M.N.; Ayied, H.A.M.; Awan, A.Z.; Almutairi, A.F.; Bamakhrama, S.A.; Almushari, B.S.; et al. Assessment of Knowledge of Monkeypox Viral Infection among the General Population in Saudi Arabia. Pathogens 2022, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Wilkins, K.; Hughes, C.; Damon, I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods 2010, 169, 223–227. [Google Scholar] [CrossRef]
- Farahat, R.A.; Abdelaal, A.; Shah, J.; Ghozy, S.; Sah, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; McHugh, T.D. Monkeypox outbreaks during COVID-19 pandemic: Are we looking at an independent phenomenon or an overlapping pandemic? Ann. Clin. Microbiol. Antimicrob. 2022, 21, 26. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Mileto, D.; Riva, A.; Cutrera, M.; Moschese, D.; Mancon, A.; Meroni, L.; Giacomelli, A.; Bestetti, G.; Rizzardini, G.; Gismondo, M.R.; et al. New challenges in human monkeypox outside Africa: A review and case report from Italy. Travel. Med. Infect. Dis. 2022, 49, 102386. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Farfour, E.; Zucman, D. Monkeypox virus: A novel sexually transmitted disease? A case report from France. Travel. Med. Infect. Dis. 2022, 49, 102394. [Google Scholar] [CrossRef] [PubMed]
- Simões, P.; Bhagani, S. A viewpoint: The 2022 monkeypox outbreak. J. Virus Erad. 2022, 8, 100078. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.M.; Escribano, P.C. Infección por virus de la viruela del mono. Caso clínico. Actas Dermosifiliogr. 2022. [Google Scholar] [CrossRef]
- Heskin, J.; Belfield, A.; Milne, C.; Brown, N.; Walters, Y.; Scott, C.; Bracchi, M.; Moore, L.S.; Mughal, N.; Rampling, T.; et al. Transmission of monkeypox virus through sexual contact—A novel route of infection. J. Infect. 2022, 81, 647–679. [Google Scholar] [CrossRef]
- Alpalhão, M.; Frade, J.V.; Sousa, D.; Patrocínio, J.; Garrido, P.; Correia, C.; Brazão, C.; Mancha, D.; Borrego, M.; Filipe, P. Monkeypox: A new (sexually transmissible) epidemic? J. Eur. Acad. Dermatol. Venereol. 2022, 36, e1016–e1017. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Yorita, K.L.; Kuehnert, M.J.; Davidson, W.B.; Huhn, G.D.; Holman, R.C.; Damon, I.K. Clinical manifestations of human monkeypox influenced by route of infection. J. Infect. Dis. 2006, 194, 773–780. [Google Scholar] [CrossRef]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef]
- Singhal, T.; Kabra, S.K.; Lodha, R. Monkeypox: A Review. Indian J. Pediatr. 2022, 89, 955–960. [Google Scholar] [CrossRef]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Da Silva Fontoura, D.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: Descriptive case series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef]
- de Nicolas-Ruanes, B.; Vivancos, M.J.; Azcarraga-Llobet, C.; Moreno, A.M.; Rodriguez-Dominguez, M.; Berna-Rico, E.D.; Garcia-Mouronte, E.; Carron-Herrero, A.; McGee, A.; Galan, J.C.; et al. Monkeypox virus case with maculopapular exanthem and proctitis during the Spanish outbreak in 2022. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e658–e660. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; DPhil, T.R.; Beadsworth, M.B.; Duncan, C.J.; et al. NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Kumar, D.; Nagar, P.; Maurya, A.; Vengat, M.; Jain, P. Clinical manifestations of human monkeypox infection and implications for outbreak strategy. Health Sci. Rev. 2022, 5, 100055. [Google Scholar] [CrossRef] [PubMed]
- About Monkeypox. Available online: https://www.cdc.gov/poxvirus/monkeypox/about.html (accessed on 1 August 2022).
- Sookaromdee, P.; Wiwanitkit, V. Mouth sores and monkeypox: A consideration. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 593. [Google Scholar] [CrossRef]
- Iamaroon, A. Oral manifestations of monkeypox: Brief review. Dent. Med. Probl. 2022, 59, 483–487. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Pandya, V.S.; Mehta, V.; Miraj, M.; Alasiry, S.M.; Alanazy, W.; Uthup, T.T.; Shaik, R.A.; D’Amico, C.; Mancini, M.; Gorassini, F.; et al. Monkeypox: An Unfamiliar Virus-Clinical and Epidemiological Characteristics, Diagnosis, and Treatment with Special Emphasis on Oral Health. Diagnostics 2022, 12, 2749. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrogio, F.; Laface, C.; De Caro, A.P.; Loconsole, D.; Centrone, F.; Lettini, T.; Cazzato, G.; Bonamonte, D.; Foti, C.; Chironna, M.; et al. Peri-oral Monkeypox Virus Infection: A Clinical Report with Confirmatory Polymerase Chain Reaction Findings. Vaccines 2023, 11, 36. https://doi.org/10.3390/vaccines11010036
Ambrogio F, Laface C, De Caro AP, Loconsole D, Centrone F, Lettini T, Cazzato G, Bonamonte D, Foti C, Chironna M, et al. Peri-oral Monkeypox Virus Infection: A Clinical Report with Confirmatory Polymerase Chain Reaction Findings. Vaccines. 2023; 11(1):36. https://doi.org/10.3390/vaccines11010036
Chicago/Turabian StyleAmbrogio, Francesca, Carmelo Laface, Anna Paola De Caro, Daniela Loconsole, Francesca Centrone, Teresa Lettini, Gerardo Cazzato, Domenico Bonamonte, Caterina Foti, Maria Chironna, and et al. 2023. "Peri-oral Monkeypox Virus Infection: A Clinical Report with Confirmatory Polymerase Chain Reaction Findings" Vaccines 11, no. 1: 36. https://doi.org/10.3390/vaccines11010036
APA StyleAmbrogio, F., Laface, C., De Caro, A. P., Loconsole, D., Centrone, F., Lettini, T., Cazzato, G., Bonamonte, D., Foti, C., Chironna, M., & Romita, P. (2023). Peri-oral Monkeypox Virus Infection: A Clinical Report with Confirmatory Polymerase Chain Reaction Findings. Vaccines, 11(1), 36. https://doi.org/10.3390/vaccines11010036









