Risk of Infection and Duration of Protection after the Booster Dose of the Anti-SARS-CoV-2 Vaccine BNT162b2 among Healthcare Workers in a Large Teaching Hospital in Italy: Results of an Observational Study
Abstract
:1. Introduction
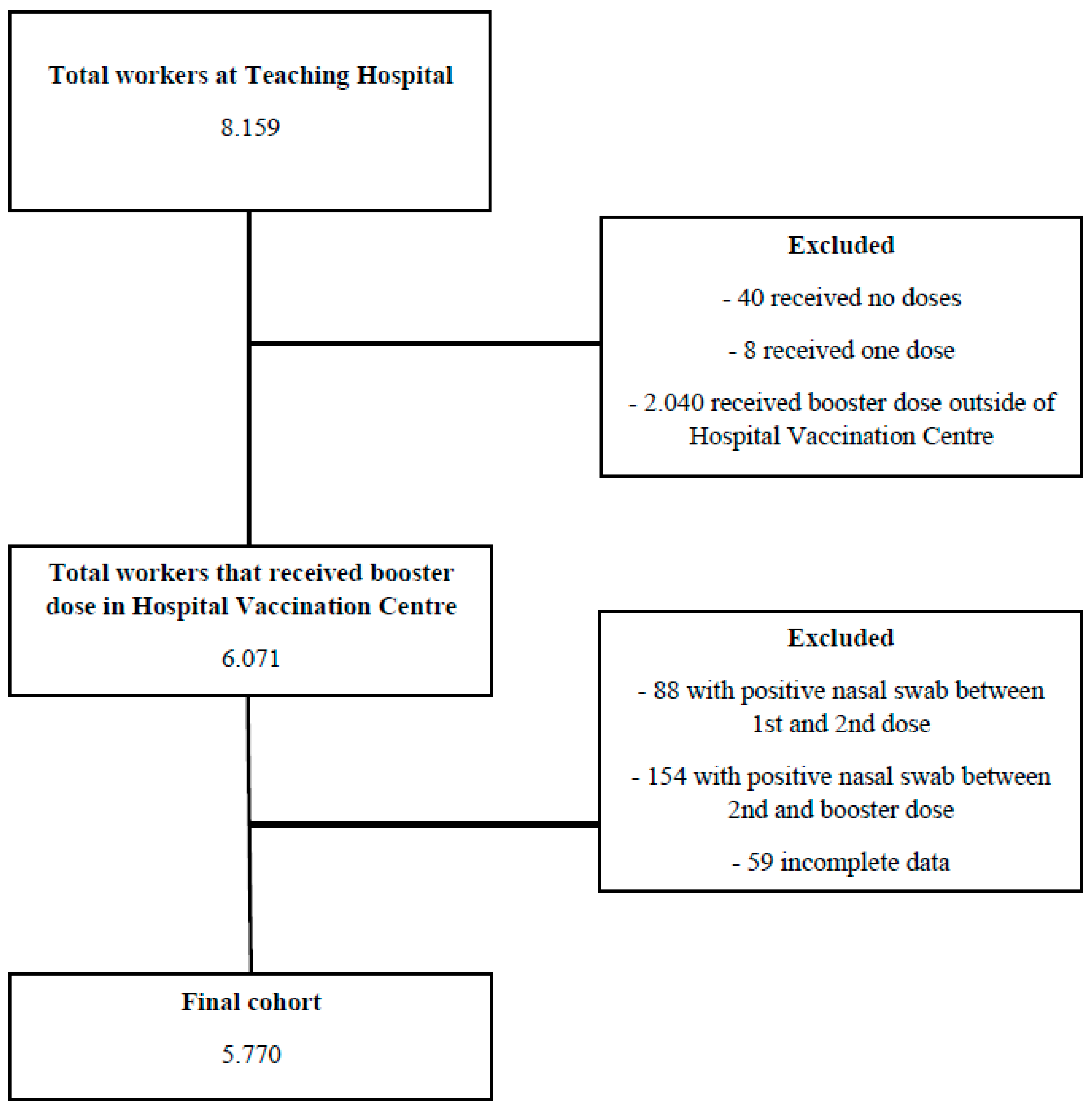
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Sources
2.3. Statistical Analysis
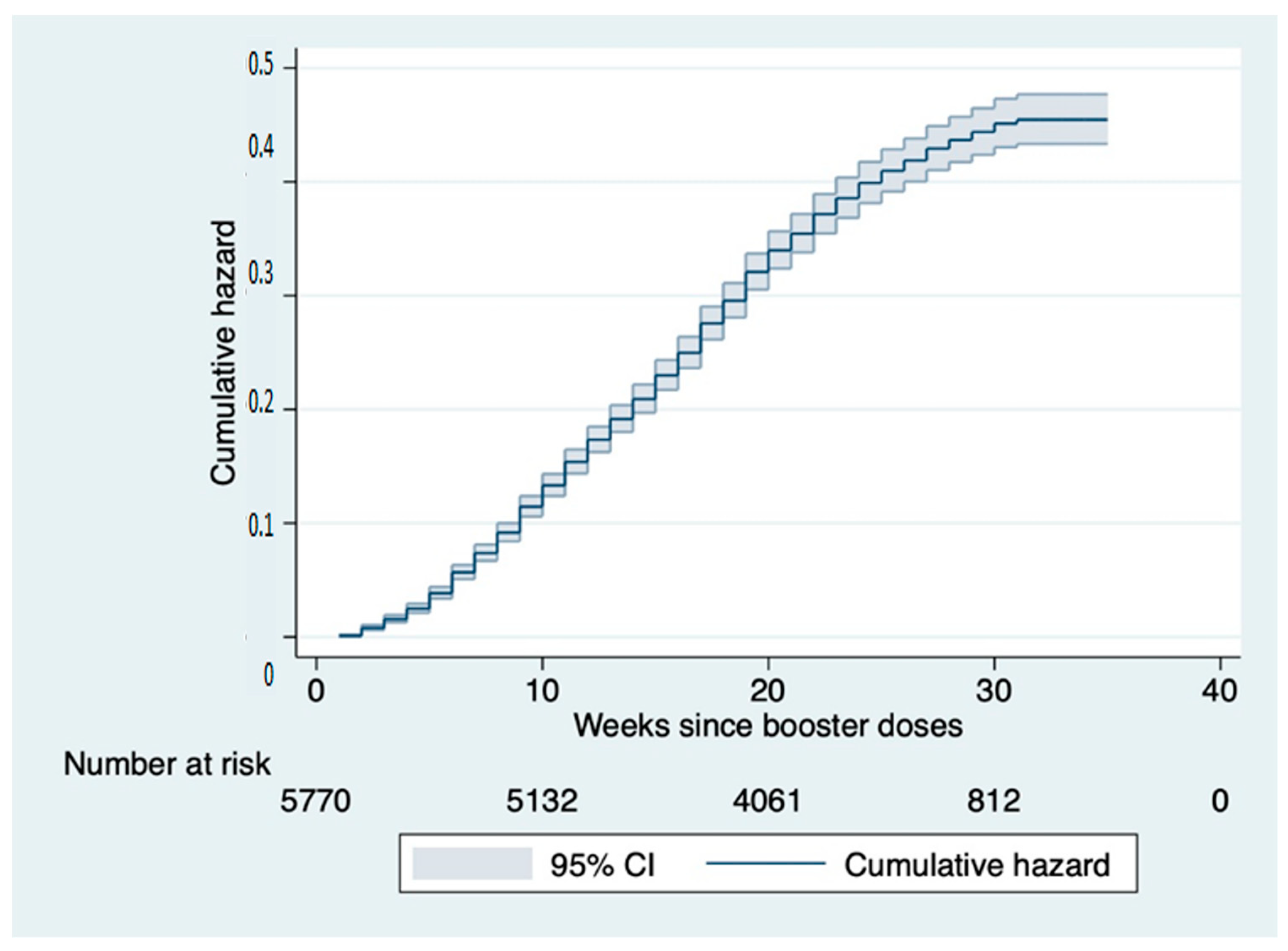
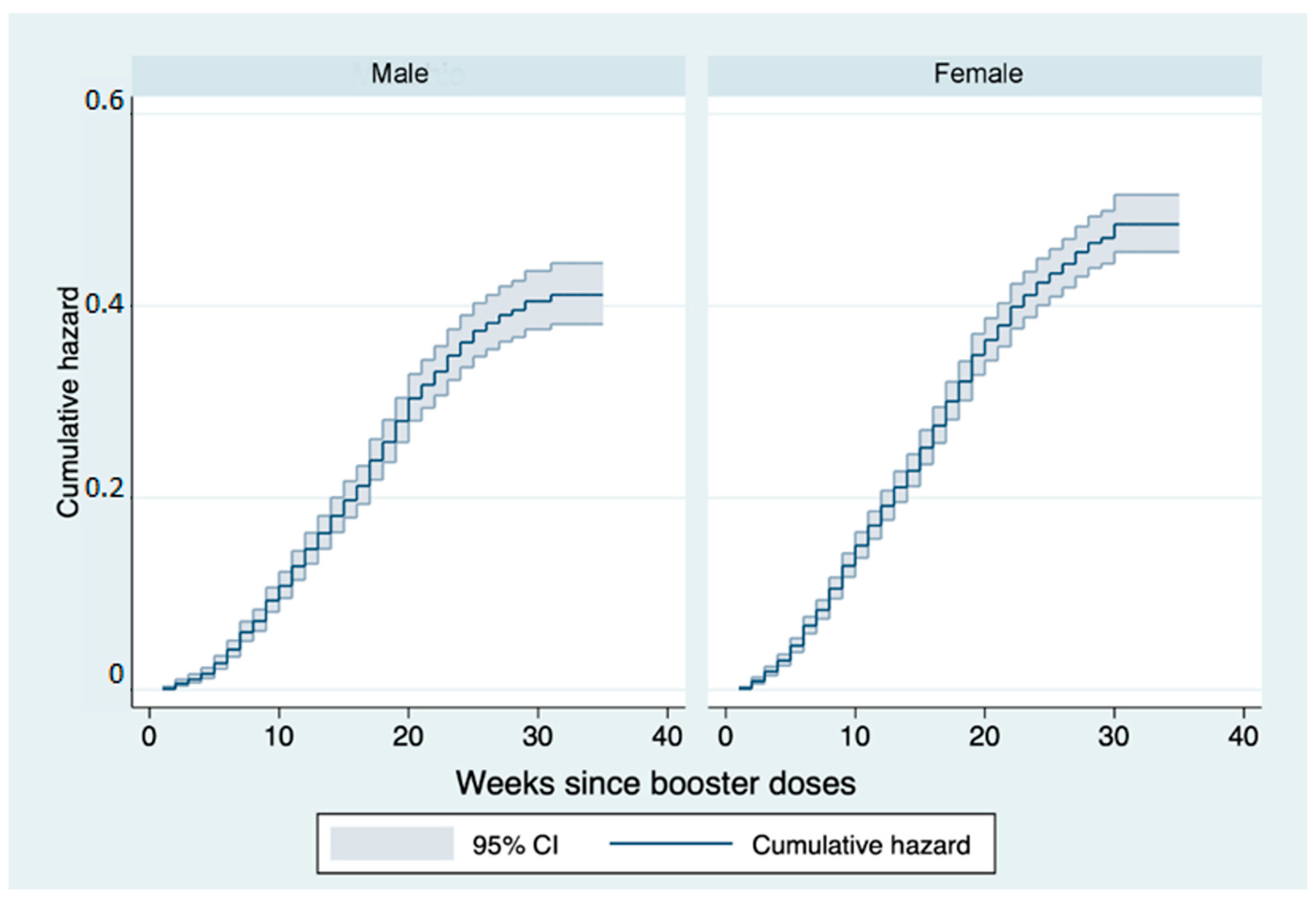
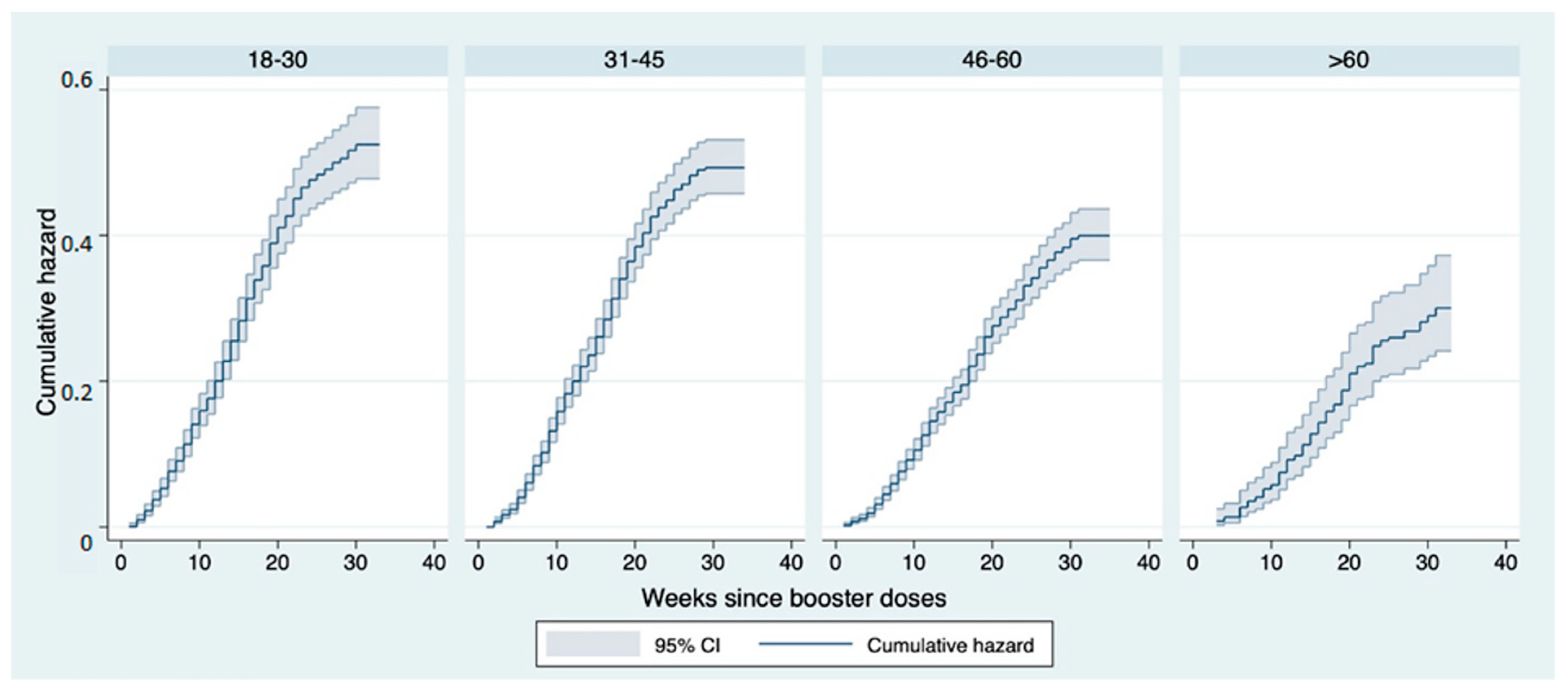
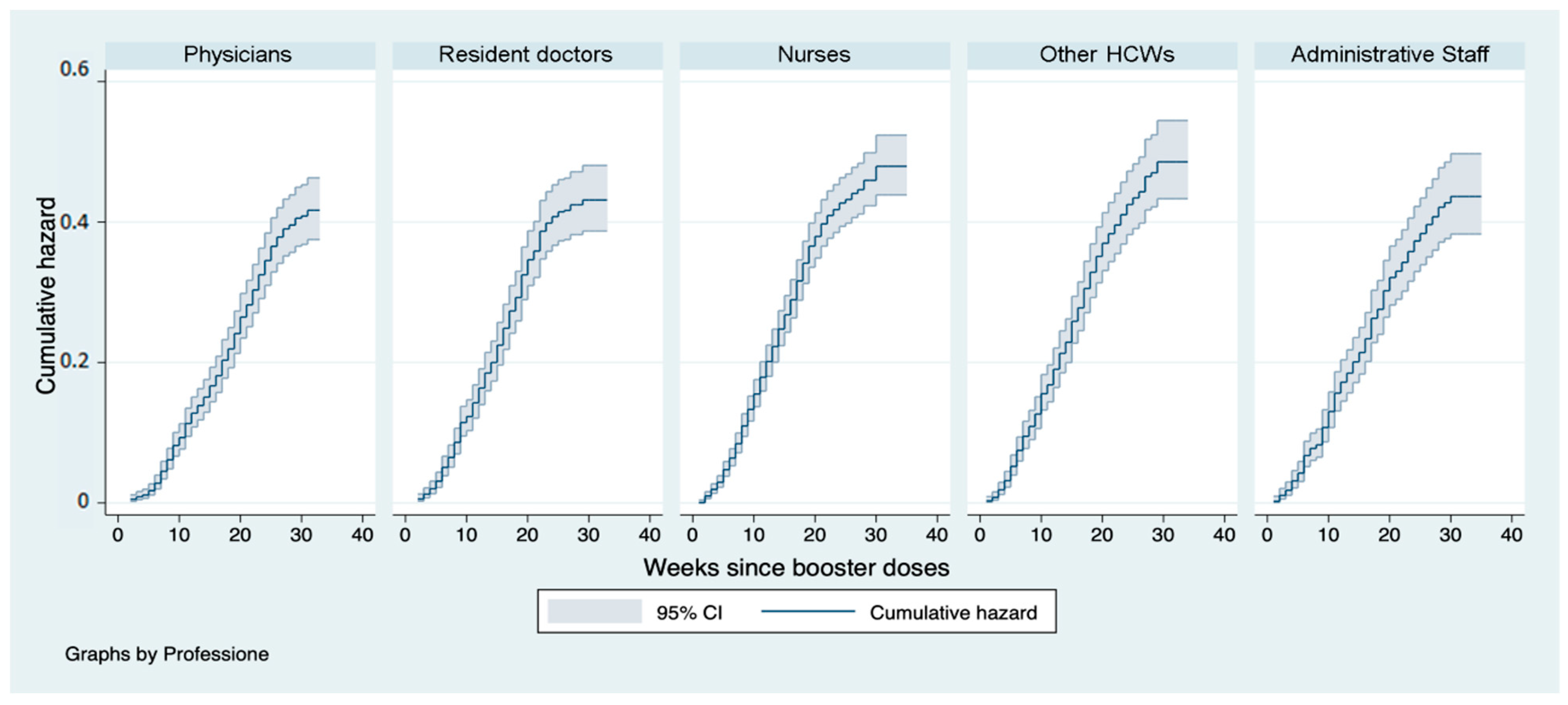
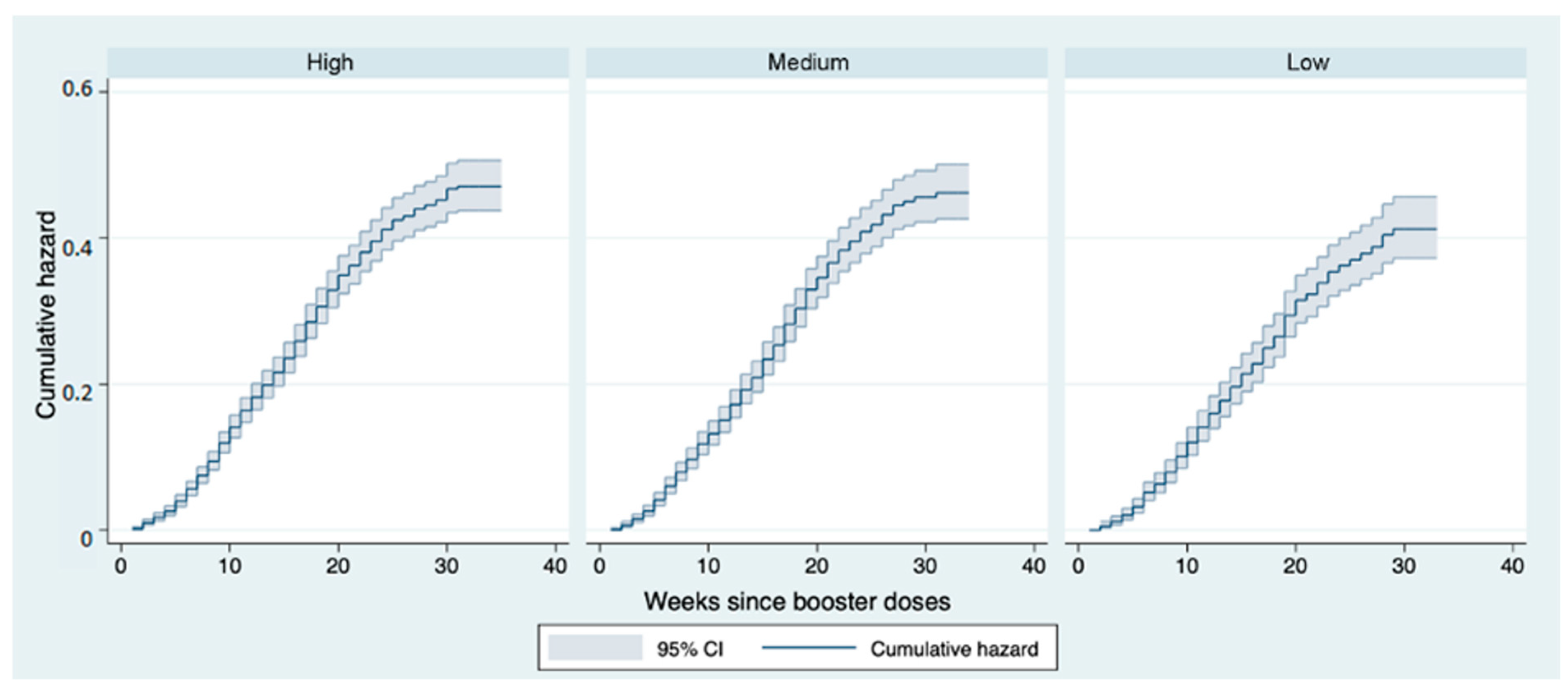
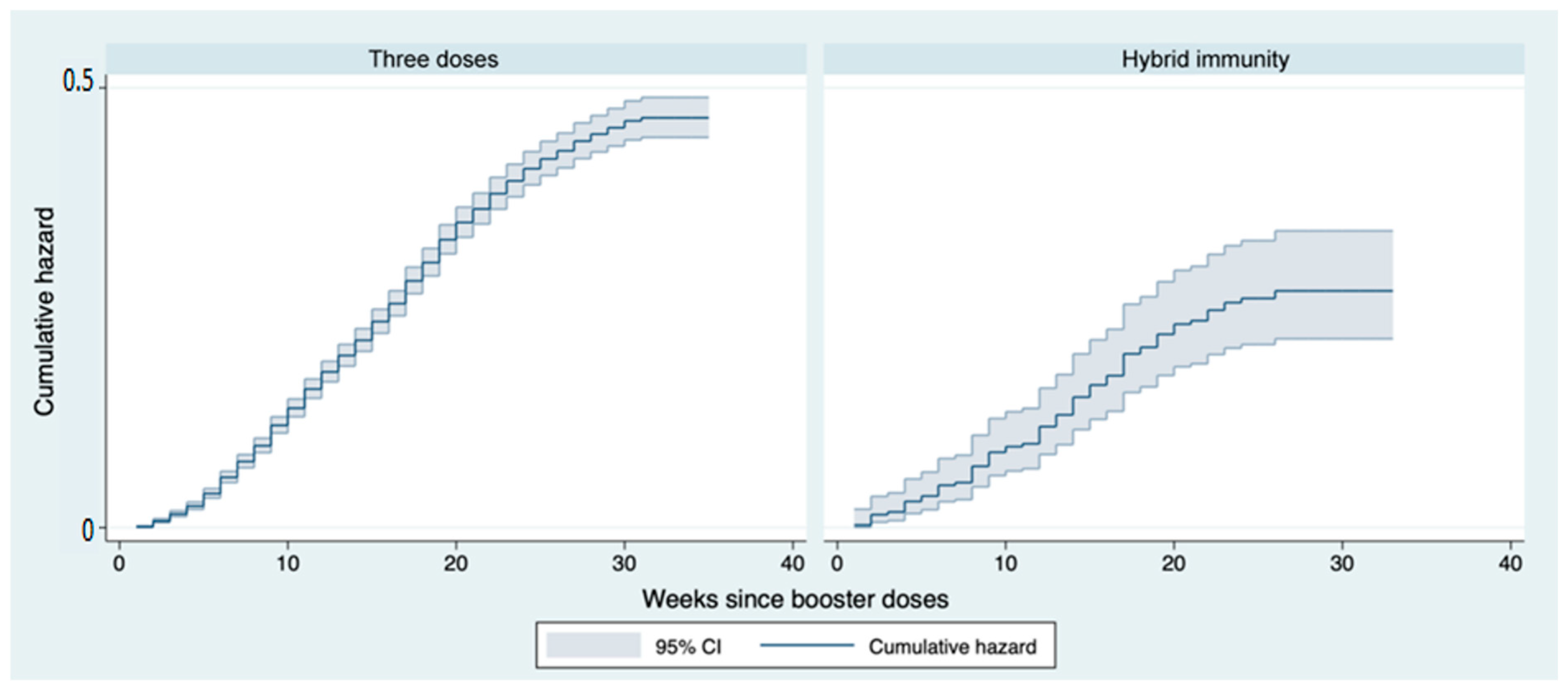
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Italian National Health Institute. COVID-19: Sorveglianza, Impatto Delle Infezioni Ed Efficacia Vaccinale; Aggiornamento Nazionale 18/05/2022. 2022. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_18-maggio-2022.pdf (accessed on 18 May 2022).
- Italian National Health Institute. Piano Nazionale Di Vaccinazione COVID-19. Available online: https://www.epicentro.iss.it/vaccini/COVID-19-piano-vaccinazione (accessed on 15 November 2022).
- Fabiani, M.; Puopolo, M.; Morciano, C.; Spuri, M.; Spila Alegiani, S.; Filia, A.; D’ancona, F.; del Manso, M.; Riccardo, F.; Tallon, M.; et al. Effectiveness of MRNA Vaccines and Waning of Protection against SARS-CoV-2 Infection and Severe COVID-19 during Predominant Circulation of the Delta Variant in Italy: Retrospective Cohort Study. BMJ 2022, 376. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the MRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Urdiales, A.; Alegiani, S.S.; Fabiani, M.; Pezzotti, P.; Filia, A.; Massari, M.; Riccardo, F.; Tallon, M.; Proietti, V.; del Manso, M.; et al. Risk of SARS-CoV-2 Infection and Subsequent Hospital Admission and Death at Different Time Intervals since First Dose of COVID-19 Vaccine Administration, Italy, 27 December 2020 to Mid-April 2021. Eur. Surveill. 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 MRNA COVID19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Angel, Y.; Spitzer, A.; Henig, O.; Saiag, E.; Sprecher, E.; Padova, H.; Ben-Ami, R. Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers. JAMA 2021, 325, 2457. [Google Scholar] [CrossRef]
- Spitzer, A.; Angel, Y.; Marudi, O.; Zeltser, D.; Saiag, E.; Goldshmidt, H.; Goldiner, I.; Stark, M.; Halutz, O.; Gamzu, R.; et al. Association of a Third Dose of BNT162b2 Vaccine With Incidence of SARS-CoV-2 Infection Among Health Care Workers in Israel. JAMA 2022, 327, 341. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Pascucci, D.; Nurchis, M.C.; Sapienza, M.; Castrini, F.; Beccia, F.; D’ambrosio, F.; Grossi, A.; Castagna, C.; Pezzullo, A.M.; Zega, M.; et al. Evaluation of the Effectiveness and Safety of the BNT162b2 COVID-19 Vaccine in the Vaccination Campaign among the Health Workers of Fondazione Policlinico Universitario Agostino Gemelli IRCCS. Int. J. Environ. Res. Public Health 2021, 18, 11098. [Google Scholar] [CrossRef]
- Nurchis, M.C.; Lontano, A.; Pascucci, D.; Sapienza, M.; Marziali, E.; Castrini, F.; Messina, R.; Regazzi, L.; Causio, F.A.; di Pilla, A.; et al. COVID-19 Vaccination Campaign among the Health Workers of Fondazione Policlinico Universitario Agostino Gemelli IRCCS: A Cost-Benefit Analysis. Int. J. Environ. Res. Public Health 2022, 19, 7848. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Zurac, S.; Vladan, C.; Dinca, O.; Constantin, C.; Neagu, M. Immunogenicity Evaluation after BNT162b2 Booster Vaccination in Healthcare Workers. Sci. Rep. 2022, 12, 12716. [Google Scholar] [CrossRef] [PubMed]
- Poukka, E.; Baum, U.; Palmu, A.A.; Lehtonen, T.O.; Salo, H.; Nohynek, H.; Leino, T. Cohort Study of COVID-19 Vaccine Effectiveness among Healthcare Workers in Finland, December 2020–October 2021. Vaccine 2022, 40, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection against COVID-19 by BNT162b2 Booster across Age Groups. N. Engl. J. Med. 2021, 385, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Wald, A. Booster Vaccination to Reduce SARS-CoV-2 Transmission and Infection. JAMA 2022, 327, 327. [Google Scholar] [CrossRef]
- Fast, H.E.; Zell, E.; Murthy, B.P.; Murthy, N.; Meng, L.; Scharf, L.G.; Black, C.L.; Shaw, L.; Chorba, T.; Harris, L.Q. Booster and Additional Primary Dose COVID-19 Vaccinations Among Adults Aged ≥65 Years—United States, 13 August 2021–19 November 2021. MMWR Morb Mortal. Wkly Rep. 2021, 70, 1735–1739. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Avvio Della Somministrazione Di Dosi “Booster” Nell’ambito Della Campagna Di Vaccinazione Anti SARS-CoV-2/COVID-19. 2021. Available online: https://fidas.it/wp/wp-content/uploads/2022/08/2021_0033643_Avvio-della-somministrazione-di-dosi-booster-nellambito-della-campagna-di-vaccinazione-anti-SARS-CoV-2-COVID-19.pdf (accessed on 18 May 2022).
- Italian Ministry of Health. Aggiornamento Delle Indicazioni Sulla Somministrazione Di Dosi Addizionali e Di Dosi «booster» Nell’ambito Della Campagna Di Vaccinazione Anti SARS-CoV-2/COVID-19. 2021. Available online: https://www.rcovid19.it/aggiornamento-delle-indicazioni-sulla-somministrazione-di-dosi-addizionali-e-booster-nellambito-della-campagna-vaccinale-anti-covid-19/ (accessed on 18 May 2022).
- Italian Ministry of Health. Aggiornamento Indicazioni Su Intervallo Temporale Tra La Somministrazione Della Dose “Booster” (Di Richiamo) e Il Completamento Del Ciclo Primario Nell’ambito Della Campagna Di Vaccinazione Anti SARS-CoV-2/COVID-19. 2021. Available online: https://www.usr.sicilia.it/index.php/tutte-le-news/5856-circolare-ministero-della-salute-riguardante-aggiornamento-indicazioni-su-intervallo-temporale-tra-la-somministrazione-della-dose-booster-di-richiamo-e-il-completamento-del-ciclo-primario-nell-ambito-della-campagna-di-vaccinazione-anti-sars-cov-2-covid-19 (accessed on 18 May 2022).
- Italian Ministry of Health. Ulteriore Estensione Della Platea Vaccinale Destinataria Della Dose Di Richiamo (“Booster”) Nell’ambito Della Campagna Di Vaccinazione Anti SARS-CoV-2/COVID-19; Gazzetta Ufficiale: Italy, 2022. [Google Scholar]
- Italian Ministry of Health. Estensione Della Platea Vaccinale Destinataria Della Seconda Dose Di Richiamo (Second Booster) Nell’ambito Della Campagna Di Vaccinazione Anti-SARS-CoV-2/COVID-19; Gazzetta Ufficiale: Italy, 2021. [Google Scholar]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of MRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639. [Google Scholar] [CrossRef]
- Debes, A.K.; Xiao, S.; Egbert, E.R.; Caturegli, P.; Sitaras, I.; Pekosz, A.; Milstone, A.M. Comparison of Total and Neutralizing SARS-CoV-2 Spike Antibodies against Omicron and Other Variants in Paired Samples after Two or Three Doses of MRNA Vaccine. medRxiv 2022. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; et al. Duration of MRNA Vaccine Protection against SARS-CoV-2 Omicron BA.1 and BA.2 Subvariants in Qatar. Nat. Commun. 2022, 13, 3082. [Google Scholar] [CrossRef]
- Cadeddu, C.; Rosano, A.; Villani, L.; Coiante, G.B.; Minicucci, I.; Pascucci, D.; de Waure, C. Planning and Organization of the COVID-19 Vaccination Campaign: An Overview of Eight European Countries. Vaccines 2022, 10, 1631. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Overview of the Implementation of COVID-19 Vaccination Strategies and Deployment Plans in the EU/EEA. 21 April 2022. Stockholm: ECDC. 2022. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Overview-of-the-implementation-of-COVID-19-vaccination-strategies-and-deployment-plans-in-the-EU-EEA-April-2022.pdf (accessed on 18 May 2022).
- Seale, H.; Dyer, C.E.F.; Abdi, I.; Rahman, K.M.; Sun, Y.; Qureshi, M.O.; Dowell-Day, A.; Sward, J.; Islam, M.S. Improving the Impact of Non-Pharmaceutical Interventions during COVID-19: Examining the Factors That Influence Engagement and the Impact on Individuals. BMC Infect Dis. 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Decreto-Legge 26 Novembre 2021, n. 172—Misure Urgenti per Il Contenimento Dell’epidemia Da COVID-19 e per Lo Svolgimento in Sicurezza Delle Attività Economiche e Sociali. 2021. Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=84133&completo=true (accessed on 18 May 2022).
- Decreto Del Presidente Del Consiglio Dei Ministri 02/03/2022—Aggiornamento Delle Modalità Di Verifica Dell’obbligo Vaccinale e Del Green Pass; Gazzetta Ufficiale: Italy, 2022; Available online: https://www.asseprim.it/web/news-contenuti/news-per-imprese/contratti-lavoro/Aggiornamento-delle-modalita-di-verifica-dellobbligo-vaccinale-e-del-green-pass/ (accessed on 18 May 2022).
- Italian Ministry of Health. Aggiornamento Sull’uso Dei Test Antigenici e Molecolari per La Rilevazione Di SARS-CoV-2. 2021. Available online: https://www.quotidianosanita.it/allegati/allegato2672387.pdf (accessed on 18 May 2022).
- Italian Ministry of Health. Circolare Ministero Della Salute 32884-21/07/2021—Aggiornamento Indicazioni Sulla Vaccinazione Dei Soggetti Che Hanno Avuto Un’infezione Da SARS-CoV-2; Gazzetta Ufficiale: Italy, 2021. [Google Scholar]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 Reinfection and COVID-19 Hospitalisation in Individuals with Natural and Hybrid Immunity: A Retrospective, Total Population Cohort Study in Sweden. Lancet Infect Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.M. COVID Vaccine plus Infection Can Lead to Months of Immunity. Nature 2022. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Hybrid Immunity. Science (1979) 2021, 372, 1392–1393. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.-C.; Yazici, S.; Christa, C.; et al. Three Exposures to the Spike Protein of SARS-CoV-2 by Either Infection or Vaccination Elicit Superior Neutralizing Immunity to All Variants of Concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Urbanowicz, R.A.; Tsoleridis, T.; Jackson, H.J.; Cusin, L.; Duncan, J.D.; Chappell, J.G.; Tarr, A.W.; Nightingale, J.; Norrish, A.R.; Ikram, A.; et al. Two Doses of the SARS-CoV-2 BNT162b2 Vaccine Enhance Antibody Responses to Variants in Individuals with Prior SARS-CoV-2 Infection. Sci. Transl. Med. 2021, 13, eabj0847. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef]
- Ferrara, P.; Ponticelli, D.; Magliuolo, R.; Borrelli, M.; Schiavone, B.; Mantovani, L.G. Time-Varying Effect of Hybrid Immunity on the Risk of Breakthrough Infection after Booster Dose of MRNA COVID-19 Vaccine: The MOSAICO Study. Vaccines 2022, 10, 1353. [Google Scholar] [CrossRef]
- Italian National Health Institute Gender Differences in COVID-19: The Importance of Sex-Disaggregated Data. Available online: https://www.epicentro.iss.it/en/coronavirus/SARS-CoV-2-gender-differences-importance-sex-disaggregated-data (accessed on 15 November 2022).
- Doerre, A.; Doblhammer, G. The Influence of Gender on COVID-19 Infections and Mortality in Germany: Insights from Age- and Gender-Specific Modeling of Contact Rates, Infections, and Deaths in the Early Phase of the Pandemic. PLoS ONE 2022, 17, e0268119. [Google Scholar] [CrossRef]
- Modenese, A.; Casolari, L.; Rossi, G.; della Vecchia, E.; Glieca, F.; D’Elia, C.; Garavini, D.; Righi, E.; Mariani, S.; Venturelli, L.; et al. Factors Associated with SARS-CoV-2 Infection Risk among Healthcare Workers of an Italian University Hospital. Healthcare 2021, 9, 1495. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and Mucosal IgA Responses Are Variably Induced in Response to SARS-CoV-2 MRNA Vaccination and Are Associated with Protection against Subsequent Infection. Mucosal. Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Perlman, S. Age-Related Susceptibility to Coronavirus Infections: Role of Impaired and Dysregulated Host Immunity. J. Clin. Invest. 2020, 130, 6204–6213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, Y.; Tan, G.; Dong, X.; Wang, B.; Lin, J.; Yan, Y.; Liu, G.; Akdis, M.; Akdis, C.A.; et al. Clinical, Radiological, and Laboratory Characteristics and Risk Factors for Severity and Mortality of 289 Hospitalized COVID-19 Patients. Allergy 2021, 76, 533–550. [Google Scholar] [CrossRef] [PubMed]
| Occupational Risk | Departments | Frequency of Collection of Swabs for SARS-CoV-2 (Days) |
|---|---|---|
| High | Emergency room sectors (general, pediatric, obstetrics); Organisational units; services and operative units (emergency department, radiodiagnostics, surgical digestive endoscopy service, operating theatres, resuscitation, intensive care, emergency medicine, emergency surgery, track units, etc.); COVID in-patient units. | 14 |
| Moderate | All the in-patient units and the staff working in them (haematology, radiotherapy, gynecological oncology, general liver transplant surgery, child neuropsychiatry, digestive surgery endocrine surgery). | 21 |
| Low | Paying wards; outpatient clinics; DH; services not included in the previous points; administrative functions. | 35 |
| Variables | N (%) |
|---|---|
| Age | 42 (21) * |
| Sex | |
| M | 2307 (40) |
| F | 3463 (60) |
| Age class | |
| 18–30 | 1404 (25) |
| 31–45 | 1976 (34) |
| 46–60 | 2017 (35) |
| >60 | 373 (6) |
| Professional category | |
| Physicians | 1151 (20) |
| Resident doctors | 1045 (18) |
| Nurses | 1717 (30) |
| Other HCWs ** | 1018 (18) |
| Administrative staff | 839 (14) |
| Occupational risk | |
| High | 2408 (42) |
| Moderate | 2003 (35) |
| Low | 1359 (23) |
| Infection | |
| Before primary schedule (hybrid immunity) | 326 (6) |
| After booster dose | 1994 (35) |
| Haz. Ratio | P > z | IC 95% | |
|---|---|---|---|
| Sex | |||
| M | Ref. | ||
| F | 1.12 | 0.012 | (1.02–1.24) |
| Age class | |||
| 18–30 | Ref. | ||
| 31–45 | 0.86 | 0.034 | (0.76–0.98) |
| 46–60 | 0.63 | 0.001 | (0.55–0.72) |
| >60 | 0.50 | 0.001 | (0.39–0.64) |
| Professional category | |||
| Physicians | Ref. | ||
| Resident doctors | 0.82 | 0.069 | (0.68–1.02) |
| Nurses | 1.08 | 0.283 | (0.93–1.24) |
| Other HCWs | 1.15 | 0.058 | (0.99–1.34) |
| Administrative staff | 1.16 | 0.118 | (0.96–1.40) |
| Occupational risk | |||
| High | Ref. | ||
| Moderate | 1.00 | 0.862 | (0.90–1.12) |
| Low | 0.91 | 0.235 | (0.79–1.05) |
| Immunity | |||
| 3 doses | Ref. | ||
| Hybrid | 0.59 | 0.001 | (0.47–0.74) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascucci, D.; Grossi, A.; Lontano, A.; Marziali, E.; Nurchis, M.C.; Grassi, V.M.; Raponi, M.; Vetrugno, G.; Capelli, G.; Calabrò, G.E.; et al. Risk of Infection and Duration of Protection after the Booster Dose of the Anti-SARS-CoV-2 Vaccine BNT162b2 among Healthcare Workers in a Large Teaching Hospital in Italy: Results of an Observational Study. Vaccines 2023, 11, 25. https://doi.org/10.3390/vaccines11010025
Pascucci D, Grossi A, Lontano A, Marziali E, Nurchis MC, Grassi VM, Raponi M, Vetrugno G, Capelli G, Calabrò GE, et al. Risk of Infection and Duration of Protection after the Booster Dose of the Anti-SARS-CoV-2 Vaccine BNT162b2 among Healthcare Workers in a Large Teaching Hospital in Italy: Results of an Observational Study. Vaccines. 2023; 11(1):25. https://doi.org/10.3390/vaccines11010025
Chicago/Turabian StylePascucci, Domenico, Adriano Grossi, Alberto Lontano, Eleonora Marziali, Mario Cesare Nurchis, Vincenzo Maria Grassi, Matteo Raponi, Giuseppe Vetrugno, Giovanni Capelli, Giovanna Elisa Calabrò, and et al. 2023. "Risk of Infection and Duration of Protection after the Booster Dose of the Anti-SARS-CoV-2 Vaccine BNT162b2 among Healthcare Workers in a Large Teaching Hospital in Italy: Results of an Observational Study" Vaccines 11, no. 1: 25. https://doi.org/10.3390/vaccines11010025
APA StylePascucci, D., Grossi, A., Lontano, A., Marziali, E., Nurchis, M. C., Grassi, V. M., Raponi, M., Vetrugno, G., Capelli, G., Calabrò, G. E., Staiti, D., Sanguinetti, M., Damiani, G., & Laurenti, P. (2023). Risk of Infection and Duration of Protection after the Booster Dose of the Anti-SARS-CoV-2 Vaccine BNT162b2 among Healthcare Workers in a Large Teaching Hospital in Italy: Results of an Observational Study. Vaccines, 11(1), 25. https://doi.org/10.3390/vaccines11010025








