Recombinant GPEHT Fusion Protein Derived from HTLV-1 Proteins with Alum Adjuvant Induces a High Immune Response in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Engineering
2.2. Plasmid Construction, Expression, and Purification of GPEHT Protein
2.3. Protein Purification and Dialysis
2.4. Animals
2.5. Experimental Groups and Immunization
2.6. Spleen Cell Suspension and Cytokine Analysis
2.7. Specific Total IgG, IgG1, and IgG2a Isotypes
2.8. Statistical Analysis
3. Results
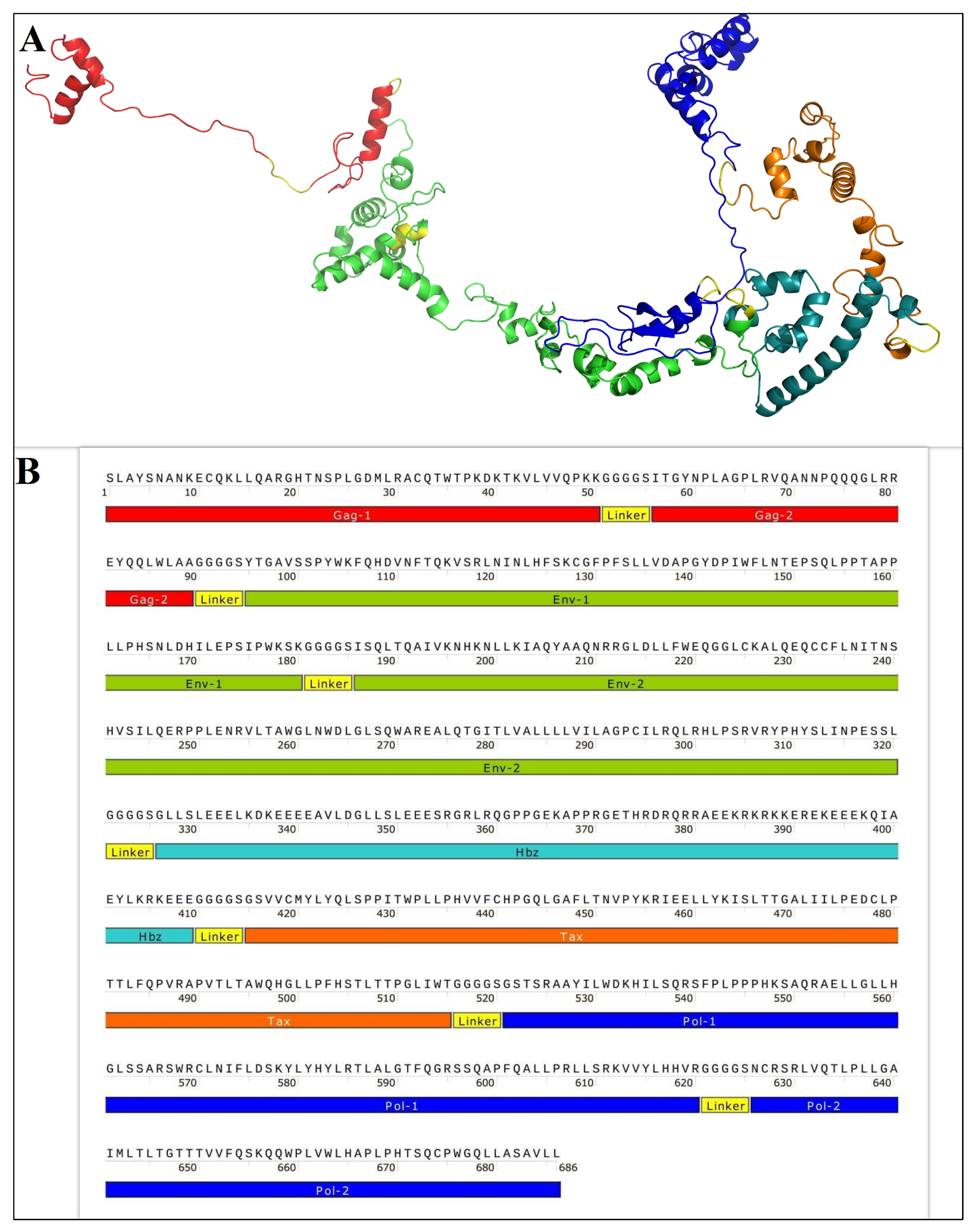
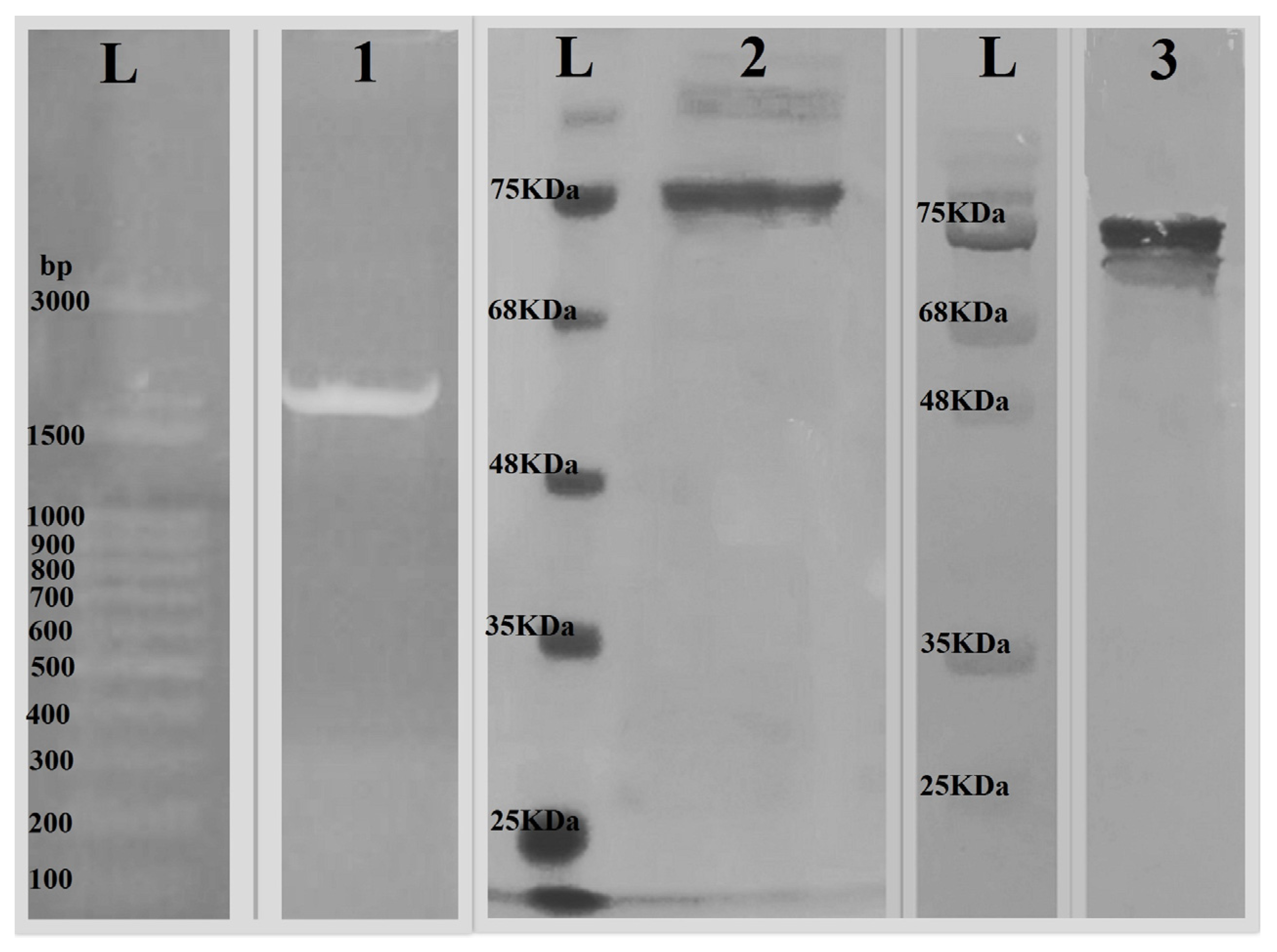
3.1. Codon Optimization and Cloning
3.2. Expression, Purification, and Characterization of the GPEHT Recombinant Protein
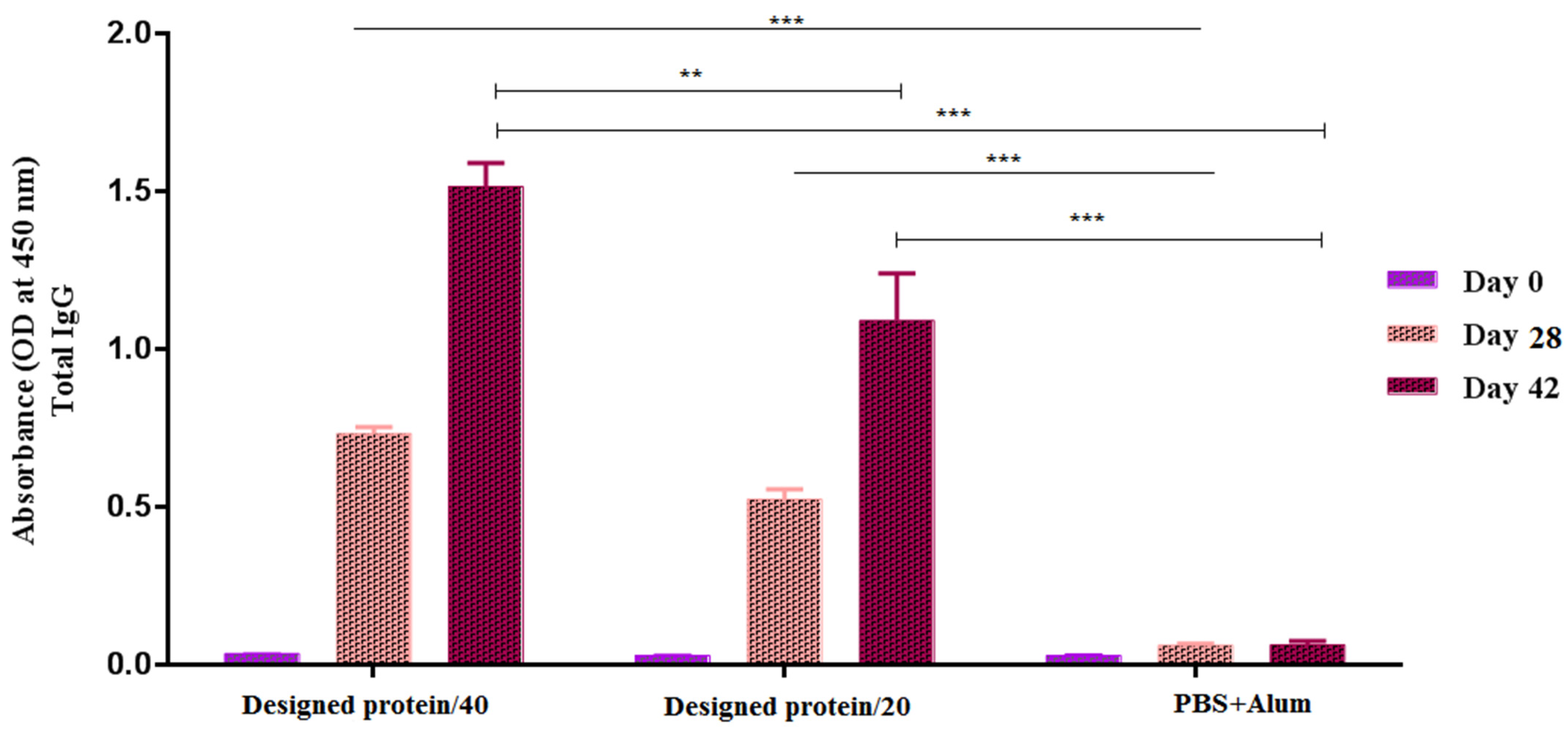
3.3. Analysis of the Humoral Immune Responses
3.4. Measurement of Antibody Isotype Responses
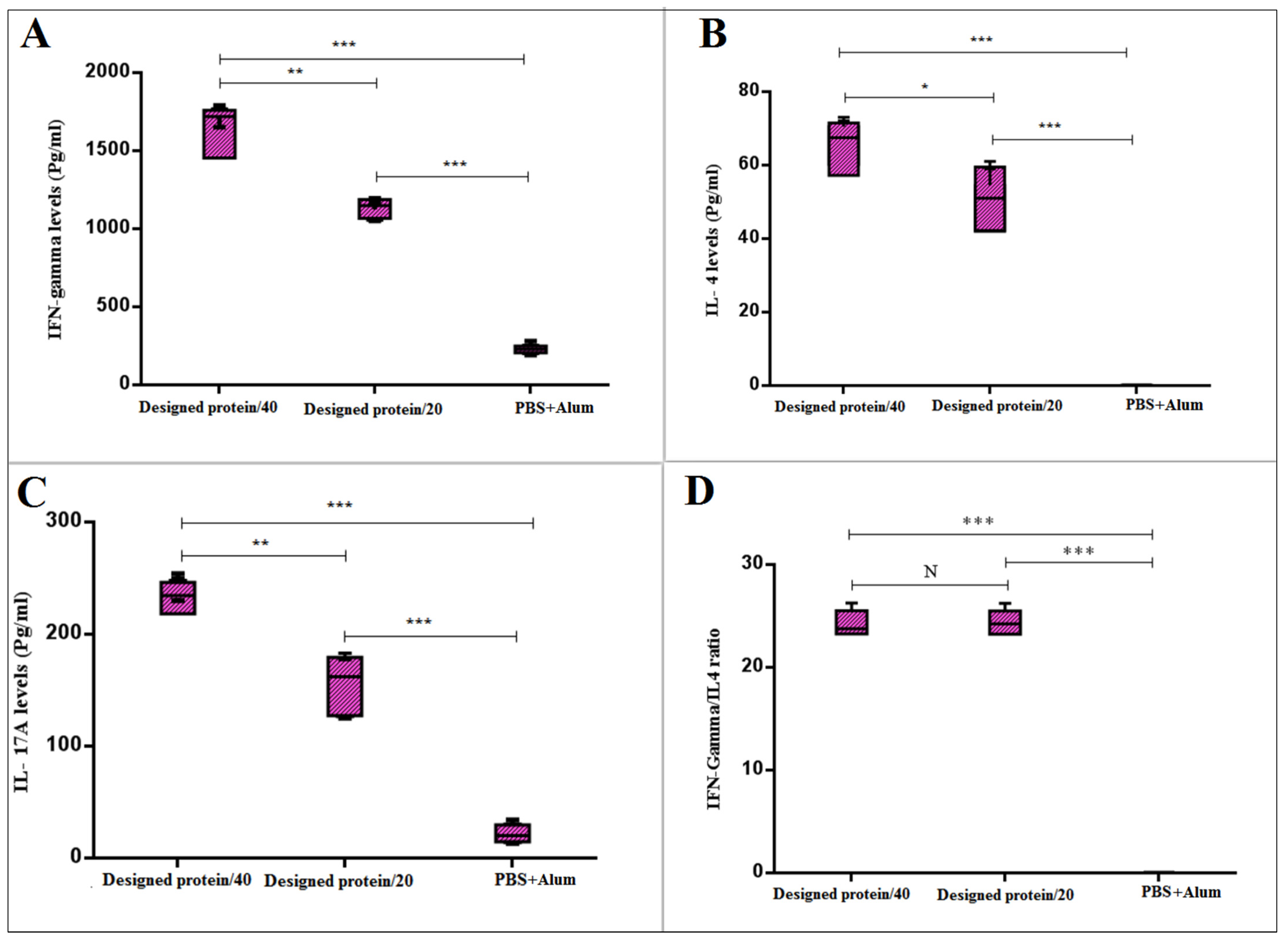
3.5. IL-17, IL-4, and IFN-γ Cytokines of Experimental Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bangham, C.R. Human T cell leukemia virus type 1: Persistence and pathogenesis. Annu. Rev. Immunol. 2018, 36, 43–71. [Google Scholar] [CrossRef]
- Matsuoka, M.; Yasunaga, J.-I. Human T-cell leukemia virus type 1: Replication, proliferation and propagation by Tax and HTLV-1 bZIP factor. Curr. Opin. Virol. 2013, 3, 684–691. [Google Scholar] [CrossRef] [Green Version]
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangham, C.R.; Araujo, A.; Yamano, Y.; Taylor, G.P. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat. Rev. Dis. Prim. 2015, 1, 1–17. [Google Scholar]
- Phillips, A.A.; Harewood, J.C. Adult T cell leukemia-lymphoma (ATL): State of the art. Curr. Hematol. Malig. Rep. 2018, 13, 300–307. [Google Scholar] [CrossRef]
- Eusebio-Ponce, E.; Anguita, E.; Paulino-Ramirez, R.; Candel, F.J. HTLV-1 infection: An emerging risk. Pathogenesis, epidemiology, diagnosis and associated diseases. Rev. Española Quimioter. 2019, 32, 485. [Google Scholar]
- Bandeira, L.M.; Uehara, S.N.; Puga, M.A.; Rezende, G.R.; Vicente, A.C.; Domingos, J.A.; do Lago, B.V.; Niel, C.; Motta-Castro, A.R. HTLV-1 intrafamilial transmission among Japanese immigrants in Brazil. J. Med. Virol. 2018, 90, 351–357. [Google Scholar] [CrossRef]
- Tamiya, S.; Matsuoka, M.; Etoh, K.I.; Watanabe, T.; Kamihira, S.; Yamaguchi, K.; Takatsuki, K. Two types of defective human T-lymphotropic virus type I provirus in adult T-cell leukemia. Blood 1996, 88, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, M.; Inoue, J.; Yoshida, M.; Seiki, M. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988, 7, 519–523. [Google Scholar] [CrossRef]
- Le Blanc, I.; Grange, M.P.; Delamarre, L.; Rosenberg, A.R.; Blot, V.; Pique, C.; Dokhelar, M.C. HTLV-1 structural proteins. Virus Res. 2001, 78, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ernzen, K.J.; Panfil, A.R. Regulation of HTLV-1 transformation. Biosci. Rep. 2022, 42, BSR20211921. [Google Scholar] [CrossRef]
- Robek, M.D.; Wong, F.-H.; Ratner, L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J. Virol. 1998, 72, 4458–4462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Nisbet, J.W.; Bartoe, J.T.; Ding, W.; Lairmore, M.D. Human T-Lymphotropic Virus Type 1 p30IIFunctions as a Transcription Factor and Differentially Modulates CREB-Responsive Promoters. J. Virol. 2000, 74, 11270–11277. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Matsuoka, M. HBZ and its roles in HTLV-1 oncogenesis. Front. Microbiol. 2012, 3, 247. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Satou, Y.; Sugata, K.; Miyazato, P.; Green, P.L.; Imamura, T.; Matsuoka, M. HTLV-1 bZIP factor enhances TGF-β signaling through p300 coactivator. Blood J. Am. Soc. Hematol. 2011, 118, 1865–1876. [Google Scholar]
- Ma, G.; Yasunaga, J.-I.; Matsuoka, M. Multifaceted functions and roles of HBZ in HTLV-1 pathogenesis. Retrovirology 2016, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panfil, A.R.; Dissinger, N.J.; Howard, C.M.; Murphy, B.M.; Landes, K.; Fernandez, S.A.; Green, P.L. Functional comparison of HBZ and the related APH-2 protein provides insight into human T-cell leukemia virus type 1 pathogenesis. J. Virol. 2016, 90, 3760–3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra-Kaushik, S.; Harding, J.; Hess, J.; Schreiber, R.; Ratner, L. Enhanced tumorigenesis in HTLV-1 tax-transgenic mice deficient in interferon-gamma. Blood 2004, 104, 3305–3311. [Google Scholar] [CrossRef]
- Grassmann, R.; Aboud, M.; Jeang, K.-T. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 2005, 24, 5976–5985. [Google Scholar] [CrossRef] [Green Version]
- Hieshima, K.; Nagakubo, D.; Nakayama, T.; Shirakawa, A.K.; Jin, Z.; Yoshie, O. Tax-inducible production of CC chemokine ligand 22 by human T cell leukemia virus type 1 (HTLV-1)-infected T cells promote preferential transmission of HTLV-1 to CCR4-expressing CD4+ T cells. J. Immunol. 2008, 180, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.S.; Petrow-Sadowski, C.; Bertolette, D.C.; Huang, Y.; Ruscetti, F.W. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 2005, 79, 12692–12702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.M.; Blot, V.; Janvier, S.; Arnulf, B.; van Endert, P.M.; Heveker, N.; Pique, C.; et al. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 2006, 80, 6844–6854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manel, N.; Kim, F.J.; Kinet, S.; Taylor, N.; Sitbon, M.; Battini, J.L. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 2003, 115, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.J.; Maksimova, V.; Ratner, L.; Panfil, A.R. The Past, Present, and Future of a Human T-Cell Leukemia Virus Type 1 Vaccine. Front. Microbiol. 2022, 13, 897346. [Google Scholar] [CrossRef] [PubMed]
- Frangione-Beebe, M.; Rose, R.; Kaumaya, P.; Schwendeman, S. Microencapsulation of a synthetic peptide epitope for HTLV-1 in biodegradable poly (D, L-lactide-co-glycolide) microspheres using a novel encapsulation technique. J. Microencapsul. 2001, 18, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.; Beebe, M.; Kaumaya, P. Structural and immunogenicity analysis of chimeric B-cell epitope constructs derived from the gp46 and gp21 subunits of the envelope glycoproteins of HTLV-1. J. Pept. Res. 2004, 63, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Kazanji, M.; Heraud, J.M.; Merien, F.; Pique, C.; Gessain, A.; Jacobson, S. Chimeric peptide vaccine composed of B-and T-cell epitopes of human T-cell leukemia virus type 1 induces humoral and cellular immune responses and reduces the proviral load in immunized squirrel monkeys (Saimiri sciureus). J. Gen. Virol. 2006, 87, 1331–1337. [Google Scholar] [CrossRef]
- Jahantigh, H.R.; Stufano, A.; Lovreglio, P.; Rezaee, S.A.; Ahmadi, K. In silico identification of epitope-based vaccine candidates against HTLV-1. J. Biomol. Struct. Dyn. 2022, 40, 6737–6754. [Google Scholar] [CrossRef]
- Ferrando, R.M.; Lay, L.; Polito, L. Gold nanoparticle-based platforms for vaccine development. Drug Discov. Today Technol. 2020, 38, 57–67. [Google Scholar] [CrossRef]
- Raza, M.T.; Mizan, S.; Yasmin, F.; Akash, A.S.; Shahik, S.M. Epitope-based universal vaccine for Human T-lymphotropic virus-1 (HTLV-1). PLoS ONE 2021, 16, e0248001. [Google Scholar] [CrossRef]
- Somvanshi, P.; Khisty, S. Peptide-based DNA delivery system. Med. Nov. Technol. Devices 2021, 11, 100091. [Google Scholar] [CrossRef]
- Kaur, R.; Arora, N.; Rawat, S.S.; Keshri, A.K.; Singh, N.; Show, S.K.; Kumar, P.; Mishra, A.; Prasad, A. Immunoinformatics driven construction of multi-epitope vaccine candidate against Ascaris lumbricoides using its entire immunogenic epitopes. Expert Rev. Vaccines 2021, 20, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Shafifar, M.; Mozhgani, S.H.; Pashabayg, K.R.; Mosavat, A.; Karbalaei, M.; Norouzi, M.; Rezaee, S.A. Selective APC-targeting of a novel Fc-fusion multi-immunodominant recombinant protein (tTax-tEnv: mFcγ2a) for HTLV-1 vaccine development. Life Sci. 2022, 308, 120920. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.S. Adjuvants and Antigen-Delivery Systems for Subunit Vaccines against Tuberculosis; MDPI: Basel, Switzerland, 2021; p. 972. [Google Scholar]
- Xu, L.; Ma, Z.; Li, Y.; Pang, Z.; Xiao, S. Antibody dependent enhancement: Unavoidable problems in vaccine development. Adv. Immunol. 2021, 151, 99–133. [Google Scholar]
- Woodland, D.L.; Hogan, R.J.; Zhong, W. Cellular immunity and memory to respiratory virus infections. Immunol. Res. 2001, 24, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Rocamonde, B.; Carcone, A.; Mahieux, R.; Dutartre, H. HTLV-1 infection of myeloid cells: From transmission to immune alterations. Retrovirology 2019, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kabiri, M.; Sankian, M.; Sadri, K.; Tafaghodi, M. Robust mucosal and systemic responses against HTLV-1 by delivery of multi-epitope vaccine in PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2018, 133, 321–330. [Google Scholar] [CrossRef]
- Arase, H.; Arase, N.; Saito, T. Interferon gamma production by natural killer (NK) cells and NK1. 1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 1996, 183, 2391–2396. [Google Scholar] [CrossRef] [Green Version]
- Lupo, K.B.; Matosevic, S. Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers 2019, 11, 769. [Google Scholar] [CrossRef] [Green Version]
- Lanigan, L.G.; Hildreth, B.E., III; Dirksen, W.P.; Simmons, J.K.; Martin, C.K.; Werbeck, J.L.; Thudi, N.K.; Papenfuss, T.L.; Boyaka, P.N.; Toribio, R.E.; et al. In Vivo Tumorigenesis, Osteolytic Sarcomas, and Tumorigenic Cell Lines from Transgenic Mice Expressing the Human T-Lymphotropic Virus Type 1 (HTLV-1) Tax Viral Oncogene. Am. J. Pathol. 2021, 191, 335–352. [Google Scholar] [CrossRef]
- Aleebrahim-Dehkordi, E.; Molavi, B.; Mokhtari, M.; Deravi, N.; Fathi, M.; Fazel, T.; Mohebalizadeh, M.; Koochaki, P.; Shobeiri, P.; Hasanpour-Dehkordi, A. T helper type (Th1/Th2) responses to SARS-CoV-2 and influenza A (H1N1) virus: From cytokines produced to immune responses. Transpl. Immunol. 2022, 70, 101495. [Google Scholar] [CrossRef] [PubMed]
- Futsch, N.; Prates, G.; Mahieux, R.; Casseb, J.; Dutartre, H. Cytokine networks dysregulation during HTLV-1 infection and associated diseases. Viruses 2018, 10, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Cooper, D.K.; Wang, H.; Chen, P.; He, C.; Cai, Z.; Mou, L.; Luan, S.; Gao, H. Potential pathological role of pro-inflammatory cytokines (IL-6, TNF-α, and IL-17) in xenotransplantation. Xenotransplantation 2019, 26, e12502. [Google Scholar] [CrossRef] [PubMed]
- Saghafi, N.; Elham, A.; Mofrad, M.H. Th17 Cells and Tregs in HTLV-1 Infection. J. Clin. Nurs. Res. 2022, 6, 122–127. [Google Scholar] [CrossRef]
- Carvalho, N.B.; de Lourdes Bastos, M.; Souza, A.S.; Netto, E.M.; Arruda, S.; Santos, S.B.; Carvalho, E.M. Impaired TNF, IL-1β, and IL-17 production and increased susceptibility to Mycobacterium tuberculosis infection in HTLV-1 infected individuals. Tuberculosis 2018, 108, 35–40. [Google Scholar] [CrossRef]
- Doyen, V.; Corazza, F.; Nhu Thi, H.; Le Chi, T.; Truyens, C.; Nagant, C.; Tran Thi Mong, H.; Fils, J.F.; Thi Ngoc Huynh, P.; Michel, O. Hookworm treatment induces a decrease of suppressive regulatory T cell associated with a Th2 inflammatory response. PLoS ONE 2021, 16, e0252921. [Google Scholar] [CrossRef]
- Mills, K.H. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2022, 23, 38–54. [Google Scholar] [CrossRef]
- Hasanzadeh, S.; Habibi, M.; Shokrgozar, M.A.; Ahangari Cohan, R.; Ahmadi, K.; Asadi Karam, M.R.; Bouzari, S. In silico analysis and in vivo assessment of a novel epitope-based vaccine candidate against uropathogenic Escherichia coli. Sci. Rep. 2020, 10, 16258. [Google Scholar] [CrossRef]

| Characteristics | GPEHT |
|---|---|
| Number of amino acids | 686 |
| Molecular weight (dalton) | 76.1598 |
| Total number of negatively charged residues (Asp + Glu) | 51 |
| Total number of positively charged residues (Arg + Lys) | 75 |
| Theoretical isoelectric point (pI) | 9.51 |
| Estimated half-life | 1.9 h in mammalian reticulocytes, >20 h in yeast, and >10 h in E. coli |
| Aliphatic index | 94.27 |
| Grand average hydropathy | −0.283 |
| Antigenicity | 0.4885 |
| Allergenicity | Non-allergenic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahantigh, H.R.; Stufano, A.; Koohpeyma, F.; Nikbin, V.S.; Shahosseini, Z.; Lovreglio, P. Recombinant GPEHT Fusion Protein Derived from HTLV-1 Proteins with Alum Adjuvant Induces a High Immune Response in Mice. Vaccines 2023, 11, 115. https://doi.org/10.3390/vaccines11010115
Jahantigh HR, Stufano A, Koohpeyma F, Nikbin VS, Shahosseini Z, Lovreglio P. Recombinant GPEHT Fusion Protein Derived from HTLV-1 Proteins with Alum Adjuvant Induces a High Immune Response in Mice. Vaccines. 2023; 11(1):115. https://doi.org/10.3390/vaccines11010115
Chicago/Turabian StyleJahantigh, Hamid Reza, Angela Stufano, Farhad Koohpeyma, Vajihe Sadat Nikbin, Zahra Shahosseini, and Piero Lovreglio. 2023. "Recombinant GPEHT Fusion Protein Derived from HTLV-1 Proteins with Alum Adjuvant Induces a High Immune Response in Mice" Vaccines 11, no. 1: 115. https://doi.org/10.3390/vaccines11010115
APA StyleJahantigh, H. R., Stufano, A., Koohpeyma, F., Nikbin, V. S., Shahosseini, Z., & Lovreglio, P. (2023). Recombinant GPEHT Fusion Protein Derived from HTLV-1 Proteins with Alum Adjuvant Induces a High Immune Response in Mice. Vaccines, 11(1), 115. https://doi.org/10.3390/vaccines11010115








