Acceptance and Preference for COVID-19 Vaccine among Japanese Residents at Early Stage of the Epidemic in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Instrument
2.3. Statistical Analysis
3. Results
3.1. COVID-19 Vaccine Acceptance in Japan’s Participants
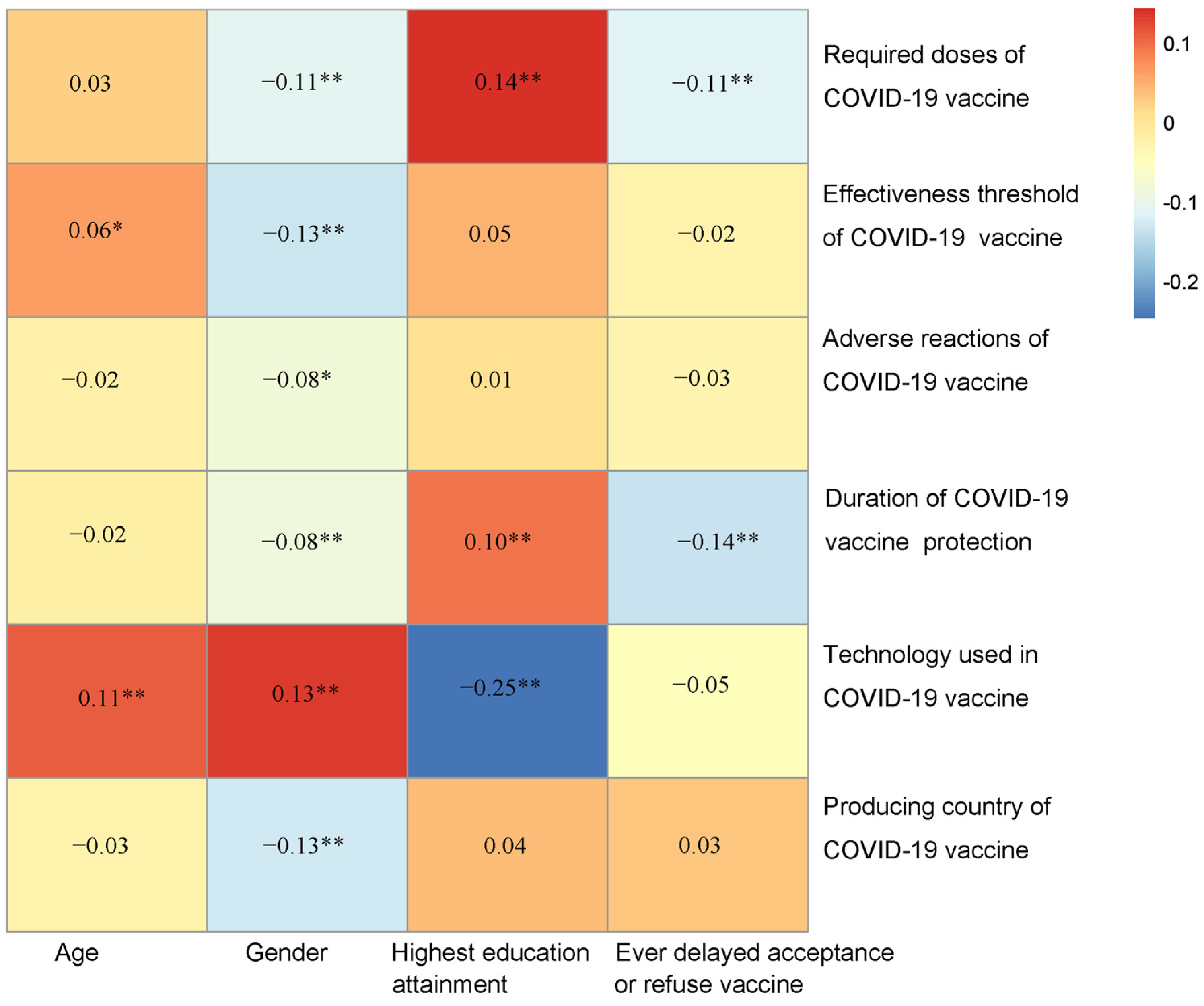
3.2. Effect of Demographic Characteristics on the Attitudes towards COVID-19 Vaccines
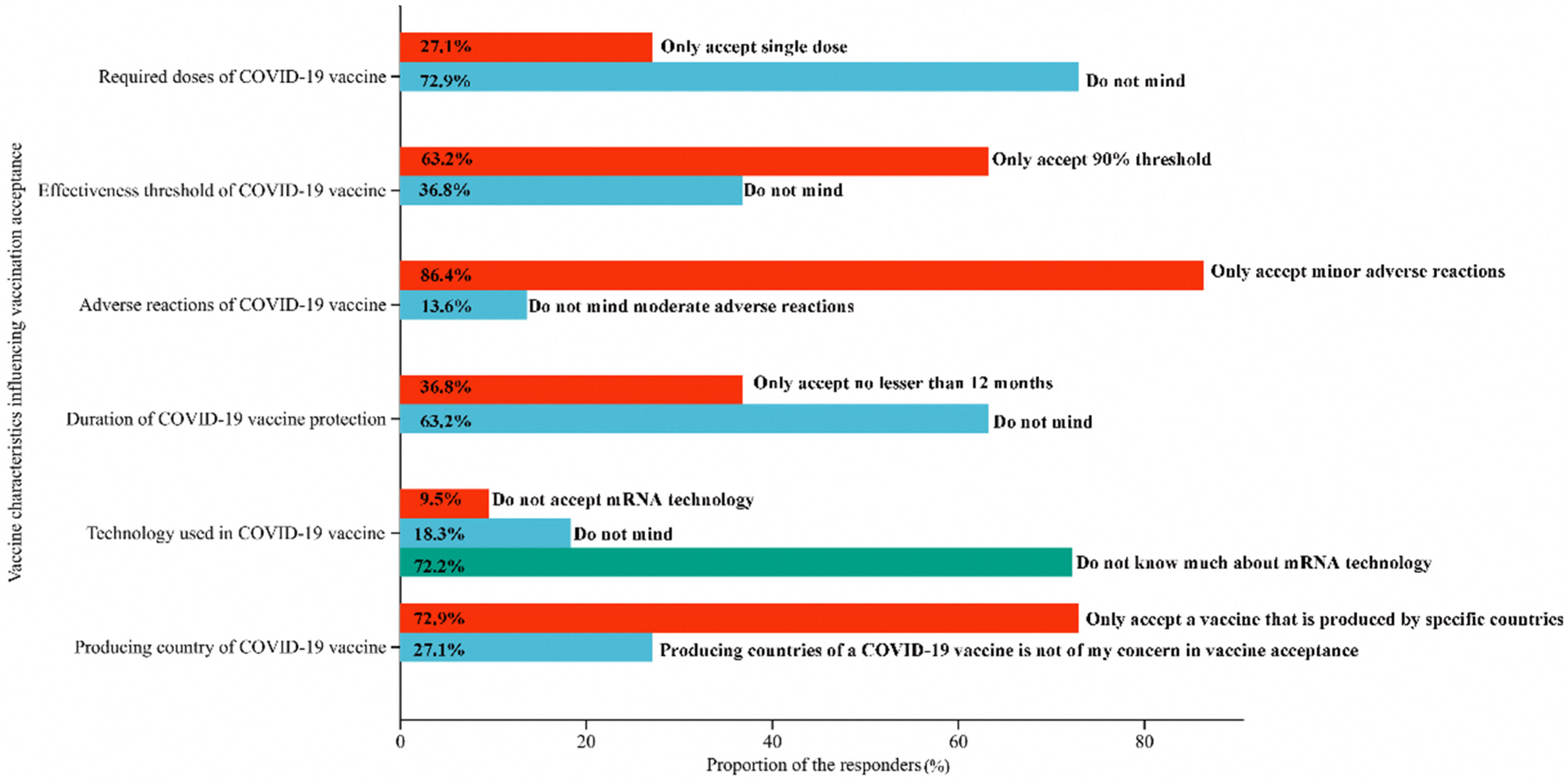
3.3. Effects of Demographic Characteristics on the Vaccine Choice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Fact Sheet: “A Comprehensive View of Global Deaths Directly and Indirectly Associated with the COVID-19 Pandemic”. 2022; Global Excess Deaths Associated with COVID-19, January 2020–December 2021. Available online: https://www.who.int/data/stories/global-excess-deaths-associated-with-covid-19-january-2020-december-2021 (accessed on 31 March 2022).
- Nomura, S.; Eguchi, A.; Tanoue, Y.; Yoneoka, D.; Kawashima, T.; Suzuki, M.; Hashizume, M. Excess deaths from COVID-19 in Japan and 47 prefectures from January through June 2021. Public Health 2022, 203, 15–18. [Google Scholar] [CrossRef] [PubMed]
- VIPER Group COVID-19 Vaccine Development and Approvals Tracker Team. COVID-19 Vaccine Development and Approvals Tracker. 2020. Available online: https://covid19.trackvaccines.org/ (accessed on 31 March 2022).
- Our World In Data. Coronavirus (COVID-19) Vaccinations. 2022. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 31 March 2022).
- WHO. Ten Threats to Global Health in 2019. Available online: https://www.who.int/vietnam/news/feature-stories/detail/ten-threats-to-global-health-in-2019 (accessed on 31 March 2022).
- MacDonald, N.E.; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. European Parliament Resolution of 19 April 2018 on Vaccine Hesitancy and Drop in Vaccination Rates in Europe (2017/2951 RSP). Available online: https://www.europarl.europa.eu/doceo/document/TA-8-2018-0188_EN.pdf (accessed on 31 March 2022).
- Abdool Karim, S.S.; de Oliveira, T. New SARS-CoV-2 variants—Clinical, public health, and vaccine implications. N. Engl. J. Med. 2021, 384, 1866–1868. [Google Scholar] [CrossRef]
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef]
- Schwarzinger, M.; Watson, V.; Arwidson, P.; Alla, F.; Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: A survey experiment based on vaccine characteristics. Lancet Public Health 2021, 6, 210–221. [Google Scholar] [CrossRef]
- Callaway, E. Russia’s fast-track coronavirus vaccine draws outrage over safety. Nature 2020, 584, 334–335. [Google Scholar] [CrossRef]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines 2021, 9, 16. [Google Scholar] [CrossRef]
- Kreps, S.; Prasad, S.; Brownstein, J.S.; Hswen, Y.; Garibaldi, B.T.; Zhang, B.; Kriner, D.L. Factors Associated with US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. Open 2020, 3, e2025594. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- Wong, L.P.; Alias, H.; Danaee, M.; Ahmed, J.; Lachyan, A.; Cai, C.Z.; Lin, Y.; Hu, Z.; Tan, S.Y.; Lu, Y.; et al. COVID-19 vaccination intention and vaccine characteristics influencing vaccination acceptance: A global survey of 17 countries. Infect. Dis. Poverty 2021, 10, 122–136. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, A.; Simas, C.; Karafillakis, E.; Paterson, P.; Larson, H.J. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: A large-scale retrospective temporal modelling study. Lancet 2020, 396, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Lorini, C.; Santomauro, F.; Donzellini, M.; Capecchi, L.; Bechini, A.; Boccalini, S.; Bonanni, P.; Bonaccorsi, G. Health literacy and vaccination: A systematic review. Hum. Vaccines Immunother. 2018, 14, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Clarke, R.M.; Jarrett, C.; Eckersberger, E.; Levine, Z.; Schulz, W.S.; Paterson, P. Measuring trust in vaccination: A systematic review. Hum. Vaccines Immunother. 2018, 14, 1599–1609. [Google Scholar] [CrossRef]
- Steens, A.; Stefanoff, P.; Daae, A.; Vestrheim, D.F.; Bergsaker, M.A.R. High overall confidence in childhood vaccination in Norway, slightly lower among the unemployed and those with a lower level of education. Vaccine 2020, 38, 4536–4541. [Google Scholar] [CrossRef]
- Taylor, V.M.; Seng, P.; Acorda, E.; Sawn, L.; Li, L. Hepatitis B Knowledge and Practices Among Cambodian Immigrants. J. Cancer Educ. 2009, 24, 100–104. [Google Scholar] [CrossRef]
- Cheung, J.; Lee, T.K.; Teh, C.-Z.; Wang, C.Y.; Kwan, W.P.; Yoshida, E.M. Cross-sectional study of Hepatitis B Awareness among Chinese and Southeast Asian Canadians in the Vancouver-Richmond community. Can. J. Gastroenterol. 2005, 19, 245–249. [Google Scholar] [CrossRef]
- Wai, C.-T.; Mak, B.; Chua, W.; Tan, M.-H.; Ng, S.; Cheok, A.; Wong, M.-L.; Lim, S.-G. Misperceptions among patients with chronic hepatitis B in Singapore. World J. Gastroenterol. 2005, 11, 5002–5005. [Google Scholar] [CrossRef]
- WHO. Safety of COVID-19 Vaccines. Available online: https://www.who.int/news-room/feature-stories/detail/safety-of-covid-19-vaccines (accessed on 31 March 2021).
- Amit, S.; Regev-Yochay, G.; Afek, A.; Kreiss, Y.; Leshem, E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021, 397, 875–877. [Google Scholar] [CrossRef]
- Abbasi, J. COVID-19 and mRNA Vaccines—First Large Test for a New Approach. JAMA 2020, 324, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Doshi, P. Will COVID-19 vaccines save lives? Current trials aren’t designed to tell us. BMJ 2020, 371, m4037. [Google Scholar] [CrossRef] [PubMed]


| Socio-Demographic | N | |
|---|---|---|
| Age, years | ||
| 18–29 | 182 | 17.6% |
| 30–39 | 200 | 19.3% |
| 40–49 | 207 | 20.0% |
| 50–59 | 167 | 16.1% |
| 60–69 | 281 | 27.1% |
| Gender | ||
| Male | 456 | 44.0% |
| Female | 581 | 56.0% |
| Highest education level | ||
| Secondary school and below | 237 | 22.9% |
| Certificate/A-level/diploma | 199 | 19.2% |
| Bachelor’s degree | 417 | 40.2% |
| Postgraduate degree | 184 | 17.7% |
| Ever delayed acceptance of or refused vaccine despite availability of vaccine service | ||
| No | 876 | 84.5% |
| Yes | 161 | 15.5% |
| Demographic | Extremely Likely | Likely | Unlikely | Extremely Unlikely | p |
|---|---|---|---|---|---|
| Age, years | 0.007 * | ||||
| 18–29 | 48 (26.4%) | 83 (45.6%) | 44 (24.2%) | 7 (3.8%) | |
| 30–39 | 34 (17.0%) | 89 (44.5%) | 67 (33.5%) | 10 (5.0%) | |
| 40–49 | 29 (14.0%) | 87 (42.0%) | 80 (38.6%) | 11 (5.3%) | |
| 50–59 | 35 (21.0%) | 72 (43.1%) | 55 (32.9%) | 5 (3.0%) | |
| 60 and above | 73 (26.0%) | 129 (45.9%) | 74 (26.3%) | 5 (1.8%) | |
| Gender | <0.001 | ||||
| Male | 124 (27.2%) | 225 (49.3%) | 82 (18.0%) | 25 (5.5%) | |
| Female | 95 (16.4%) | 235 (40.4%) | 238 (41.0%) | 13 (2.2%) | |
| Highest education level | 0.053 | ||||
| Secondary school and below | 38 (16.0%) | 104 (43.9%) | 81 (34.2%) | 14 (5.9%) | |
| Certificate/A-level/diploma | 35 (17.6%) | 85 (42.7%) | 71 (35.7%) | 8 (4.0%) | |
| Bachelor’s degree | 99 (23.7%) | 191 (45.8%) | 117 (28.1%) | 10 (2.4%) | |
| Postgraduate degree | 47 (25.5%) | 80 (43.5%) | 51 (27.7%) | 6 (3.3%) | |
| Ever delayed acceptance of or refuse vaccine despite availability of vaccine service | <0.001 | ||||
| No | 201 (22.9%) | 407 (46.5%) | 243 (27.7%) | 25 (2.9%) | |
| Yes | 18 (11.2%) | 53 (32.9%) | 77 (47.8%) | 13 (8.1%) | |
| Demographic | Participants (N = 1037) | Extremely Unlikely/Unlikely vs. Extremely Likely/Likely to Accept COVID-19 Vaccination | |
|---|---|---|---|
| Univariate OR (95% CI) | Multivariate OR (95% CI) | ||
| Age, years | |||
| 18–29 | 182 | 1.00 (reference) | 1.00 (reference) |
| 30–39 | 200 | 1.61 (1.04, 2.48) * | 1.63 (1.04, 2.58) * |
| 40–49 | 207 | 2.01 (1.32, 3.09) * | 1.88 (1.20, 2.58) * |
| 50–59 | 167 | 1.44 (0.91, 2.26) | 1.25 (0.77, 2.03) |
| 60 and above | 281 | 1.00 (0.66, 1.52) | 0.80 (0.51, 1.26) |
| Gender | |||
| Male | 456 | 1.00 (reference) | 1.00 (reference) |
| Female | 581 | 2.48 (1.89, 3.26) * | 2.66 (1.99, 3.58) * |
| Highest education attainment | |||
| Secondary school and below | 237 | 1.00 (reference) | 1.00 (reference) |
| Certificate/A-level/diploma | 199 | 0.98 (0.66, 1.44) | 0.82 (0.53, 1.23) |
| Bachelor’s degree | 417 | 0.65 (0.46, 0.91) * | 0.73 (0.51, 1.05) |
| Postgraduate degree | 184 | 0.67 (0.44, 1.00) | 0.64 (0.40, 1.00) |
| Ever delayed acceptance of or refuse vaccine despite availability of vaccine service | |||
| No | 876 | 1.00 (reference) | 1.00 (reference) |
| Yes | 161 | 2.87 (2.04, 4.06) * | 3.49 (2.42, 5.05) * |
| Demographic | Required Doses of COVID-19 Vaccine a | Effectiveness Threshold of COVID-19 Vaccine b | Adverse Reactions of COVID-19 Vaccine c | Duration of COVID-19 Vaccine Protection d | Technology Used in COVID-19 Vaccine e | Producing Country of COVID-19 Vaccine f |
|---|---|---|---|---|---|---|
| Multivariate OR (95% CI) | Multivariate OR (95% CI) | Multivariate OR (95% CI) | Multivariate OR (95% CI) | Multivariate OR (95% CI) | Multivariate OR (95% CI) | |
| Age, years | ||||||
| 18–29 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 30–39 | 1.14 (0.72, 1.82) | 0.83 (0.53, 1.28) | 0.98 (0.55, 1.71) | 1.03 (0.67, 1.58) | 0.69 (0.36, 1.29) | 1.25 (0.80, 1.95) |
| 40–49 | 0.81 (0.50, 1.30) | 0.58 (0.37, 0.89) * | 1.31 (0.72, 2.39) | 0.86 (0.56, 1.33) | 0.81 (0.43, 1.50) | 1.52 (0.96, 2.41) |
| 50–59 | 0.80 (0.48, 1.31) | 0.46 (0.29, 0.73) * | 1.43 (0.75, 2.78) | 0.77 (0.48, 1.22) | 0.40 (0.17, 0.87) * | 1.38 (0.85, 2.26) |
| 60 and above | 0.64 (0.40, 1.00) | 0.62 (0.41, 0.94) * | 1.08 (0.62, 1.85) | 0.97 (0.65, 1.46) | 0.44 (0.22, 0.86) * | 1.13 (0.74, 1.73) |
| Gender | ||||||
| Male | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Female | 1.56 (1.15, 2.10) * | 1.61 (1.23, 2.10) * | 1.54 (1.06, 2.23) * | 1.42 (1.08, 1.86) * | 1.04 (0.67, 1.63) | 1.68 (1.26, 2.24) * |
| Highest education level | ||||||
| Secondary school and below | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Certificate/A-level/diploma | 0.87 (0.57, 1.31) | 1.43 (0.94, 2.18) | 1.20 (0.66, 2.21) | 0.78 (0.52, 1.16) | 0.99 (0.47, 2.03) | 1.49 (0.94, 2.38) |
| Bachelor’s degree | 0.64 (0.44, 0.91) * | 0.80 (0.57, 1.13) | 0.92 (0.57, 1.47) | 0.66 (0.47, 0.93) * | 0.85 (0.46, 1.60) | 1.14 (0.79, 1.65) |
| Postgraduate degree | 0.31 (0.18, 0.51) * | 0.89 (0.58, 1.36) | 1.27 (0.70, 2.36) | 0.60 (0.39, 0.91) * | 2.19 (1.17, 4.17) * | 0.92 (0.59, 1.43) |
| Ever delayed acceptance of or refused vaccine despite availability of vaccine service | ||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 2.06 (1.42, 2.96) * | 1.23 (0.86, 1.78) | 1.33 (0.80, 2.34) | 2.17 (1.54, 3.07) | 2.11 (1.25, 3.45) | 0.87 (0.60, 1.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Cai, G.; Fan, Y.; Arima, K.; Lin, Y.; Wong, L.; Zhang, Z.; Yamamoto, T.; Morita, K.; Yoshikawa, A.; et al. Acceptance and Preference for COVID-19 Vaccine among Japanese Residents at Early Stage of the Epidemic in Japan. Vaccines 2023, 11, 157. https://doi.org/10.3390/vaccines11010157
Wu J, Cai G, Fan Y, Arima K, Lin Y, Wong L, Zhang Z, Yamamoto T, Morita K, Yoshikawa A, et al. Acceptance and Preference for COVID-19 Vaccine among Japanese Residents at Early Stage of the Epidemic in Japan. Vaccines. 2023; 11(1):157. https://doi.org/10.3390/vaccines11010157
Chicago/Turabian StyleWu, Jiwen, Guoxi Cai, Yi Fan, Kazuhiko Arima, Yulan Lin, Liping Wong, Zhuo Zhang, Taro Yamamoto, Kouichi Morita, Akira Yoshikawa, and et al. 2023. "Acceptance and Preference for COVID-19 Vaccine among Japanese Residents at Early Stage of the Epidemic in Japan" Vaccines 11, no. 1: 157. https://doi.org/10.3390/vaccines11010157
APA StyleWu, J., Cai, G., Fan, Y., Arima, K., Lin, Y., Wong, L., Zhang, Z., Yamamoto, T., Morita, K., Yoshikawa, A., Lu, Y., & Aoyagi, K. (2023). Acceptance and Preference for COVID-19 Vaccine among Japanese Residents at Early Stage of the Epidemic in Japan. Vaccines, 11(1), 157. https://doi.org/10.3390/vaccines11010157







