Optimization of Elderly Influenza and Pneumococcal Immunization Programs in Beijing, China Using Health Economic Evaluations: A Modeling Study
Abstract
1. Introduction
2. Methods
2.1. Model Overview
2.2. Vaccination Strategies
2.3. Epidemiological and Clinical Inputs
2.4. Health State Utilities
2.5. Costs
2.6. Decision Rules
- Simulate costs and QALYs associated with each strategy;
- Sequence the strategies by costs in increasing order;
- Determine whether each strategy iss dominated by the next strategy starting from the least expensive strategy. Repeat this step, until there are no dominated strategies;
- Determine whether each strategy (e.g., strategy k) is extensively dominated by the next strategy by comparing the ICER of the next strategy (strategy k + 1) in relation to the previous strategy (strategy k − 1) to that of the running ICER (ICERs of strategy k vs. strategy k − 1). If the ICER of strategy k + 1 vs. k − 1 is smaller than the running ICER, then strategy k is extensively dominated. This is repeated, until there are no extensively dominated strategies;
- Identify the strategy with the ICER is immediately below the WTP threshold as the optimal strategy;
- If ICERs are not applicable, the NMB of the optimal strategy in reference to the current policy (strategy 3) is calculated.
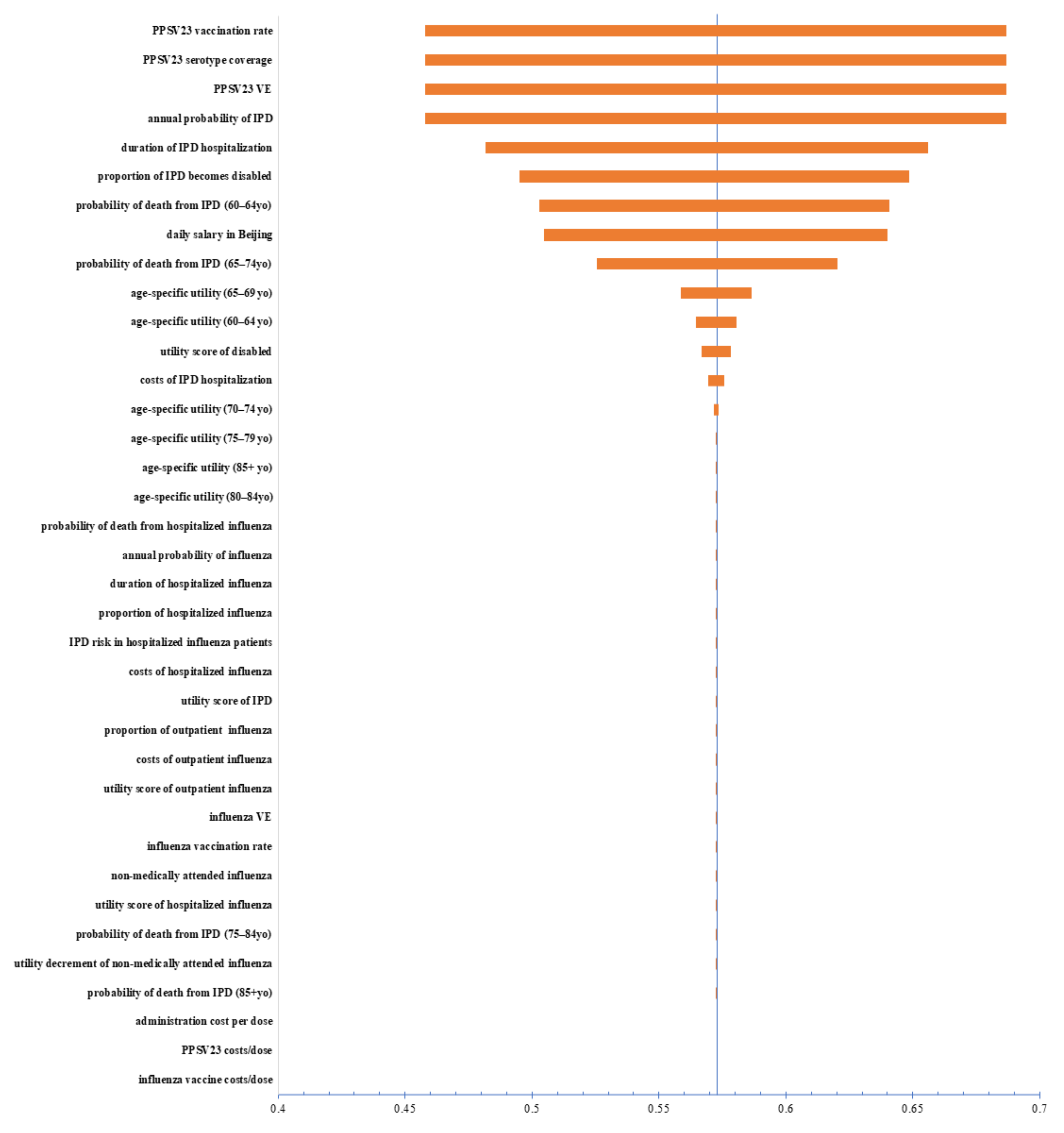
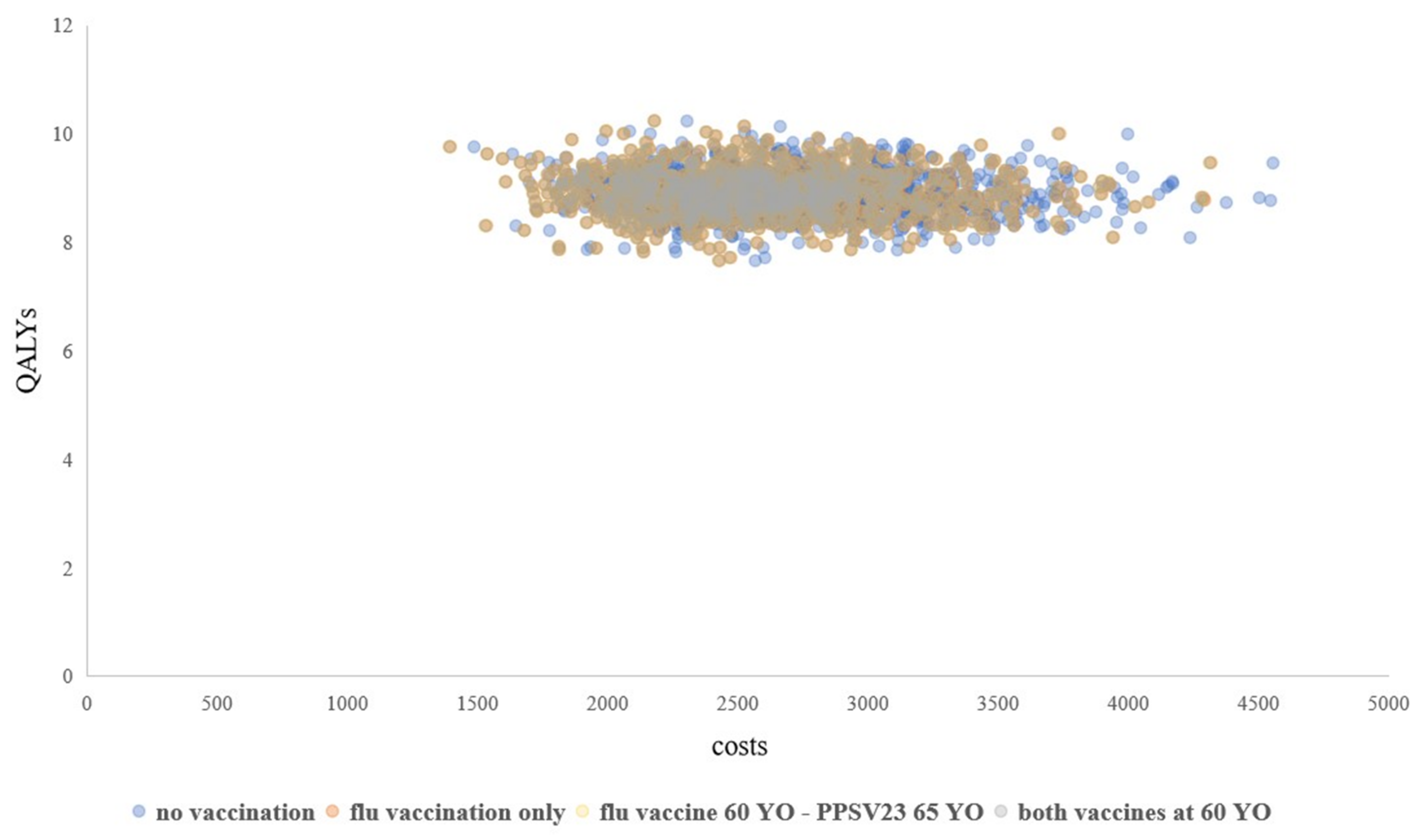
2.7. Sensitivity Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Liu, Y.; Wu, P.; Peng, Z.; Wang, X.; Chen, T.; Wong, J.Y.T.; Yang, J.; Bond, H.S.; Wang, L.; et al. Influenza-associated excess respiratory mortality in China, 2010–2015: A population-based study. Lancet Public Health 2019, 4, e473–e481. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Han, Y.; Yu, C.; Guo, Y.; Pei, P.; Yang, L.; Chen, Y.; Du, H.; Sun, D.; Pang, Y.; et al. The hospitalization burden of all-cause pneumonia in China: A population-based study, 2009–2017. Lancet Reg. Health. West. Pac. 2022, 22, 100443. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Silk, B.J.; Li, W.; Fleischauer, A.T.; Xing, X.; Jiang, X.; Yu, H.; Olsen, S.J.; Cohen, A.L. Pneumonia incidence and mortality in Mainland China: Systematic review of Chinese and English literature, 1985–2008. PLoS ONE 2010, 5, e11721. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 2006, 19, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–2021 Influenza Season. MMWR. Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef]
- Matanock, A.; Lee, G.; Gierke, R.; Kobayashi, M.; Leidner, A.; Pilishvili, T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 1069–1075. [Google Scholar] [CrossRef]
- Li, X.; Leng, S.X. Influenza immunization among Chinese seniors: Urgent calling for improving vaccination coverage, education, and research. Aging Med. 2020, 3, 12–15. [Google Scholar] [CrossRef]
- Lu, E.Y.; Chen, H.H.; Zhao, H.; Ozawa, S. Health and economic impact of the pneumococcal conjugate vaccine in hindering antimicrobial resistance in China. Proc. Natl. Acad. Sci. USA 2021, 118, e2004933118. [Google Scholar] [CrossRef]
- Zhang, Y.; Muscatello, D.J.; Wang, Q.; Yang, P.; Wu, J.; MacIntyre, C.R. Overview of influenza vaccination policy in Beijing, China: Current status and future prospects. J. Public Health Policy 2017, 38, 366–379. [Google Scholar] [CrossRef]
- Lu, X.; Lu, J.; Zhang, L.; Mei, K.; Guan, B.; Lu, Y. Gap between willingness and behavior in the vaccination against influenza, pneumonia, and herpes zoster among Chinese aged 50–69 years. Expert Rev. Vaccines 2021, 20, 1147–1152. [Google Scholar] [CrossRef]
- National Immunization Advisory Committee Technical Working Group. Technical guidelines for seasonal influenza vaccination in China (2020–2021). Chin. J. Epidemiol. 2020, 54, 1035–1059. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, L.; Xie, M.; Li, X.; Recommendations for Influenza, Streptococcus pneumoniae Vaccination in Elderly People in China Writing Group, Geriatric Respiratory Group and Chinese Society of Geriatrics. Recommendations for influenza and Streptococcus pneumoniae vaccination in elderly people in China. Aging Med. 2020, 3, 4–14. [Google Scholar] [CrossRef]
- Influenza and Streptococcus pneumoniae Vaccination Chinese Experts Recommendation Writing group; Chinese Medical Association Gerontology Branch Respiratory Group. Recommendation of influenza and Streptococcus pneumoniae vaccination n the elderly. Chin. J. Geriatr. Res. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Choi, M.J.; Shin, G.; Kang, D.; Lim, J.O.; Kim, Y.K.; Choi, W.S.; Yun, J.W.; Noh, J.Y.; Song, J.Y.; Kim, W.J.; et al. Cost-Effectiveness of Influenza Vaccination Strategies in Adults: Older Adults Aged ≥ 65 Years, Adults Aged 50–64 Years, and At-Risk Adults Aged 19–64 Years. Vaccines 2022, 10, 445. [Google Scholar] [CrossRef]
- Ding, H.; Huang, J.; Ngai, C.H.; Sun, Q.; Kwok, K.O.; Wang, H.H.; Chong, M.; Wong, M.C. The cost-effectiveness of starting 23-valent pneumococcal polysaccharide vaccine and influenza vaccination at 50 vs. 65 years: A comparative modelling study. Vaccine 2022, 40, 1282–1288. [Google Scholar] [CrossRef]
- Chen, D.; Ye, Z.; Pi, Z.; Mizukami, S.; Aoyagi, K.; Jiang, Y. Cost-effectiveness of dual influenza and pneumococcal vaccination among the elderly in Shenzhen, China. Vaccine 2021, 39, 2237–2245. [Google Scholar] [CrossRef]
- Neumann, P.J.; Sanders, G.D.; Russell, L.B.; Siegel, J.E.; Ganiats, T.G. Cost-Effectiveness in Health and Medicine; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- National Bureau of Statistics of China. China Statistical Yearbook 2019; China Statistics Press: Beijing, China, 2020.
- Smith, K.J.; Lee, B.Y.; Nowalk, M.P.; Raymund, M.; Zimmerman, R.K. Cost-effectiveness of dual influenza and pneumococcal vaccination in 50-year-olds. Vaccine 2010, 28, 7620–7625. [Google Scholar] [CrossRef]
- Smith, K.J.; Zimmerman, R.K.; Lin, C.J.; Nowalk, M.P.; Ko, F.S.; McEllistrem, M.C.; Roberts, M.S. Alternative strategies for adult pneumococcal polysaccharide vaccination: A cost-effectiveness analysis. Vaccine 2008, 26, 1420–1431. [Google Scholar] [CrossRef]
- Zhu, D.; Lv, M.; Bai, Y.; Wu, J.; He, P. Cost-effectiveness analysis of quadrivalent seasonal influenza vaccines in Beijing: A modeling analysis. Vaccine 2022, 40, 994–1000. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, M.; Wang, S.; Wu, F.; Yan, Q.; Yang, Q.; Li, Y.; Guo, X.; Fu, C.; Shi, Y.; et al. Vaccination coverage with the pneumococcal and influenza vaccine among persons with chronic diseases in Shanghai, China, 2017. BMC Public Health 2020, 20, 359. [Google Scholar] [CrossRef]
- Somes, M.P.; Turner, R.M.; Dwyer, L.J.; Newall, A.T. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: A systematic review and meta-analysis. Vaccine 2018, 36, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Dushoff, J.; Plotkin, J.B.; Levin, S.A.; Earn, D.J. Dynamical resonance can account for seasonality of influenza epidemics. Proc. Natl. Acad. Sci. USA 2004, 101, 16915–16916. [Google Scholar] [CrossRef] [PubMed]
- Xing, N.; Cheung, W.Y.; Jiang, M.; You, J.H.S. Standing orders program of pneumococcal vaccination for hospitalized elderly patients in Hong Kong: A cost-effectiveness analysis. Am. J. Infect. Control 2019, 47, 1302–1308. [Google Scholar] [CrossRef]
- Gray, A.M.; Clarke, P.M.; Wolstenholme, J.L.; Wordsworth, S. Applied Methods of Cost-Effectiveness Analysis in Healthcare; OUP Oxford: New York City, NY, USA, 2011. [Google Scholar]
- Beck, J.R.; Kassirer, J.P.; Pauker, S.G. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am. J. Med. 1982, 73, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Darvishian, M.; van den Heuvel, E.R.; Bissielo, A.; Castilla, J.; Cohen, C.; Englund, H.; Gefenaite, G.; Huang, W.T.; la Bastide-van Gemert, S.; Martinez-Baz, I.; et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: An individual participant data meta-analysis of test-negative design case-control studies. Lancet. Respir. Med. 2017, 5, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Kraicer-Melamed, H.; O’Donnell, S.; Quach, C. The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: A systematic review and meta-analysis. Vaccine 2016, 34, 1540–1550. [Google Scholar] [CrossRef]
- Shami, J.J.P.; Pathadka, S.; Chan, E.W.; Hui, J.; Sato, R.; Patil, S.; Li, X. Evaluating the cost-effectiveness of a sequential pneumococcal vaccination compared to single-dose vaccination strategy for adults in Hong Kong. Hum. Vaccines Immunother. 2020, 16, 1937–1944. [Google Scholar] [CrossRef]
- Molinari, N.A.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef]
- Caldwell, R.; Roberts, C.S.; An, Z.; Chen, C.I.; Wang, B. The health and economic impact of vaccination with 7-valent pneumococcal vaccine (PCV7) during an annual influenza epidemic and influenza pandemic in China. BMC Infect. Dis. 2015, 15, 284. [Google Scholar] [CrossRef]
- World Health Organization. Life Tables by Country (GHE: Life tables). Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-ghe-life-tables-by-country (accessed on 20 October 2020).
- Jiang, M.; Li, P.; Wang, W.; Zhao, M.; Atif, N.; Zhu, S.; Fang, Y. Cost-effectiveness of quadrivalent versus trivalent influenza vaccine for elderly population in China. Vaccine 2020, 38, 1057–1064. [Google Scholar] [CrossRef]
- Wu, D.B.; Roberts, C.S.; Huang, Y.C.; Chien, L.; Fang, C.H.; Chang, C.J. A retrospective study to assess the epidemiological and economic burden of pneumococcal diseases in adults aged 50 years and older in Taiwan. J. Med. Econ. 2014, 17, 312–319. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J.; Johannesson, M.; Kind, P.; Xu, L.; Zhang, Y.; Burström, K. Population health status in China: EQ-5D results, by age, sex and socio-economic status, from the National Health Services Survey 2008. Qual. Life Res. 2011, 20, 309–320. [Google Scholar] [CrossRef]
- Yang, J.; Jit, M.; Zheng, Y.; Feng, L.; Liu, X.; Wu, J.T.; Yu, H. The impact of influenza on the health related quality of life in China: An EQ-5D survey. BMC Infect. Dis. 2017, 17, 686. [Google Scholar] [CrossRef]
- Sisk, J.E.; Whang, W.; Butler, J.C.; Sneller, V.P.; Whitney, C.G. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: Role of comorbid conditions and race. Ann. Intern. Med. 2003, 138, 960–968. [Google Scholar] [CrossRef]
- Yang, J.; Jit, M.; Leung, K.S.; Zheng, Y.-M.; Feng, L.-Z.; Wang, L.-P.; Lau, E.H.Y.; Wu, J.T.; Yu, H.-J. The economic burden of influenza-associated outpatient visits and hospitalizations in China: A retrospective survey. Infect. Dis. Poverty 2015, 4, 44. [Google Scholar] [CrossRef]
- Han, X.; Chen, L.; Wang, Y.; Li, H.; Wang, H.; Xing, X.; Zhang, C.; Suo, L.; Wang, J.; Yu, G.; et al. Cost Effectiveness of Different Initial Antimicrobial Regimens for Elderly Community-Acquired Pneumonia Patients in General Ward. Infect. Drug Resist. 2021, 14, 1845–1853. [Google Scholar] [CrossRef]
- Annoucement of The result of the Bididng of Inactivated Trivalent Influenza Vaccines for Beijing Municipal Immunization Programs. Available online: http://www.ccgp.gov.cn/cggg/dfgg/cjgg/202012/t20201210_15604218.htm (accessed on 21 May 2021).
- Information of Pharmaceutical Bids. Available online: https://data.yaozh.com/yaopinzhongbiao (accessed on 17 May 2021).
- Beijing Bureau of Statistics. Beijing Statistical Yearbook 2020. Available online: http://nj.tjj.beijing.gov.cn/nj/main/2020-tjnj/zk/e/indexce.htm (accessed on 15 May 2021).
- Liu, G.; Wu, J.; Sun, L.; Wu, J.; Dong, C. China Guidelines for Pharmacoeconomic Evaluations 2019; Chinese Pharmaceutical Association: Beijing, China, 2019. [Google Scholar]
- Fielding, J.E.; Grant, K.A.; Papadakis, G.; Kelly, H.A. Estimation of type- and subtype-specific influenza vaccine effectiveness in Victoria, Australia using a test negative case control method, 2007–2008. BMC Infect. Dis. 2011, 11, 170. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. Consumer Price Indices, Healthcare. Available online: http://data.stats.gov.cn/english/adv.htm?m=advquery&cn=A01 (accessed on 17 May 2021).
- Jiang, Y.; Ye, Z.; Chen, D.; Shu, Y. Dual influenza and pneumococcal vaccination was associated with lower short-term risks of all-cause and acute respiratory hospitalizations among the elderly in Shenzhen, China: A retrospective cohort study. Emerg. Microbes Infect. 2020, 9, 2578–2587. [Google Scholar] [CrossRef]
- You, J.H.; Wong, W.C.; Ip, M.; Lee, N.L.; Ho, S.C. Cost-effectiveness analysis of influenza and pneumococcal vaccination for Hong Kong elderly in long-term care facilities. J. Epidemiol. Community Health 2009, 63, 906–911. [Google Scholar] [CrossRef]
- Olsen, S.J.; Winn, A.K.; Budd, A.P.; Prill, M.M.; Steel, J.; Midgley, C.M.; Kniss, K.; Burns, E.; Rowe, T.; Foust, A.; et al. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic—United States, 2020–2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1013–1019. [Google Scholar] [CrossRef]
- Walker, D.G.; Hutubessy, R.; Beutels, P. WHO Guide for standardisation of economic evaluations of immunization programmes. Vaccine 2010, 28, 2356–2359. [Google Scholar] [CrossRef] [PubMed]

| Inputs | Base-Case Value | Value Change for OWSA | Distribution for PSA | Reference |
|---|---|---|---|---|
| Clinical inputs | ||||
| Influenza vaccination rate | 0.19 | ±20% | beta | [21] |
| Annual probability of influenza infection in the unvaccinated elderly | 0.23 | ±20% | beta | [22] |
| Annual probability of IPD in the unvaccinated elderly per 100,000 | 8.3 | ±20% | beta | [25] |
| Influenza vaccine effectiveness | 32% | ±20% | beta | [28] |
| PPSV23 effectiveness | 50% | ±20% | beta | [29] |
| PPSV23 serotype coverage | 0.77 | ±20% | beta | [30] |
| Proportion of non-medically attended influenza | 0.34 | - | - | Estimated |
| Proportion of outpatient influenza | 0.62 | ±20% | beta | [31] |
| Proportion of hospitalized influenza | 0.04 | ±20% | beta | [31] |
| Probability of hospitalized influenza patients to develop IPD | 0.10 | ±20% | beta | [19] |
| Proportion of IPD patients to become disabled | 0.07 | ±20% | beta | [32] |
| Probability of recovery from influenza hospitalization | 0.78 | - | - | Estimated |
| Probability of recovery from IPD | 0.54 | - | - | Estimated |
| Annual background mortality rate death per 1000 (60–64 years old) | 2.0 | ±20% | beta | [33] |
| Annual probability of natural death per 1000 (70–74 years old) | 5.9 | ±20% | beta | [33] |
| Probability of death from hospitalized influenza | 0.12 | ±20% | beta | [34] |
| Probability of death from IPD | 0.16 | ±20% | beta | [35] |
| Mortality ratio of the disabled | 1.1 | - | [19] | |
| Utility scores | ||||
| 60–64 years old | 0.74 | ±10% | beta | [36] |
| 65–69 years old | 0.71 | ±10% | beta | |
| 70–74 years old | 0.69 | ±10% | beta | |
| 75–79 years old | 0.68 | ±10% | beta | |
| 80–84 years old | 0.66 | ±10% | beta | |
| Utility decrement of non-medically attended influenza | 0.05 | ±10% | beta | Assumed |
| Utility score of outpatient influenza | 0.61 | ±10% | beta | [37] |
| Utility score of hospitalized influenza | 0.56 | ±10% | beta | [37] |
| Utility score of IPD | 0.20 | ±10% | beta | [38] |
| Utility score of disabled | 0.40 | ±10% | beta | [19] |
| Duration | ||||
| Length of non-medical/outpatient influenza (days) | 6.2 | - | - | [37] |
| Length of hospitalized influenza (days) | 9 | ±20% | gamma | [39] |
| Length of IPD (days) | 12 | ±20% | gamma | [40] |
| Costs (2020 CNY) | ||||
| Price of influenza vaccine per dose | 28 | ±20% | gamma | [41] |
| Price of PPSV23 per dose | 200 | ±20% | gamma | [42] |
| Vaccine administration costs per dose | 25 | ±20% | gamma | [21] |
| Non-medically attended influenza cost | 100 | ±20% | gamma | Estimated |
| Outpatient influenza cost | 421 | ±20% | gamma | [21] |
| Hospitalized influenza cost | 15,870 | ±20% | gamma | [21] |
| IPD hospitalization cost | 12,460 | ±20% | gamma | [40] |
| Daily labor compensation rate in Beijing | 656 | ±20% | gamma | [43] |
| Average life expectancy | ||||
| 60–64 years old | 21 | - | - | [33] |
| 70–74 years old | 13 | - | - | [33] |
| Annual discount rate | 5% | - | - | [44] |
| Strategy NO. | Strategy | Costs (CNY) | QALYs | ICER | NMB (CNY) |
|---|---|---|---|---|---|
| 1 | No vaccination | 2746.1 | 8.900666 | Dominated * | NA |
| 2 | Flu vaccine for people ≥ 60 YO | 2589.2 | 8.905088 | Dominated * | NA |
| 3 | Flu vaccine for people ≥ 60 YO and PPSV23 for people ≥ 65 YO | 2585.0 | 8.905136 | Dominated * | Reference strategy |
| 4 | Both vaccines for people ≥ 60 YO | 2584.6 | 8.905139 | Optimal | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi, Z.; Aoyagi, K.; Arima, K.; Wu, X.; Ye, Z.; Jiang, Y. Optimization of Elderly Influenza and Pneumococcal Immunization Programs in Beijing, China Using Health Economic Evaluations: A Modeling Study. Vaccines 2023, 11, 161. https://doi.org/10.3390/vaccines11010161
Pi Z, Aoyagi K, Arima K, Wu X, Ye Z, Jiang Y. Optimization of Elderly Influenza and Pneumococcal Immunization Programs in Beijing, China Using Health Economic Evaluations: A Modeling Study. Vaccines. 2023; 11(1):161. https://doi.org/10.3390/vaccines11010161
Chicago/Turabian StylePi, Zhenfei, Kiyoshi Aoyagi, Kazuhiko Arima, Xiaoliang Wu, Zhaojia Ye, and Yawen Jiang. 2023. "Optimization of Elderly Influenza and Pneumococcal Immunization Programs in Beijing, China Using Health Economic Evaluations: A Modeling Study" Vaccines 11, no. 1: 161. https://doi.org/10.3390/vaccines11010161
APA StylePi, Z., Aoyagi, K., Arima, K., Wu, X., Ye, Z., & Jiang, Y. (2023). Optimization of Elderly Influenza and Pneumococcal Immunization Programs in Beijing, China Using Health Economic Evaluations: A Modeling Study. Vaccines, 11(1), 161. https://doi.org/10.3390/vaccines11010161






