Understanding the Barriers and Attitudes toward Influenza Vaccine Uptake in the Adult General Population: A Rapid Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Eligibility Criteria
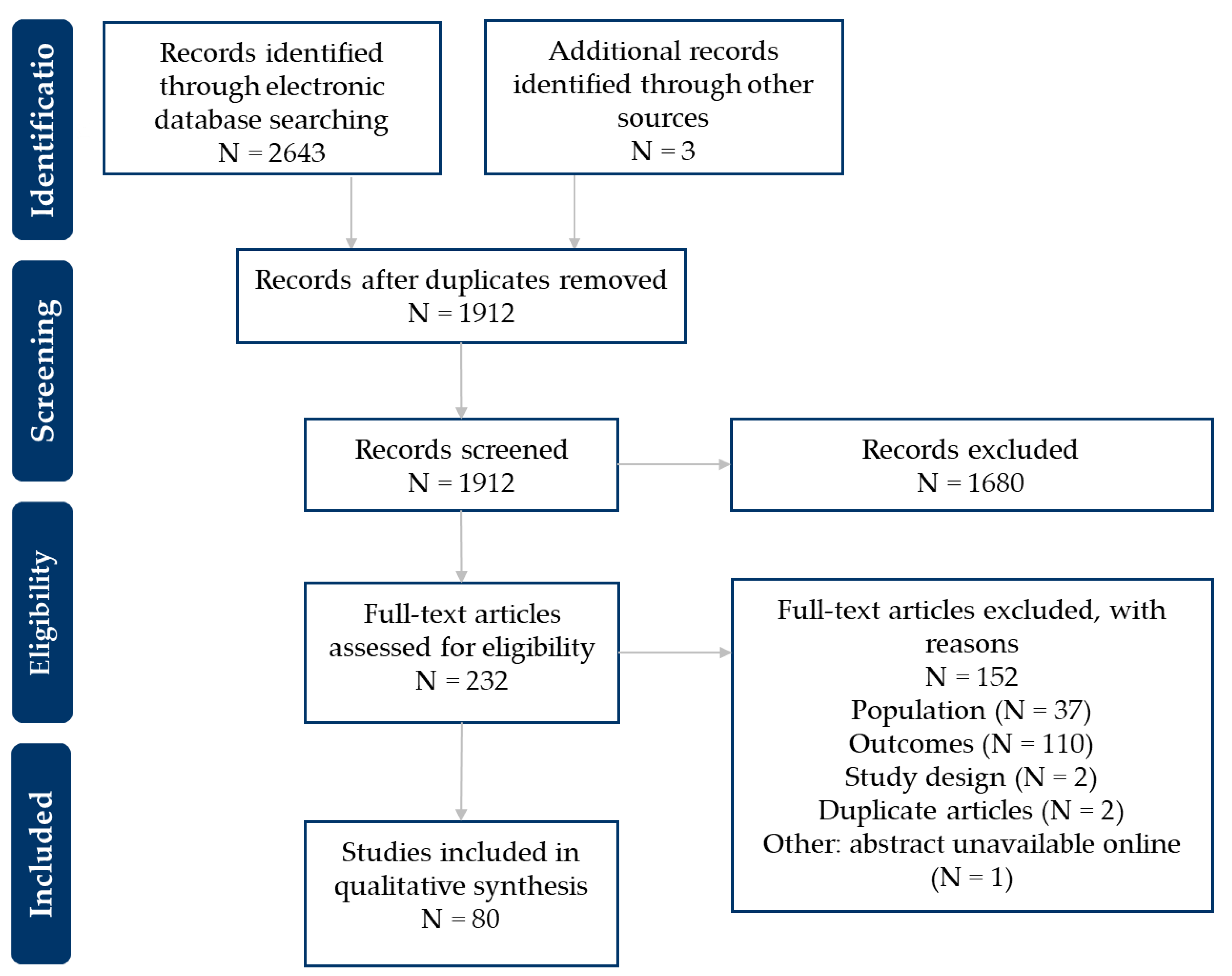
2.3. Study Selection and Data Extraction
3. Results
3.1. Summary of Results
3.2. Study Characteristics
3.3. Patient Characteristics
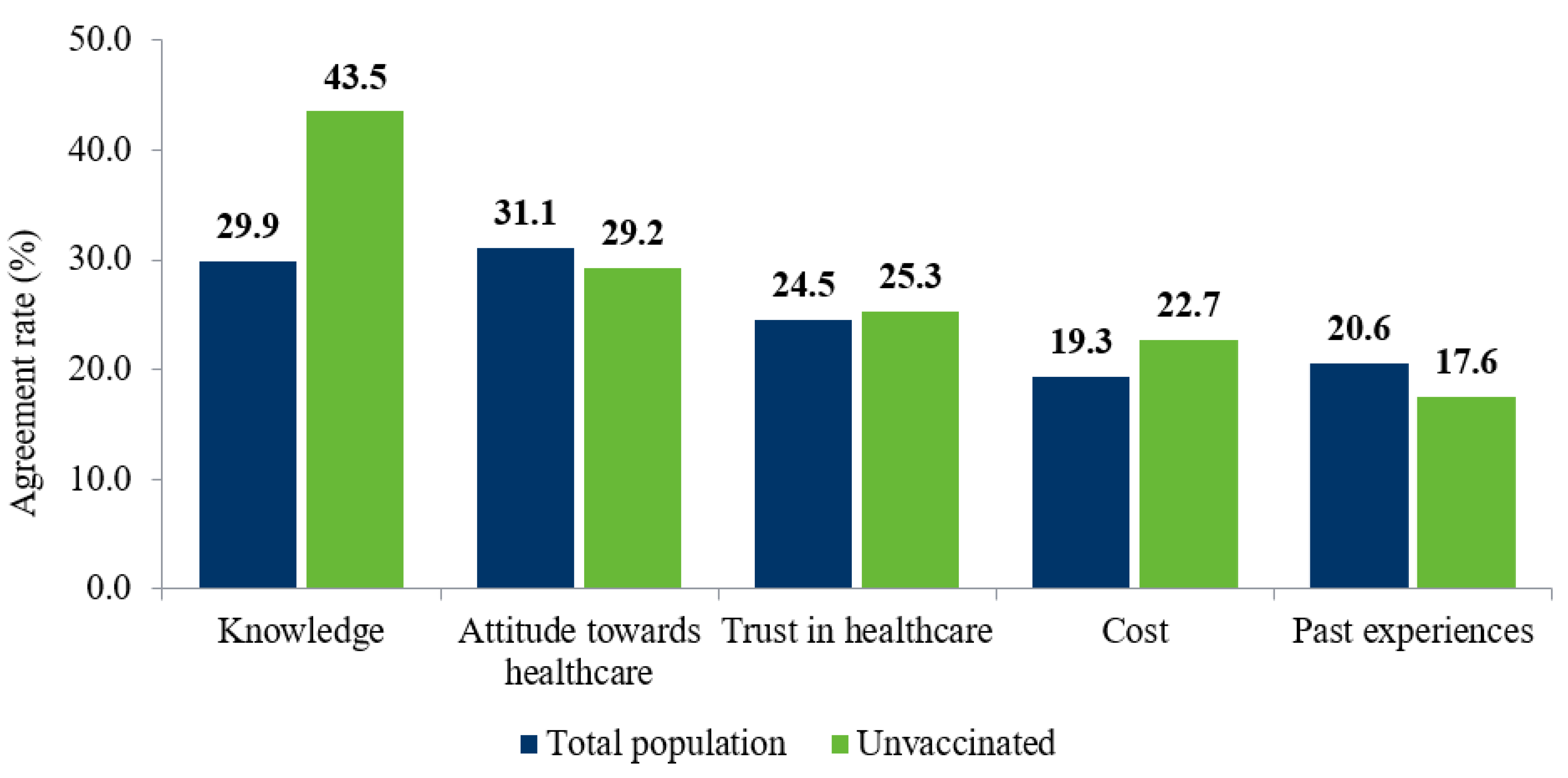
3.4. Barriers to Vaccination
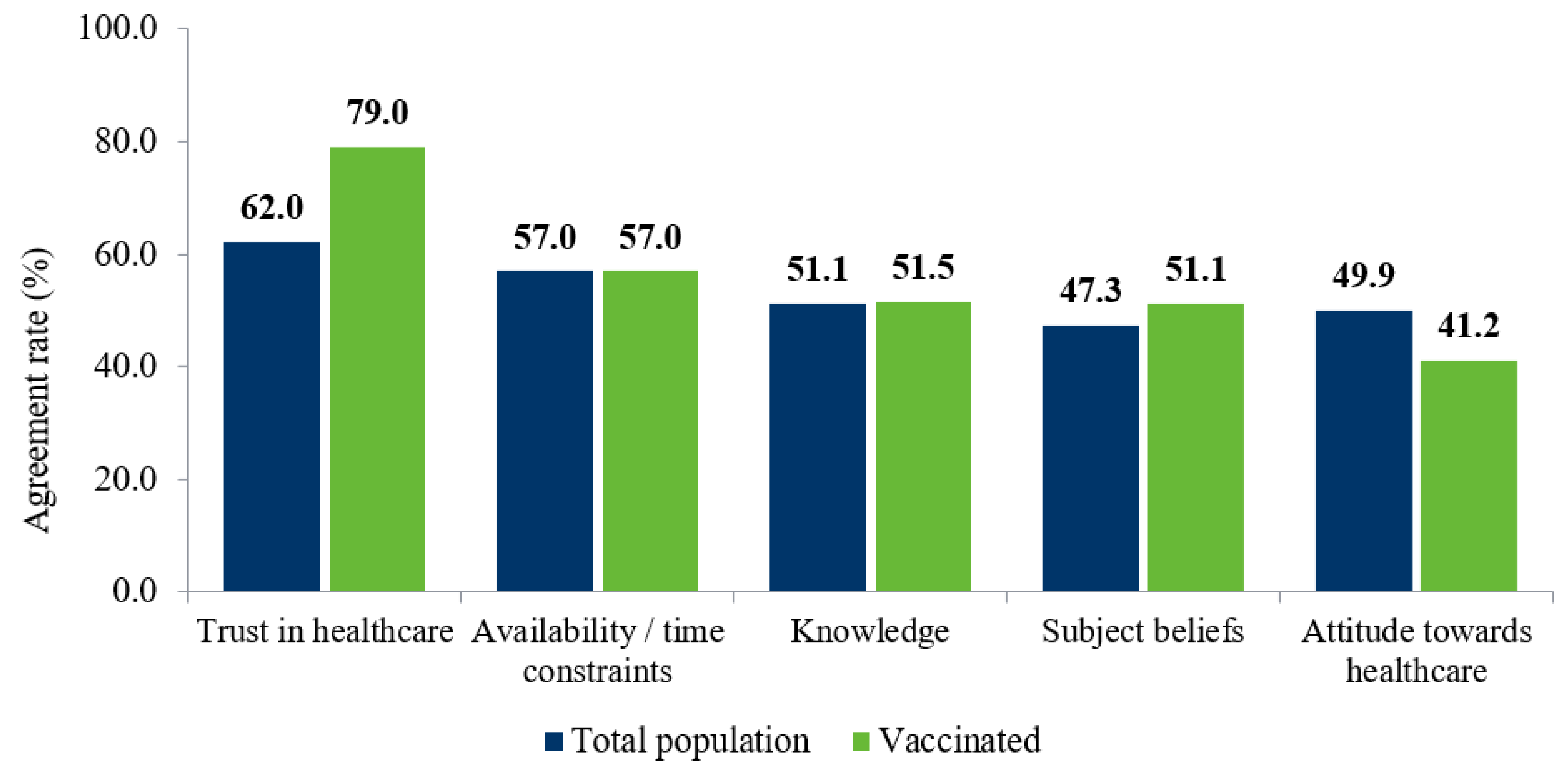
3.5. Promoters of Vaccination
3.6. Intent to Vaccinate
3.7. Strategies to Overcome Barriers
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, K.A.; Ostrowsky, J.T.; Kraigsley, A.M.; Mehr, A.J.; Bresee, J.S.; Friede, M.H.; Gellin, B.G.; Golding, J.P.; Hart, P.J.; Moen, A.; et al. A Research and Development (R&D) roadmap for influenza vaccines: Looking toward the future. Vaccine 2021, 39, 6573–6584. [Google Scholar] [PubMed]
- WHO. Global Influenza Strategy 2019–2030. Available online: https://apps.who.int/iris/bitstream/handle/10665/311184/9789241515320-eng.pdf?sequence=18&isAllowed=ycoll (accessed on 8 September 2022).
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Prim. 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Macias, A.E.; McElhaney, J.E.; Chaves, S.S.; Nealon, J.; Nunes, M.C.; Samson, S.I.; Seet, B.T.; Weinke, T.; Yu, H. The disease burden of influenza beyond respiratory illness. Vaccine 2021, 39, A6–A14. [Google Scholar] [CrossRef] [PubMed]
- Honce, R.; Schultz-Cherry, S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- World Health Organization. Influenza. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 9 August 2022).
- Centers for Disease Control and Prevention. Who Should and Who Should NOT Get a Flu Vaccine. Available online: https://www.cdc.gov/flu/prevent/whoshouldvax.htm#:~:text=All%20persons%20aged%206%20months,of%20dveloing%20serious%20flu%20complications.&text=People%20who%20can%20get%20the,are%20appropriate%20for%20most%20people. (accessed on 9 August 2022).
- World Health Organisation. Influenza Vaccination Coverage. Available online: https://immunizationdata.who.int/pages/coveage/flu.html?CODE=USA+GBR+FRA+ESP+DEU+ITA+CHN+BRA+CAN+MEX+JPN+SAU+ZAF+AUS&ANTIGEN=FLU_ALL&YEAR= (accessed on 9 August 2022).
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.L. Barriers of influenza vaccination intention and behavior–a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar] [CrossRef]
- Lindley, M.C.; Wortley, P.M.; Winston, C.A.; Bardenheier, B.H. The role of attitudes in understanding disparities in adult influenza vaccination. Am. J. Prev. Med. 2006, 31, 281–285. [Google Scholar] [CrossRef]
- Brewer, L.I.; Ommerborn, M.J.; Nguyen, A.L.; Clark, C.R. Structural inequities in seasonal influenza vaccination rates. BMC Public Health 2021, 21, 1166. [Google Scholar] [CrossRef]
- Mahmud, S.M.; Xu, L.; Hall, L.L.; Puckrein, G.; Thommes, E.; Loiacono, M.M.; Chit, A. Effect of race and ethnicity on influenza vaccine uptake among older US Medicare beneficiaries: A record-linkage cohort study. Lancet Healthy Longev. 2021, 2, e143–e153. [Google Scholar] [CrossRef]
- Garritty, C.; Gartlehner, G.; Nussbaumer-Streit, B.; King, V.J.; Hamel, C.; Kamel, C.; Affengruber, L.; Stevens, A. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J. Clin. 2021, 130, 13–22. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020. [Google Scholar]
- De Perio, M.A.; Wiegand, D.M.; Brueck, S.E. Influenza vaccination coverage among school employees: Assessing knowledge, attitudes, and behaviors. J. Sch. Health 2014, 84, 586–592. [Google Scholar] [CrossRef]
- Alzeer, A.A.; Alfantoukh, L.A.; Theneyan, A.; Bin Eid, F.; Almangour, T.A.; Alshememry, A.K.; Alhossan, A.M. The influence of demographics on influenza vaccine awareness and hesitancy among adults visiting educational hospital in Saudi Arabia. Saudi Pharm. J. 2021, 29, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.A.; Aljammaz, K.I.; Alrashed, A.A. Knowledge, Attitude, and Barriers Influencing Seasonal Influenza Vaccination Uptake. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 7653745. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, M.; Kotera, Y.; Tsuda, K.; Takahashi, K.; Hamaki, T.; Kusumi, E.; Kami, M.; Tanimoto, T. Influenza vaccination uptake and attitudes among adult cancer patients in Japan: A web-based questionnaire survey before the 2020/2021 season. Hum. Vaccines Immunother. 2021, 17, 5509–5513. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Watanabe, T. Changes in Vaccine Hesitancy in Japan across Five Months during the COVID-19 Pandemic and Its Related Factors. Vaccines 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.P.; Ng, S.K.; Tong, E.T.; Chan, S.S.; Coker, R. Factors associated with uptake of influenza vaccine in people aged 50 to 64 years in Hong Kong: A case-control study. BMC Public Health 2015, 15, 617. [Google Scholar] [CrossRef]
- Iwasa, T.; Wada, K. Reasons for and against receiving influenza vaccination in a working age population in Japan: A national cross-sectional study. BMC Public Health 2013, 13, 647. [Google Scholar]
- Yan, S.; Wang, Y.; Zhu, W.; Zhang, L.; Gu, H.; Liu, D.; Zhu, A.; Xu, H.; Hao, L.; Ye, C.; et al. Barriers to influenza vaccination among different populations in Shanghai. Hum. Vaccines Immunother. 2021, 17, 1403–1411. [Google Scholar] [CrossRef]
- Bertoldo, G.; Pesce, A.; Pepe, A.; Pelullo, C.P.; Di Giuseppe, G.; Group, C.W. Seasonal influenza: Knowledge, attitude and vaccine uptake among adults with chronic conditions in Italy. PLoS ONE 2019, 14, e0215978. [Google Scholar] [CrossRef]
- Genovese, C.; Costantino, C.; Odone, A.; Trimarchi, G.; La Fauci, V.; Mazzitelli, F.; D’Amato, S.; Squeri, R.; The Covid-Risk Perception Group. A Knowledge, Attitude, and Perception Study on Flu and COVID-19 Vaccination during the COVID-19 Pandemic: Multicentric Italian Survey Insights. Vaccines 2022, 10, 142. [Google Scholar] [CrossRef]
- Hidalgo, M.L.S.; Abbamonte, J.M.; Regalini, L.; Schlesinger, M.; Alcaide, M.L.; Dickinson, G.M. 1650. Knowledge and Attitudes Toward Influenza Vaccination Among Hispanics: A Survey Conducted in Latin American Consulates in South Florida. Open Forum Infect. Dis. 2019, 6, S603. [Google Scholar] [CrossRef]
- Alabbad, A.A.; Alsaad, A.K.; Al Shaalan, M.A.; Alola, S.; Albanyan, E.A. Prevalence of influenza vaccine hesitancy at a tertiary care hospital in Riyadh, Saudi Arabia. J. Infect. Public Health 2018, 11, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, A.R.; Law, A.V. Will they, or won’t they? Examining patients’ vaccine intention for flu and COVID-19 using the Health Belief Model. Res. Soc. Adm. Pharm. 2021, 17, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Aljamili, A.A. Knowledge and practice toward seasonal influenza vaccine and its barriers at the community level in Riyadh, Saudi Arabia. J. Fam. Med. Prim. Care 2020, 9, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Valerio, V.; Hudson, M.; Wang, M.; Bernatsky, S.; Hazel, E.M.; Ward, B.; Colmegna, I. Influenza Vaccine Hesitancy and Its Determinants Among Rheumatology Patients. ACR Open Rheumatol. 2022, 4, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Luz, P.M.; Johnson, R.E.; Brown, H.E. Workplace availability, risk group and perceived barriers predictive of 2016–17 influenza vaccine uptake in the United States: A cross-sectional study. Vaccine 2017, 35, 5890–5896. [Google Scholar] [CrossRef]
- Roy, M.; Sherrard, L.; Dubé, È.; Gilbert, N.L. Determinants of non-vaccination against seasonal influenza. Health Rep. 2018, 29, 12–22. [Google Scholar]
- Feng, W.; Cui, J.; Li, H. Determinants of Willingness of Patients with Type 2 Diabetes Mellitus to Receive the Seasonal Influenza Vaccine in Southeast China. Int. J. Environ. Res. Public Health 2019, 16, 2203. [Google Scholar] [CrossRef]
- Cohen, V.; Jellinek-Cohen, S.P.; Likourezos, A.; Lum, D.; Zimmerman, D.E.; Willner, M.A.; Rose, J.; Marshall, J.P. Feasibility of a pharmacy-based influenza immunization program in an academic emergency department. Ann. Pharmacother. 2013, 47, 1440–1447. [Google Scholar] [CrossRef]
- Almotairy, A.M.; Sheikh, W.A.; Joraid, A.A.A.; Bajwi, A.A.; Alharbi, M.S.F.; Al-Dubai, S.A.R. Association between knowledge of influenza vaccine and vaccination status among general population attending primary health care centers in Al-Madinah, Saudi Arabia. J. Fam. Med. Prim. Care 2019, 8, 2971–2974. [Google Scholar] [CrossRef]
- Domnich, A.; Grassi, R.; Fallani, E.; Spurio, A.; Bruzzone, B.; Panatto, D.; Marozzi, B.; Cambiaggi, M.; Vasco, A.; Orsi, A.; et al. Changes in Attitudes and Beliefs Concerning Vaccination and Influenza Vaccines between the First and Second COVID-19 Pandemic Waves: A Longitudinal Study. Vaccines 2021, 9, 1016. [Google Scholar] [CrossRef]
- Trent, M.J.; Salmon, D.A.; MacIntyre, C.R. Using the health belief model to identify barriers to seasonal influenza vaccination among Australian adults in 2019. Influenza Other Respir. Viruses 2021, 15, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.D.; Akingbola, O.; Anderson, J.; Hart, J.; Chapple, A.; Woods, C.; Yeary, K.; McLean, A. Combatting a “Twin-demic”: A quantitative assessment of COVID-19 and influenza vaccine hesitancy in primary care patients. Health Promot. Perspect. 2021, 11, 179–185. [Google Scholar] [CrossRef]
- Gilstad-Hayden, K.; Durante, A.; Earnshaw, V.A.; Rosenthal, L.; Ickovics, J.R. Association of influenza vaccine uptake with health, access to health care, and medical mistreatment among adults from low-income neighborhoods in New Haven, CT: A classification tree analysis. Prev. Med. 2015, 74, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Crouse Quinn, S.; Jamison, A.M.; Freimuth, V.S.; An, J.; Hancock, G.R. Determinants of influenza vaccination among high-risk Black and White adults. Vaccine 2017, 35, 7154–7159. [Google Scholar] [CrossRef]
- Bauer, K.A.; Johnson, K.; Stephenson, J.J.; Visaria, J.; Chung, H.; York, W.; Kern, D.M.; Puzniak, L.A. Rate of preventative vaccine use and vaccine beliefs among a commercially insured population. Vaccine 2020, 38, 7087–7093. [Google Scholar] [CrossRef] [PubMed]
- Hilger, K.A.; Hilger, J.R.; Putnam, S.D.; Carstairs, S.D.; Maves, R.C. Feasibility and Patient Acceptance of Emergency Department-Based Influenza Vaccination in a Military Medical Center. Mil. Med. 2016, 181, 883–886. [Google Scholar] [CrossRef]
- Luz, P.M.; Brown, H.E.; Struchiner, C.J. Disgust as an emotional driver of vaccine attitudes and uptake? A mediation analysis. Epidemiol. Infect. 2019, 147, e182. [Google Scholar] [CrossRef]
- Cotugno, S.; Morrow, G.; Cooper, C.; Cabie, M.; Cohn, S. Impact of pharmacist intervention on influenza vaccine assessment and documentation in hospitalized psychiatric patients. Am. J. Health Syst. Pharm. 2017, 74, S90–S94. [Google Scholar] [CrossRef]
- Benjamin, S.M.; Bahr, K.O. Barriers Associated with Seasonal Influenza Vaccination among College Students. Influenza Res. Treat. 2016, 2016, 4248071. [Google Scholar] [CrossRef]
- Lytle, K.L.; Collins, S.P.; Feldstein, L.R.; Baughman, A.H.; Brown, S.M.; Casey, J.D.; Erickson, H.L.; Exline, M.C.; Files, D.C.; Gibbs, K.W.; et al. Influenza vaccine acceptance and hesitancy among adults hospitalized with severe acute respiratory illnesses, United States 2019–2020. Vaccine 2021, 39, 5271–5276. [Google Scholar] [CrossRef]
- Sandler, D.S.; Ruderman, E.M.; Brown, T.; Lee, J.Y.; Mixon, A.; Liss, D.T.; Baker, D.W. Understanding vaccination rates and attitudes among patients with rheumatoid arthritis. Am. J. Manag. Care 2016, 22, 161–167. [Google Scholar] [PubMed]
- Murray, A.R.; Caron, R.M. Influenza vaccination challenges in an at-risk student population: Considerations for health services. Int. J. Adolesc. Med. Health 2016, 30, 20160093. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Gupta, V.; Unni, E.J. Utilizing the Theory of Planned Behavior to determine the intentions to receive the influenza vaccine during COVID-19: A cross-sectional survey of US adults. Prev. Med. Rep. 2021, 23, 101417. [Google Scholar] [CrossRef]
- Quinn, S.; Jamison, A.; Musa, D.; Hilyard, K.; Freimuth, V. Exploring the Continuum of Vaccine Hesitancy Between African American and White Adults: Results of a Qualitative Study. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Gidengil, C.A.; Parker, A.M.; Zikmund-Fisher, B.J. Trends in risk perceptions and vaccination intentions: A longitudinal study of the first year of the H1N1 pandemic. Am. J. Public Health 2012, 102, 672–679. [Google Scholar] [CrossRef]
- Chen, G.; Kazmi, M.; Chen, D.; Phillips, J. Identifying associations between influenza vaccination status and access, beliefs, and sociodemographic factors among the uninsured population in Suffolk County, NY. J. Community Health 2020, 45, 1236–1241. [Google Scholar] [CrossRef]
- Mongeau, P.A.; Liu, Y.; Hashi, E.C.; Roberto, A.J. College students’ influenza vaccine hesitation: A reasoned action investigation with quantitative and qualitative data. J. Behav. Med. 2022, 45, 1236–1241. [Google Scholar] [CrossRef]
- Nunley, L.; Swanson, K.; Szabo, S.; Drees, M. The Flu Vaccine: Why Are Our Human Immunodeficiency Virus (HIV)-Infected Patients Saying No? Open Forum Infect. Dis. 2016, 3, 741. [Google Scholar] [CrossRef]
- Al Hassan, Y.T.; Fabella, E.L.; Estrella, E.D.; Al Ramadan, H.A.; Al Rajeh, A.M.; Al Saleh, F.H. Association of vaccine awareness and confidence on the influenza vaccination status of Al Ahsa, Saudi Arabia residents. Hum. Vaccines Immunother. 2021, 17, 2190–2196. [Google Scholar] [CrossRef]
- Alhawsawi, M.M.; Alghamdi, A.A.; Alzayed, B.M.; Binmugren, H.M.; Alshehri, R.A.; Abusalih, H.H. Knowledge, barriers and uptake of influenza vaccine among non-health college students at Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia. J. Public Health Res. 2020, 9, 1856. [Google Scholar] [CrossRef] [PubMed]
- Blank, P.R.; Bonnelye, G.; Ducastel, A.; Szucs, T.D. Attitudes of the general public and general practitioners in five countries towards pandemic and seasonal influenza vaccines during season 2009/2010. PLoS ONE 2012, 7, e45450. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, M.M.; Walter, D.; Falkenhorst, G.; Müters, S.; Krause, G.; Wichmann, O. Barriers to pandemic influenza vaccination and uptake of seasonal influenza vaccine in the post-pandemic season in Germany. BMC Public Health 2012, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- Casalino, E.; Ghazali, A.; Bouzid, D.; Antoniol, S.; Pereira, L.; Kenway, P.; Choquet, C. Emergency Department study group on respiratory, v. Patient’s behaviors and missed opportunities for vaccination against seasonal epidemic influenza and evaluation of their impact on patient’s influenza vaccine uptake. PLoS ONE 2018, 13, e0193029. [Google Scholar] [CrossRef]
- Dallagiacoma, G.; Allora, A.; Salvati, S.; Cocciolo, G.; Capraro, M.; Lamberti, A.; Senatore, S.; Gentile, L.; Gianfredi, V.; Laurenzi, A.; et al. Type 1 Diabetes Patients’ Practice, Knowledge and Attitudes towards Influenza Immunization. Vaccines 2021, 9, 707. [Google Scholar] [CrossRef]
- Domnich, A.; Cambiaggi, M.; Vasco, A.; Maraniello, L.; Ansaldi, F.; Baldo, V.; Bonanni, P.; Calabro, G.E.; Costantino, C.; de Waure, C.; et al. Attitudes and Beliefs on Influenza Vaccination during the COVID-19 Pandemic: Results from a Representative Italian Survey. Vaccines 2020, 8, 711. [Google Scholar] [CrossRef]
- Domnich, A.; Grassi, R.; Fallani, E.; Ciccone, R.; Bruzzone, B.; Panatto, D.; Ferrari, A.; Salvatore, M.; Cambiaggi, M.; Vasco, A.; et al. Acceptance of COVID-19 and Influenza Vaccine Co-Administration: Insights from a Representative Italian Survey. J. Pers. Med. 2022, 12, 139. [Google Scholar] [CrossRef]
- Fall, E.; Izaute, M.; Chakroun-Baggioni, N. How can the health belief model and self-determination theory predict both influenza vaccination and vaccination intention? A longitudinal study among university students. Psychol. Health 2018, 33, 746–764. [Google Scholar] [CrossRef]
- Figueroa-Parra, G.; Esquivel-Valerio, J.A.; Santoyo-Fexas, L.; Moreno-Salinas, A.; Gamboa-Alonso, C.M.; de Leon-Ibarra, A.L.; Galarza-Delgado, D.A. Knowledge and attitudes about influenza vaccination in rheumatic diseases patients. Hum. Vaccines Immunother. 2021, 17, 1420–1425. [Google Scholar] [CrossRef]
- Souza, E.P.; Teixeira Mde, S. Pandemic influenza A/H1N1 vaccination coverage, adverse reactions, and reasons for vaccine refusal among medical students in Brazil. Rev. Inst. Med. Trop. São Paulo 2012, 54, 77–82. [Google Scholar] [CrossRef]
- Guay, M.; Gosselin, V.; Petit, G.; Baron, G.; Gagneur, A. Determinants of vaccine hesitancy in Quebec: A large population-based survey. Hum. Vaccines Immunother. 2019, 15, 2527–2533. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Lv, Q.; Qi, J.; Guo, X.; Wei, Q.; Liao, Z.; Lin, Z.; Gu, J. Knowledge, attitude, and practice regarding infection and vaccination in patients with rheumatic diseases in China. Hum. Vaccines Immunother. 2019, 15, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, R.; Lopez-de-Andres, A.; Hernandez-Barrera, V.; Gomez-Campelo, P.; San Andres-Rebollo, F.J.; de Burgos-Lunar, C.; Cardenas-Valladolid, J.; Abanades-Herranz, J.C.; Salinero-Fort, M.A. Influenza vaccination in people with type 2 diabetes, coverage, predictors of uptake, and perceptions. Result of the MADIABETES cohort a 7years follow up study. Vaccine 2017, 35, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; Nishiura, H. Exploring Influenza Vaccine Uptake and Its Determinants among University Students: A Cross-Sectional Study. Vaccines 2020, 8, 52. [Google Scholar] [CrossRef]
- Khalid, S.; Fawthrop, F. 070 What are patients’ understanding of vaccination recommendations? A quality improvement project. Rheumatology 2018, 57, key075. 294. [Google Scholar] [CrossRef]
- La Vecchia, C.; Negri, E.; Alicandro, G.; Scarpino, V. Attitudes towards influenza vaccine and a potential COVID-19 vaccine in Italy and differences across occupational groups, September 2020. Med. Lav. 2020, 111, 445–448. [Google Scholar] [CrossRef]
- Lejri-El Euchi, H.; Chirpaz, E.; Foucher, A.; Sultan-Bichat, N.; Randrianjohany, A.; Poubeau, P.; Gamon, E.; Roussin, C.; Osdoit, S.; Raffray, L.; et al. Vaccination against influenza and pneumococcal infections in patients with autoimmune disorders under biological therapy: Coverage and attitudes in patients and physicians. Eur. J. Intern. Med. 2019, 69, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Maguire, P.A.; Reay, R.E.; Looi, J.C. Nothing to sneeze at-uptake of protective measures against an influenza pandemic by people with schizophrenia: Willingness and perceived barriers. Australas. Psychiatry 2019, 27, 171–178. [Google Scholar] [CrossRef]
- Nestor, L.; Fernandez, C.; Fitzgerald, M.; Zaheri, S. Vaccination guideline adherence in patients on biological therapy: A forgotten friend? Br. J. Dermatol. 2019, 181, 139–140. [Google Scholar]
- Olatunbosun, O.D.; Esterhuizen, T.M.; Wiysonge, C.S. A cross sectional survey to evaluate knowledge, attitudes and practices regarding seasonal influenza and influenza vaccination among diabetics in Pretoria, South Africa. Vaccine 2017, 35, 6375–6386. [Google Scholar] [CrossRef]
- Quezada, M.; Alvarez, J.; Macias, A.; Villalpando, M. False fear from influenza vaccine complications contributes to vaccine hesitancy and requires urgent attention in multiple sclerosis patients. A case-control study. Mult. Scler. J. 2019, 25, 616. [Google Scholar]
- Radtke, M.A.; Rustenbach, S.J.; Reusch, M.; Stromer, K.; Augustin, M. Influenza vaccination rate among patients with moderate to severe psoriasis. J. Dtsch. Dermatol. Ges. 2013, 11, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wang, R.; Liu, J. Comparison of Vaccine Acceptance Between COVID-19 and Seasonal Influenza Among Women in China: A National Online Survey Based on Health Belief Model. Front. Med. 2021, 8, 679520. [Google Scholar] [CrossRef] [PubMed]
- Uppal, N.; Tobin, S.; Cape, J.; Muller, M.; Hicks, L.K. Feasibility and utility of influenza vaccination in hematology/oncology clinics. J. Clin. Oncol. 2013, 31, 166. [Google Scholar] [CrossRef]
- Wheelock, A.; Miraldo, M.; Thomson, A.; Vincent, C.; Sevdalis, N. Evaluating the importance of policy amenable factors in explaining influenza vaccination: A cross-sectional multinational study. BMJ Open 2017, 7, e014668. [Google Scholar] [CrossRef]
- Wong, K.K.; Cohen, A.L.; Norris, S.A.; Martinson, N.A.; von Mollendorf, C.; Tempia, S.; Walaza, S.; Madhi, S.A.; McMorrow, M.L.; Variava, E.; et al. Knowledge, attitudes, and practices about influenza illness and vaccination: A cross-sectional survey in two South African communities. Influenza Other Respir. Viruses 2016, 10, 421–428. [Google Scholar] [CrossRef]
- Wu, S.; Yang, P.; Li, H.; Ma, C.; Zhang, Y.; Wang, Q. Influenza vaccination coverage rates among adults before and after the 2009 influenza pandemic and the reasons for non-vaccination in Beijing, China: A cross-sectional study. BMC Public Health 2013, 13, 636. [Google Scholar] [CrossRef] [PubMed]
- Sagor, K.H.; AlAteeq, M.A. Beliefs, attitudes and barriers associated with the uptake of the seasonal influenza vaccine among patients visiting primary healthcare clinics. Saudi Med. J. 2018, 39, 690. [Google Scholar] [CrossRef]
- Ntatsaki, E.; Tahir, A.; Jiskani, A.; Mohammed, A.; Vassiliou, V.; Watts, R. FRI0617 Uptake on flu and pneumonia vaccination at the rheumatology clinic at a uk district general hospital-are we better than 10 years ago? Ann. Rheum. Dis. 2018, 77, 831–832. [Google Scholar]
- Wu, S.; Su, J.; Yang, P.; Zhang, H.; Li, H.; Chu, Y.; Hua, W.; Li, C.; Tang, Y.; Wang, Q.; et al. Willingness to accept a future influenza A(H7N9) vaccine in Beijing, China. Vaccine 2018, 36, 491–497. [Google Scholar] [CrossRef]
- Liu, M.; Cui, T.; Wang, Q.; Han, Y.; Han, Y.; Yang, L.; Shi, N.; Yi, Y.; Jin, H. Using an extended protection motivation theory to explain vaccine hesitancy: A cross-sectional study among Chinese adults. Hum. Vaccines Immunother. 2022, 18, 2026136. [Google Scholar] [CrossRef] [PubMed]
- Fayed, A.A.; Al Shahrani, A.S.; Almanea, L.T.; Alsweed, N.I.; Almarzoug, L.M.; Almuwallad, R.I.; Almugren, W.F. Willingness to Receive the COVID-19 and Seasonal Influenza Vaccines among the Saudi Population and Vaccine Uptake during the Initial Stage of the National Vaccination Campaign: A Cross-Sectional Survey. Vaccines 2021, 9, 765. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, X.; Qu, Z.; Francis, M.R.; Han, K.; Xu, C.; Cai, E.; Shi, H.; Hou, Z. Public Interest in Distribution and Determinants of Influenza and Pneumonia Vaccination during the COVID-19 Pandemic: An Infodemiology and Cross-Sectional Study from China. Vaccines 2021, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Roldan, M.; Cano-Torres, A.O.R.J.; Camiro-Zuñiga, A.; Caro-Vega, Y.; Galindo-Fraga, A.; Huertas-Jimenez, M.; Sierra-Madero, J.; Belaunzaran-Zamudio, B. Vaccine coverage in adults living with HIV and receiving care in a tertiary care centre in Mexico City, 2005–2016. J. Int. AIDS Soc. 2018, 21, 31. [Google Scholar]
- Wada, K.; Smith, D.R. Influenza vaccination uptake among the working age population of Japan: Results from a national cross-sectional survey. PLoS ONE 2013, 8, e59272. [Google Scholar] [CrossRef]
- Werneck, G.L.; Faerstein, E. Willingness to vaccinate against influenza A (H1N1)pdm09 among Brazilian civil servants: Pro-Saude cohort study. Rev. Bras. Epidemiol. 2021, 24, e210014. [Google Scholar] [CrossRef]
- Montalti, M.; di Valerio, Z.; Rallo, F.; Squillace, L.; Costantino, C.; Tomasello, F.; Mauro, G.L.; Stillo, M.; Perrone, P.; Resi, D.; et al. Attitudes toward the SARS-CoV-2 and Influenza Vaccination in the Metropolitan Cities of Bologna and Palermo, Italy. Vaccines 2021, 9, 1200. [Google Scholar] [CrossRef]
- Rodas, J.R.; Lau, C.H.; Zhang, Z.Z.; Griffiths, S.M.; Luk, W.C.; Kim, J.H. Exploring predictors influencing intended and actual acceptability of the A/H1N1 pandemic vaccine: A cohort study of university students in Hong Kong. Public Health 2012, 126, 1007–1012. [Google Scholar] [CrossRef]
- Schlenker, E.H.; Tschetter, R.L.; Straub, H.R. Influence of the H1N1 pandemic on university students’ knowledge of influenza. South Dak. Med. 2013, 66, 449–457. [Google Scholar]
- Rempenault, C.; Barnetche, T.; Magnol, M.; Castagne, B.; Pugibet, M.; Berard, E.; Truchetet, M.E.; Vergne-Salle, P.; Tournadre, A.; Ruyssen-Witrand, A.; et al. SAT0064 vaccination rate and risk factors for non-vaccination in rheumatoid arthritis: A cross-sectional prospective multicentric observational study. Ann. Rheum. Dis. 2020, 79, 965. [Google Scholar] [CrossRef]
- Paget, J.; Danielle Iuliano, A.; Taylor, R.J.; Simonsen, L.; Viboud, C.; Spreeuwenberg, P. Estimates of mortality associated with seasonal influenza for the European Union from the GLaMOR project. Vaccine 2022, 40, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Lammert, S.M.; Rao, S.R.; Jentes, E.S.; Fairley, J.K.; Erskine, S.; Walker, A.T.; Hagmann, S.H.; Sotir, M.J.; Ryan, E.T.; LaRocque, R.C. Refusal of recommended travel-related vaccines among US international travellers in Global TravEpiNet. J. Travel Med. 2017, 24, taw075. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, T.L.; Ramirez, J.A.; Carrico, R.M. Reasons for vaccine declination in healthy individuals attending an international vaccine and travel clinic. J. Refug. Glob. Health 2019, 2, 2. [Google Scholar]
- Gallant, A.J.; Flowers, P.; Deakin, K.; Cogan, N.; Rasmussen, S.; Young, D.; Williams, L. Barriers and enablers to influenza vaccination uptake in adults with chronic respiratory conditions: Applying the behaviour change wheel to specify multi-levelled tailored intervention content. Psychol. Health 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.B.; Bell, R.A. Predictors of intention to vaccinate against COVID-19: Results of a nationwide survey. Vaccine 2021, 39, 1080–1086. [Google Scholar] [CrossRef]
- Luo, C.; Yang, Y.; Liu, Y.; Zheng, D.; Shao, L.; Jin, J.; He, Q. Intention to COVID-19 vaccination and associated factors among health care workers: A systematic review and meta-analysis of cross-sectional studies. Am. J. Infect. Control 2021, 49, 1295–1304. [Google Scholar] [CrossRef]
- Okoli, G.N.; Abou-Setta, A.M.; Neilson, C.J.; Chit, A.; Thommes, E.; Mahmud, S.M. Determinants of seasonal influenza vaccine uptake among the elderly in the United States: A systematic review and meta-analysis. Gerontol. Geriatr. Med. 2019, 5, 2333721419870345. [Google Scholar] [CrossRef]
- Fergie, J.; Howard, A.; Huang, L.; Srivastava, A. Implementation Experience with Meningococcal Serogroup B Vaccines in the United States: Impact of a Nonroutine Recommendation. Pediatr. Infect. Dis. J. 2021, 40, 269–275. [Google Scholar] [CrossRef]
- McLaughlin, J.M.; Swerdlow, D.L.; Khan, F.; Will, O.; Curry, A.; Snow, V.; Isturiz, R.E.; Jodar, L. Disparities in uptake of 13-valent pneumococcal conjugate vaccine among older adults in the United States. Hum. Vaccine Immunother. 2019, 15, 841–849. [Google Scholar] [CrossRef]
- Spencer, J.C.; Calo, W.A.; Brewer, N.T. Disparities and reverse disparities in HPV vaccination: A systematic review and meta-analysis. Prev. Med. 2019, 123, 197–203. [Google Scholar] [CrossRef]
- Carlson, S.J.; Scanlan, C.; Marshall, H.S.; Blyth, C.C.; Macartney, K.; Leask, J. Attitudes about and access to influenza vaccination experienced by parents of children hospitalised for influenza in Australia. Vaccine 2019, 37, 5994–6001. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, G.; Pieralli, F.; Innocenti, M.; Milani, C.; Del Riccio, M.; Bechini, A.; Boccalini, S.; Bonanni, P.; Lorini, C. Non-familial paid caregivers as potential flu carriers and cause of spread: The primary prevention of flu measured through their adhesion to flu vaccination campaigns-A Florentine experience. Hum. Vaccine Immunother. 2019, 15, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Gagnon, D.; Vivion, M. Public health network: Optimizing communication material to address vaccine hesitancy. Can. Commun. Dis. Rep. 2020, 46, 48. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.R.; Brewer, N.T.; Wang, J.B.; Chambers, C.D. Theory-based predictors of influenza vaccination among pregnant women. Vaccine 2012, 31, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Lim, N.A.; Chin, Y.H.; Ng, Y.P.M.; Amin, Z. Effect of COVID-19 Pandemic on Influenza Vaccination Intention: A Meta-Analysis and Systematic Review. Vaccines 2022, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Influenza Vaccination Increased During the COVID-19 Pandemic. JAMA 2021, 326, 2465. [Google Scholar] [CrossRef] [PubMed]
- Roman, P.C.; Kirtland, K.; Zell, E.R.; Jones-Jack, N.; Shaw, L.; Shrader, L.; Sprague, C.; Schultz, J.; Le, Q.; Nalla, A.; et al. Influenza Vaccinations During the COVID-19 Pandemic—11 US Jurisdictions, September–December 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 1575. [Google Scholar] [CrossRef]
- Del Riccio, M.; Lina, B.; Caini, S.; Staadegaard, L.; Wiegersma, S.; Kynčl, J.; Combadière, B.; MacIntyre, C.R.; Paget, J. Letter to the editor: Increase of influenza vaccination coverage rates during the COVID-19 pandemic and implications for the upcoming influenza season in northern hemisphere countries and Australia. Eurosurveillance 2021, 26, 2101143. [Google Scholar] [CrossRef]
- Boey, L.; Roelants, M.; Vandermeulen, C. Increased vaccine uptake and less perceived barriers toward vaccination in long-term care facilities that use multi-intervention manual for influenza campaigns. Hum. Vaccines Immunother. 2021, 17, 673–680. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. How Influenza (Flu) Vaccines Are Made. Available online: https://www.cdc.gov/flu/prevent/how-fluvaccine-made.htm#:~:text=Cell%2Dbased%20flu%20vaccine%20production,the%20flu%20vaccine%20manufacturing%20process (accessed on 12 August 2022).
- Pilkington, E.H.; Suys, E.J.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]


| Topic | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population(s) | Adults (18–64 years) eligible for the influenza vaccine in the following countries: France Germany Italy Spain United Kingdom United States Canada Mexico China Japan Brazil Saudi Arabia South Africa Taiwan Hong Kong Australia | Any other region/country. Focus on pediatric or elderly (≥65 years) populations. Focus on pregnant population. Studies reporting primarily on healthcare workers. |
| Interventions | Any the following seasonal influenza vaccinations: Recombinant vaccine Trivalent vaccine Quadrivalent vaccine Inactivated vaccine Live attenuated vaccine mRNA vaccine Intramuscular vaccine Intranasal vaccine Intradermal vaccine | N/A |
| Comparisons | Any/none | N/A |
| Outcomes | Data on barriers or attitudes to influenza uptake and/or strategies to improve uptake, including: Patient attitudes and perceptions towards vaccine (safety, efficacy) and healthcare system/professionals. Outcomes from a patient perspective Attitude towards vaccine technology. Accessibility and availability of vaccine. Vaccine hesitancy including altered vaccine schedule, or delayed acceptance. Perceived barriers (contextual, social, psychological). Barriers to seasonal influenza vaccines compared to non-seasonal vaccines (e.g., Human papillomavirus, Varicella, etc.). Direct and indirect costs as a barrier to uptake. Refusal rate. Intention to vaccinate self and/or dependents (i.e., children). Satisfaction level with vaccine. Preference to receive COVID-19 vaccine over influenza vaccine. Impact of previous vaccination experience on future uptake. | Studies not reporting barriers or attitudes towards influenza vaccinations. Studies reporting only on uptake rate with no mention of barriers/attitudes. Not reporting outcomes from patient perspective. |
| Time | Published from January 2012 to 6 May 2022. | N/A |
| Study design | Clinical studies Case control studies Cohort studies Observational studies Longitudinal studies Epidemiological studies Cross-sectional studies Systematic literature reviews Meta-analyses Real world evidence/data Database studies (medical records, claims) Patient/physician surveys/questionnaires Patient/physician preference studies | Randomized controlled trials Editorials Letters to journals Non-systematic literature reviews Conference minutes |
| Other | Human studies English language | Animal studies Duplicates Non-English language |
| Category | Question Type | Definition | Barrier Example | Promoter Example |
|---|---|---|---|---|
| Access | Access to vaccine | Questions that determine the access that a participant has to influenza vaccination | “Getting the flu vaccine required a lot of effort on my part” [16] | “Ease of access” [17] |
| Access | Availability/time constraints | Questions that refer to the participant’s availability to obtain the influenza vaccination | “I don’t have the time to get vaccinated” [18] | “Supposed to receive vaccination in the workplace” [19] |
| Access | Recommended by HCW | Questions investigating influence of recommendation by HCWs | N/A | “The specialists encouraged a vaccination.” [20] |
| Access | Transport | Questions that determine any transportation restraints of the participants | “Inconvenient to reach a vaccination location” [21] | “Convenient to reach a vaccination location” [21] |
| Cost | Cost | Questions that highlight cost as the determining factor over choice of vaccination | “Could not afford vaccination” [22] | “Vaccine was a reasonable price” [23] |
| Intent to vaccinate | Intent to vaccinate | Intent of the participant to vaccinate in the upcoming influenza season(s) | “Unwilling to receive influenza vaccination” [24] | “Willingness to Receive Influenza Vaccination” [25] |
| Knowledge | Knowledge | Questions referring to the knowledge that the participant has of influenza vaccinations | “Believed the vaccine causes influenza” [26] | “Even if infected with influenza, wanted to prevent the symptoms from becoming serious” [22] |
| Non-optional | Health exemptions | Avoidance of vaccination due to medical reasons (such as allergies) | “I am allergic to flu vaccine” [27] | N/A |
| Non-optional | Requirement (for job/religion) | Requirement of vaccination (by either employer or religion) | N/A | “It is mandatory for my work” [28] “Hajj requirement” [29] |
| Psychological | Past behaviors | Questions relating to the behavior of participants towards vaccines in previous years | “Previously rejected influenza vaccine” [30] | “I am accustomed to getting a flu shot each year” [31] |
| Psychological | Past experiences | Questions investigating the effect of past experiences with vaccination | “Bad reaction to previous shot” [32] | “Suffered from influenza last year” [33] |
| Social | Attitude towards healthcare | Questions that determine the attitude of the participant towards healthcare | “Perception of low self-risk” [34] | “The best way to avoid the complications of influenza is by using influenza vaccine” [35] |
| Social | Subjective beliefs | Questions used to determine the beliefs of a participant in relation to the influenza vaccine | “I think it is harmful” [17] | “Vaccines are crucial to guaranteeing public health and should be mandatory” [36] |
| Trust | Trust in healthcare | Questions used to determine the trust in healthcare and government guidelines that the participant has | “I don’t trust vaccines” [37] | “Influenza vaccine is safe and effective” [35] |
| Participant Demographic | US Results |
|---|---|
| Sample size, mean | 673 |
| Age, mean (range) | 44.4 years (15–94) |
| Sex: Female | 54.8% |
| Race/ethnicity (verbatim from text) | Black, White, Asian, South Asian, Hispanic or Latino, American Indian or Alaska Native, Native Hawaiian/other Pacific Islander, Mixed Race. |
| Employment status | Full-time, part-time, unemployed, retired, student, disabled. |
| Income range (USD) | <15,000 to ≥150,000 |
| Undergraduate education or above | 62.9% |
| Comorbidities (verbatim from text) | High-risk population, psychiatric patients, cardiovascular disease, rheumatoid arthritis, pulmonary disease, asthma, chronic bronchitis, cancer (all types except for skin cancer), cystic fibrosis, diabetes, epilepsy, heart attack, heart disease, high blood pressure, HIV/AIDS, kidney disease, stroke, renal dysfunction, hemoglobinopathy, immunosuppression. |
| Current season vaccination rate | 43.7% |
| Barrier | Number of Questions Investigating Barrier | Agreement Rate of Participant with Barrier (%) | ||
|---|---|---|---|---|
| Total Population | Unvaccinated | Total Population | Unvaccinated | |
| Trust | 98 | 26 | 20.6 | 14.1 |
| Knowledge | 40 | 6 | 19.3 | 32.3 |
| Costs | 28 | 10 | 15.5 | 27.3 |
| Social | 276 | 67 | 14.1 | 14.5 |
| Psychological | 89 | 16 | 13.0 | 22.0 |
| Access | 105 | 29 | 10.0 | 12.7 |
| Health | 21 | 10 | 1.8 | 2.2 |
| Promoter | Number of Questions Investigating Promoter | Agreement Rate of Participant with Promoter (%) | ||
|---|---|---|---|---|
| Total Population | Vaccinated | Total Population | Vaccinated | |
| Trust | 91 | 8 | 68.1 | 79.0 |
| Social | 125 | 26 | 47.6 | 45.5 |
| Costs | 15 | 4 | 44.1 | 41.1 |
| Knowledge | 22 | 5 | 43.8 | 51.5 |
| Access | 28 | 14 | 31.8 | 26.1 |
| Non-optional | 7 | 3 | 21.1 | 10.9 |
| Psychological | 14 | 1 | 20.3 | 5.4 |
| Strategy to Improve Influenza Vaccination Rate | Example |
|---|---|
| Education | “Increased efforts to educate college students about the risks and importance of the vaccine may serve to minimize widely held misconceptions about the vaccine.” [79]. |
| HCW to promote vaccination | “Primary care physicians should intensively promote vaccination because vaccination recommendation by a physician and information dissemination regarding vaccines and vaccination to patients significantly increase vaccination rates.” [29]. |
| Improving public knowledge | “False fear from vaccine complications is by far the most significant and the one that requires urgent attention.” [76]. |
| Costs | “There is need for a different government approach to resolving the financial deficit in Italy focused on health promotion and disease prevention.” [24]. |
| Targeting a specific sup-population | “Information strategies and vaccination campaigns need to be adapted to the characteristics of the targeted population.” [59]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welch, V.L.; Metcalf, T.; Macey, R.; Markus, K.; Sears, A.J.; Enstone, A.; Langer, J.; Srivastava, A.; Cane, A.; Wiemken, T.L. Understanding the Barriers and Attitudes toward Influenza Vaccine Uptake in the Adult General Population: A Rapid Review. Vaccines 2023, 11, 180. https://doi.org/10.3390/vaccines11010180
Welch VL, Metcalf T, Macey R, Markus K, Sears AJ, Enstone A, Langer J, Srivastava A, Cane A, Wiemken TL. Understanding the Barriers and Attitudes toward Influenza Vaccine Uptake in the Adult General Population: A Rapid Review. Vaccines. 2023; 11(1):180. https://doi.org/10.3390/vaccines11010180
Chicago/Turabian StyleWelch, Verna L., Tom Metcalf, Richard Macey, Kristen Markus, Amy J. Sears, Ashley Enstone, Jakob Langer, Amit Srivastava, Alejandro Cane, and Timothy L. Wiemken. 2023. "Understanding the Barriers and Attitudes toward Influenza Vaccine Uptake in the Adult General Population: A Rapid Review" Vaccines 11, no. 1: 180. https://doi.org/10.3390/vaccines11010180
APA StyleWelch, V. L., Metcalf, T., Macey, R., Markus, K., Sears, A. J., Enstone, A., Langer, J., Srivastava, A., Cane, A., & Wiemken, T. L. (2023). Understanding the Barriers and Attitudes toward Influenza Vaccine Uptake in the Adult General Population: A Rapid Review. Vaccines, 11(1), 180. https://doi.org/10.3390/vaccines11010180





