Amphotericin B Nano-Assemblies Circumvent Intrinsic Toxicity and Ensure Superior Protection in Experimental Visceral Leishmaniasis with Feeble Toxic Manifestation
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Animal Model and Experimental Design
2.3. Preparation of Mangifera Indica Fruit Extract
2.4. Biomimetic Synthesis of Nano-Assembled Amphotericin B (AmB-NA)
2.5. In Vitro Release Kinetics
2.6. Toxicity Assessment of the AmB-NA Formulation
2.6.1. Haemolytic Effect of AmB-NA
2.6.2. MTT Assay
2.7. Leishmania Donovani Culture
2.8. Parasiticidal Potential of As-Synthesized AmB-NA against L. donovani
2.9. Effect of Drug on Promastigote Form of the Parasite
2.9.1. Evaluation of Intracellular Reduced Thiol
2.9.2. DNA Fragmentation Assay
2.9.3. Flow Cytometric Analysis of AmB-NA Treated Promastigotes Employing Annexin-V–FITC/Propidium Iodide
2.9.4. Membrane Integrity Assay
2.9.5. Analysis of Variation in Mitochondrial Membrane Potential (ΔΨm)
2.10. Anti-Leishmanial Activity of AmB-NA in a Mouse VL Model
2.11. Soluble Leishmanial Antigen (SLA) Preparation
2.12. Assessment of the Delayed Type of Hypersensitivity (DTH) Response
2.13. Assessment of Serum Immunoglobulin (IgG) Isotype
2.14. AmB-NA Treatment Ensues in Th1/Th2 Cytokine Production
2.15. Determination of Reactive Nitrogen Species (RNS) and Reactive Oxygen Species (ROS)
2.16. Statistical Analysis
3. Results
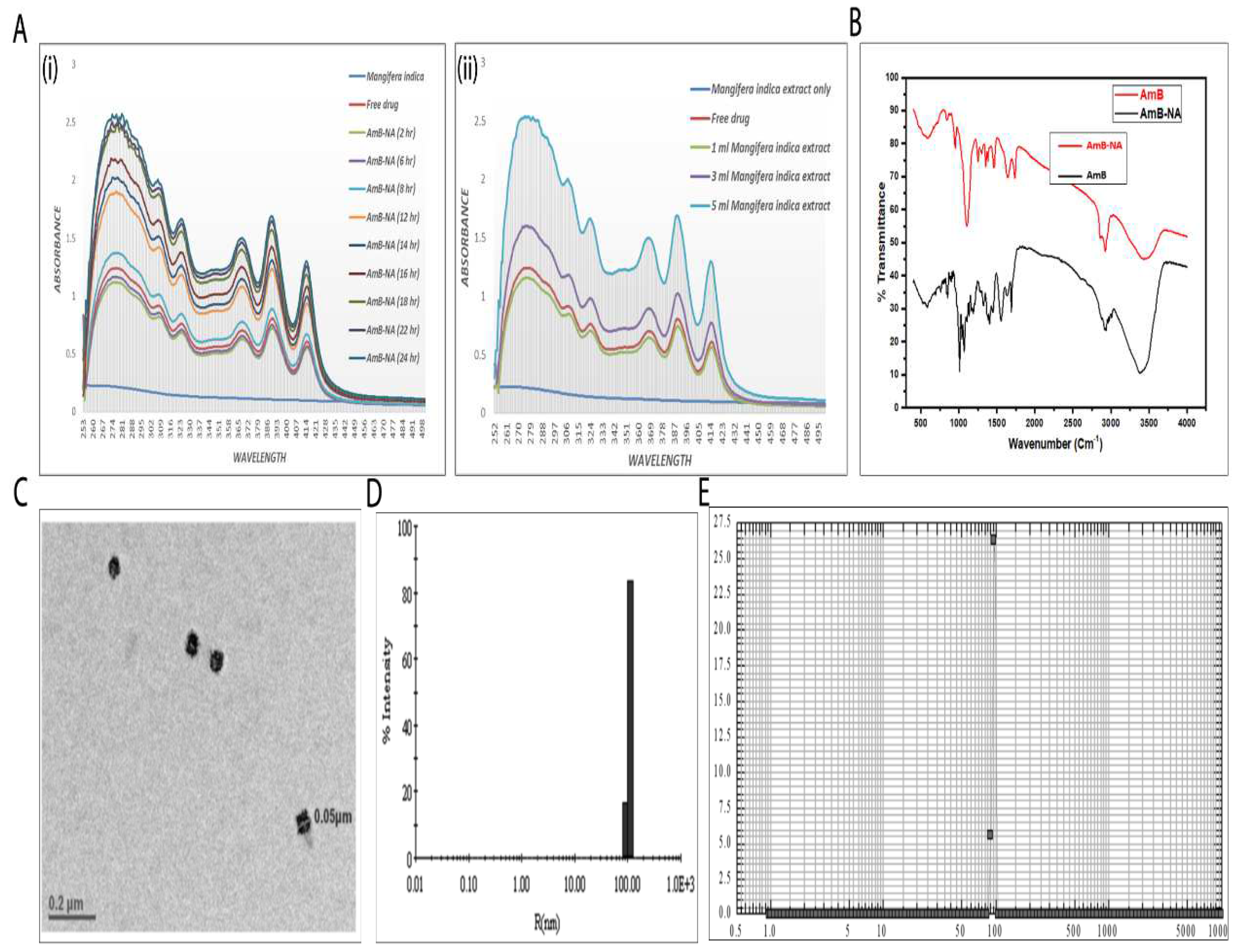
3.1. Spectroscopic Analysis of As-Synthesized AmB-NA Formulation
3.2. Physico-Chemical Characterization of AmB-NA Formulation
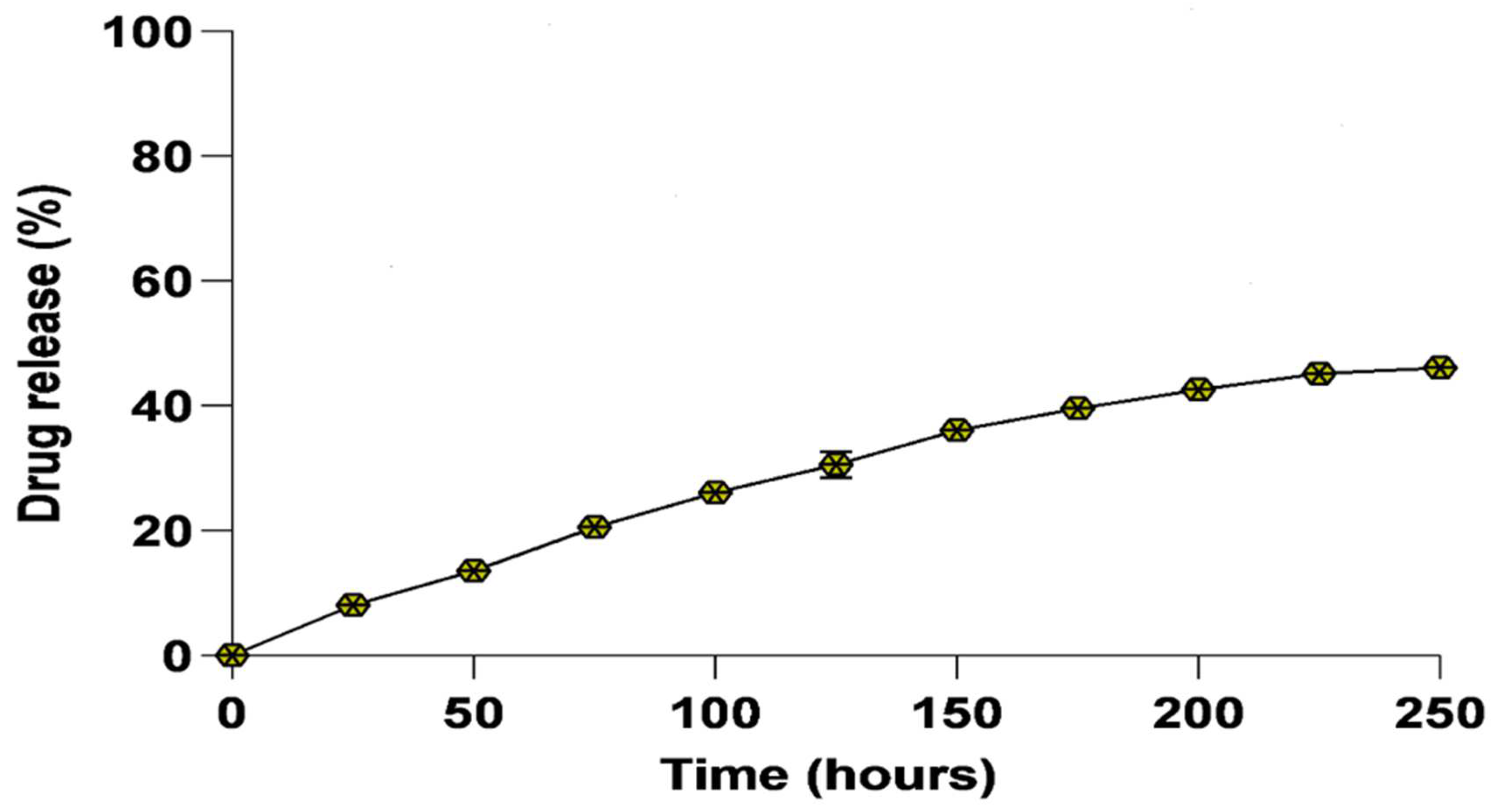
3.3. In Vitro Drug Release Kinetics
3.4. Toxicity Assessment of the AmB-NA Formulation
3.4.1. Assessment of Hemotoxic Effect Revealed Minimal Toxicity of AmB-NA on RBCs
3.4.2. AmB-NA Were Negligibly Toxic to PECs-Derived Macrophages
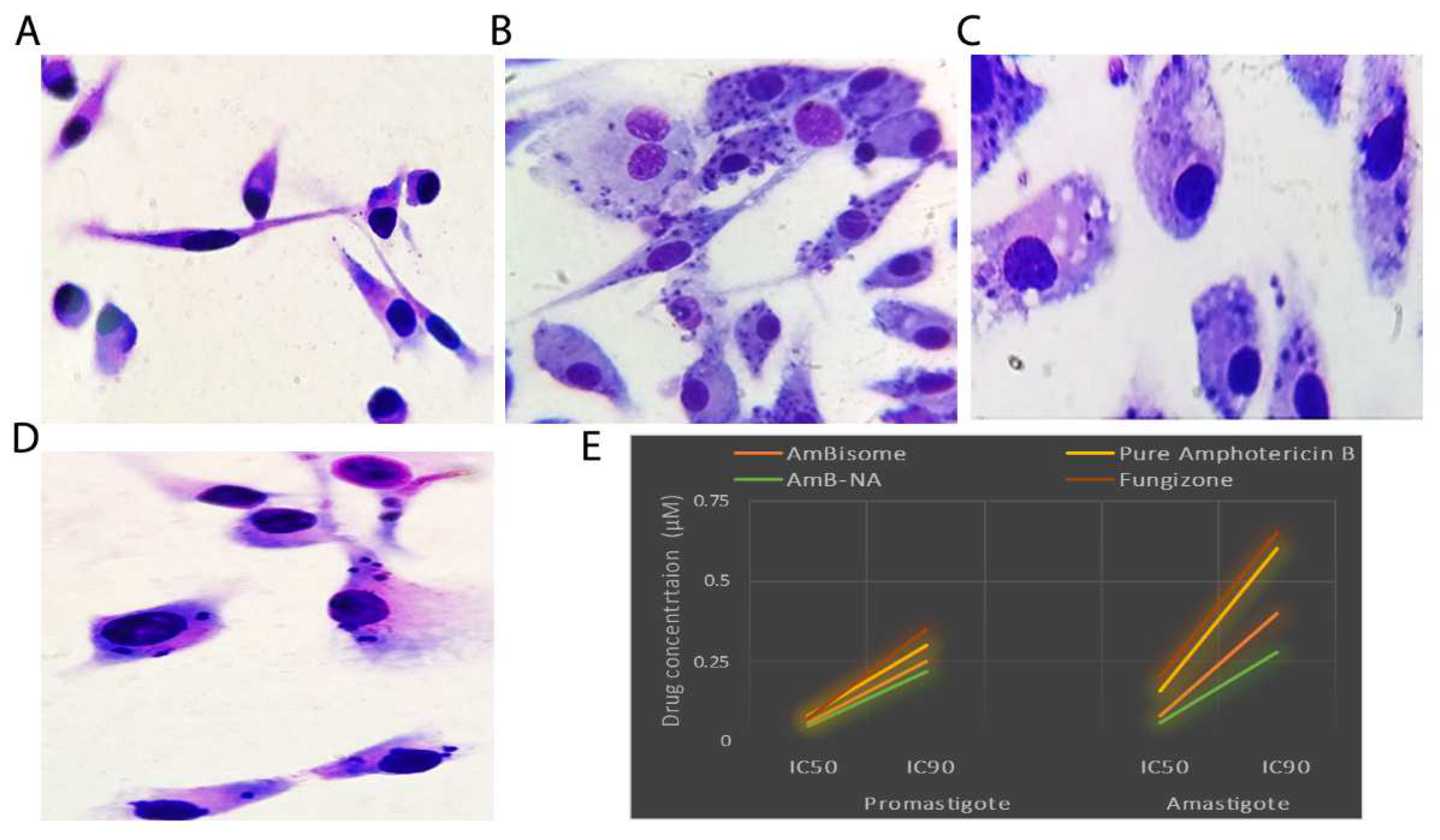
3.5. Killing Potential of AmB-NA on Leishmania-Infected PECs from BALB/c Mice
3.6. Effect of Drug on Leishmania Promastigotes
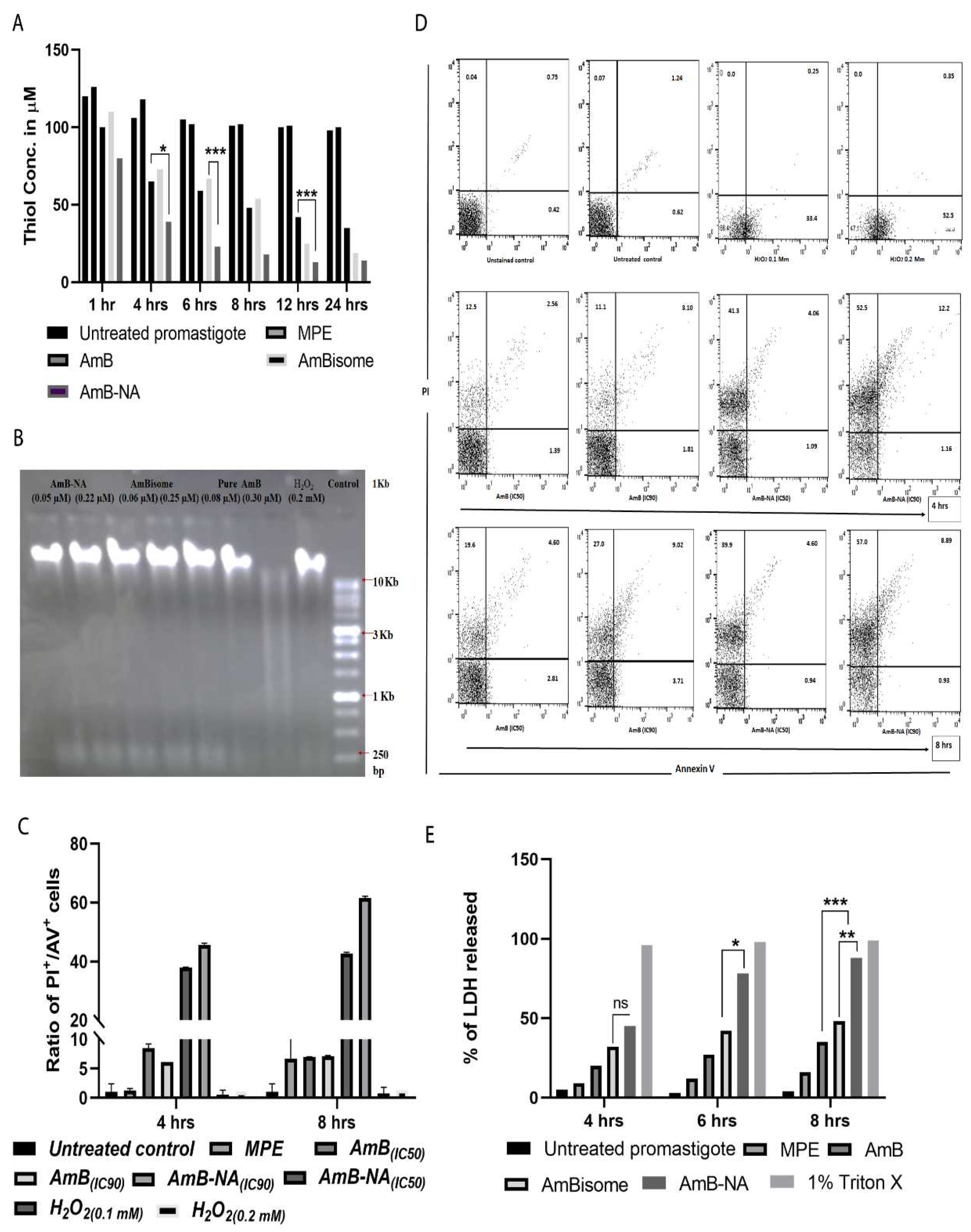
3.6.1. AmB-NA Treatment Reduced Intracellular Thiol Content
3.6.2. No Fragmentation (Apoptosis) of L. donovani DNA following Treatment with AmB-NA
3.6.3. Annexin/PI Assay Revealed Predominant Necrotic Cell Death in the Infected Host Macrophages Post AmB-NA Treatment
3.6.4. Estimation of LDH Membrane Escape in Treated Parasite Post AmB-NA Treatment
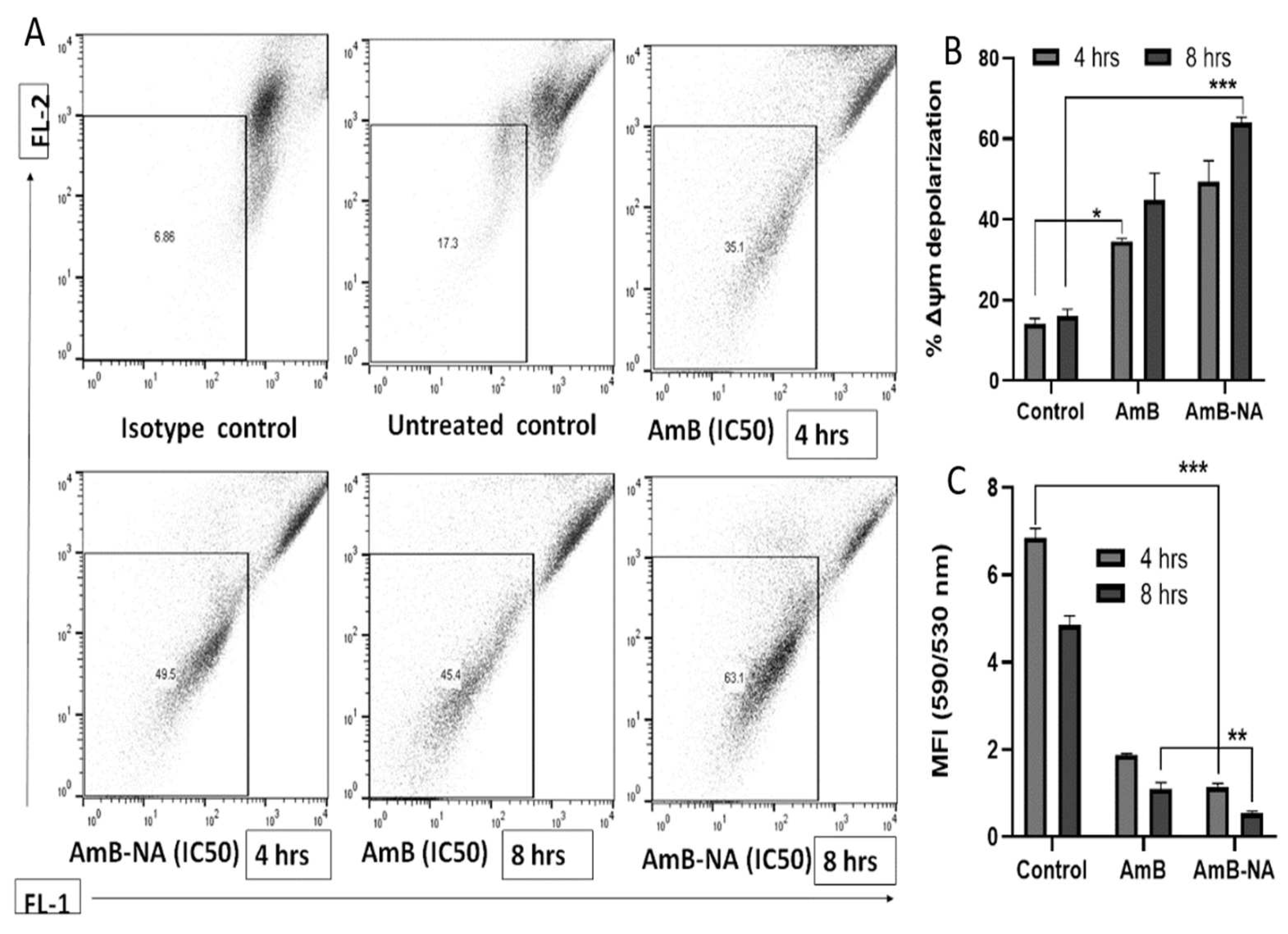
3.6.5. AmB-NA Treatment Induces Mitochondrial Membrane Depolarization
3.7. Assessment of Biochemical Parameters in a Mouse VL Model
3.8. DTH Response Post-Treatment with AmB-NA
3.9. AmB-NA-Treated Mice Induced Higher IgG2a to IgG1 Ratio
3.10. Parasite Burdens in Vital Organs of VL-Challenged Mice
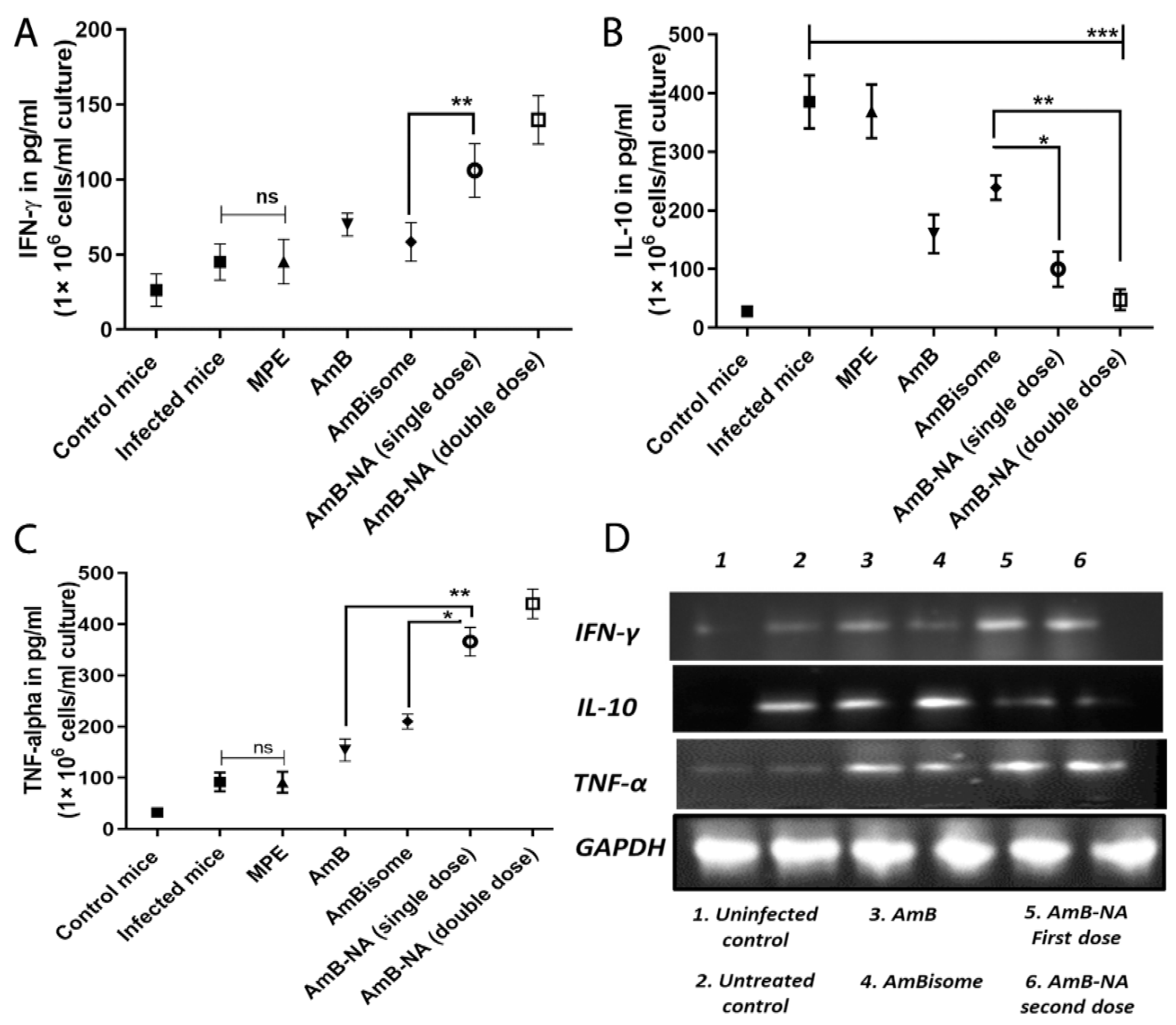
3.11. Quantitative Analysis of T-Helper Type (Th1/Th2) Cytokines
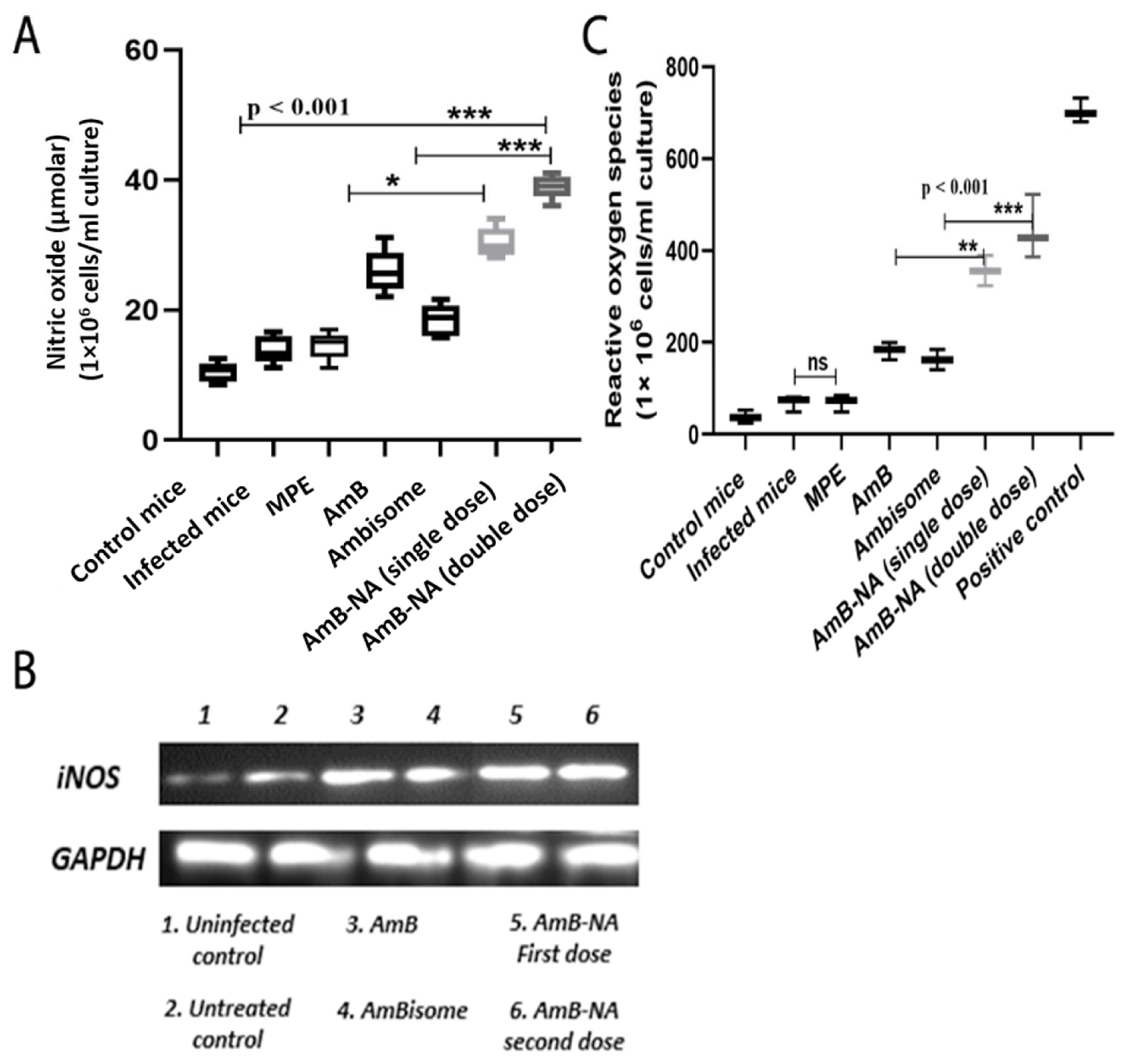
3.12. AmB-NA Administration Increases NO and ROS Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Han, S.; Chen, S.-B.; Yang, Z.-H.; Feng, Y.; Wu, W.-P. Epidemiology of Leishmania Carriers in Tan Chang County, Gansu Province, China. Front. Cell. Infect. Microbiol. 2021, 11, 645944. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Rijal, S.; Sundar, S.; Mondal, D.; Das, P.; Alvar, J.; Boelaert, M. Eliminating visceral leishmaniasis in South Asia: The road ahead. BMJ 2019, 364, k5224. [Google Scholar] [CrossRef] [PubMed]
- Akuffo, H.; Costa, C.; Van Griensven, J.; Burza, S.; Moreno, J.; Herrero, M. New insights into leishmaniasis in the immunosuppressed. PLOS Negl. Trop. Dis. 2018, 12, e0006375. [Google Scholar] [CrossRef] [PubMed]
- Leishmaniasis, WHO 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 19 October 2021).
- Balaska, S.; Fotakis, E.A.; Chaskopoulou, A.; Vontas, J. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLOS Negl. Trop. Dis. 2021, 15, e0009586. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, B.C.; Duthie, M.S.; Pereira, V.R.A. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum. Vaccines Immunother. 2019, 16, 919–930. [Google Scholar] [CrossRef]
- Khanra, S.; Juin, S.K.; Jawed, J.J.; Ghosh, S.; Dutta, S.; Nabi, S.A.; Dash, J.; Dasgupta, D.; Majumdar, S.; Banerjee, R. In vivo experiments demonstrate the potent antileishmanial efficacy of repurposed suramin in visceral leishmaniasis. PLOS Negl. Trop. Dis. 2020, 14, e0008575. [Google Scholar] [CrossRef]
- Khanra, S.; Sarraf, N.R.; Das, A.K.; Roy, S.; Manna, M. Miltefosine Resistant Field Isolate from Indian Kala-Azar Patient Shows Similar Phenotype in Experimental Infection. Sci. Rep. 2017, 7, 10330. [Google Scholar] [CrossRef]
- Ferreira, C.; Mesquita, I.; Barbosa, A.M.; Osório, N.S.; Torrado, E.; Beauparlant, C.-J.; Droit, A.; Cunha, C.; Carvalho, A.; Saha, B.; et al. Glutamine supplementation improves the efficacy of miltefosine treatment for visceral leishmaniasis. PLOS Negl. Trop. Dis. 2020, 14, e0008125. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Zia, Q.; Azhar, A.; Kamal, M.A.; Aliev, G.; Owais, M.; Ashraf, G. Super aggregated form of Amphotericin B: A novel way to increase its therapeutic index. Curr. Pharm. Des. 2016, 22, 792–803. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J.; Agarwal, D.; Rai, M.; Murray, H.W. Single-Dose Liposomal Amphotericin B for Visceral Leishmaniasis in India. N. Engl. J. Med. 2010, 362, 504–512. [Google Scholar] [CrossRef]
- Meheus, F.; Balasegaram, M.; Olliaro, P.; Sundar, S.; Rijal, S.; Faiz, A.; Boelaert, M. Cost-Effectiveness Analysis of Combination Therapies for Visceral Leishmaniasis in the Indian Subcontinent. PLOS Negl. Trop. Dis. 2010, 4, e818. [Google Scholar] [CrossRef]
- Salih, N.A.; Van Griensven, J.; Chappuis, F.; Antierens, A.; Mumina, A.; Hammam, O.; Boulle, P.; Alirol, E.; Alnour, M.; Elhag, M.S.; et al. Liposomal amphotericin B for complicated visceral leishmaniasis (kala-azar) in eastern Sudan: How effective is treatment for this neglected disease? Trop. Med. Int. Health 2014, 19, 146–152. [Google Scholar] [CrossRef]
- Kneale, M.; Bartholomew, J.S.; Davies, E.; Denning, D.W. Global access to antifungal therapy and its variable cost. J. Antimicrob. Chemother. 2016, 71, 3599–3606. [Google Scholar] [CrossRef]
- Bourgeois, N.; Lachaud, L.; Reynes, J.; Rouanet, I.; Mahamat, A.; Bastien, P. Long-term monitoring of visceral leishmaniasis in patients with AIDS: Relapse risk factors, value of polymerase chain reaction, and potential impact on secondary prophylaxis. J. Acquir. Immune Defic. Syndr. 2008, 48, 13–19. [Google Scholar] [CrossRef]
- Morizot, G.; Jouffroy, R.; Faye, A.; Chabert, P.; Belhouari, K.; Calin, R.; Charlier, C.; Miailhes, P.; Siriez, J.-Y.; Mouri, O.; et al. Antimony to Cure Visceral Leishmaniasis Unresponsive to Liposomal Amphotericin B. PLOS Negl. Trop. Dis. 2016, 10, e0004304. [Google Scholar] [CrossRef]
- Buggins, T.R.; Dickinson, P.A.; Taylor, G. The effects of pharmaceutical excipients on drug disposition. Adv. Drug Deliv. Rev. 2007, 59, 1482–1503. [Google Scholar] [CrossRef]
- Zia, Q.; Mohammad, O.; Rauf, M.A.; Khan, W.; Zubair, S. Biomimetically engineered Amphotericin B nano-aggregates circumvent toxicity constraints and treat systemic fungal infection in experimental animals. Sci. Rep. 2017, 7, 11873. [Google Scholar] [CrossRef]
- Barwicz, J.; Christian, S.; Gruda, I. Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob. Agents Chemother. 1992, 36, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Legrand, P.; Romero, E.A.; Cohen, B.E.; Bolard, J. Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob. Agents Chemother. 1992, 36, 2518–2522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, X.-L.; Gu, Y.; Huang, H.; Zhang, G.-W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.; Kumar, J.; Das, A.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.; Kim, B. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Liu, S.; Kurzrock, R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat. Rev. 2014, 40, 883–891. [Google Scholar] [CrossRef]
- Rodallec, A.; Fanciullino, R.; Lacarelle, B.; Ciccolini, J. Seek and destroy: Improving PK/PD profiles of anticancer agents with nanoparticles. Expert Rev. Clin. Pharmacol. 2018, 11, 599–610. [Google Scholar] [CrossRef]
- Du, Y.; Yin, J.; Sargent, D.J.; Mandrekar, S.J. An adaptive multi-stage phase I dose-finding design incorporating continuous efficacy and toxicity data from multiple treatment cycles. J. Biopharm. Stat. 2019, 29, 271–286. [Google Scholar] [CrossRef]
- Morozkina, S.N.; Vu, T.H.N.; Generalova, Y.E.; Snetkov, P.P.; Uspenskaya, M.V. Mangiferin as New Potential Anti-Cancer Agent and Mangiferin-Integrated Polymer Systems—A Novel Research Direction. Biomolecules 2021, 11, 79. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Chauhan, A.; Zubair, S.; Sherwani, A.; Owais, M. Aloe vera Induced Biomimetic Assemblage of Nucleobase into Nanosized Particles. PLoS ONE 2012, 7, e32049. [Google Scholar] [CrossRef]
- Rauf, M.A.; Owais, M.; Rajpoot, R.; Ahmad, F.; Khan, N.; Zubair, S. Biomimetically synthesized ZnO nanoparticles attain potent antibacterial activity against less susceptible S. aureus skin infection in experimental animals. RSC Adv. 2017, 7, 36361–36373. [Google Scholar] [CrossRef]
- Panneerselvi, V.; Shankar, K.; Muthukrishnan, P.; Prabhu, A. Mangifera indica Resin Assisted Synthesis of Nano Silver: Assessing their Photocatalytic Degradation of Methylene Blue, Anticorrosive and Antioxidant Activity. J. Clust. Sci. 2021, 33, 123–133. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Nacher, A.; Hernández, M.J.; Buso, M.O.V.; Sauri, A.R.; Díez-Sales, O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int. J. Pharm. 2019, 564, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, M.; Amoa-Bosompem, M.; Kwofie, K.D.; Agyapong, J.; Adegle, R.; Sakyiamah, M.M.; Ayertey, F.; Owusu, K.B.; Tuffour, I.; Atchoglo, P.; et al. In vitro antiprotozoan activity and mechanisms of action of selected G hanaian medicinal plants against Trypanosoma, Leishmania, and Plasmodium parasites. Phytother. Res. 2018, 32, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Haldar, N.; Basu, S.; Bhattacharya, S.; Pandey, J.N.; Biswas, M. Antileishmanial activity of Mangifera indica leaf extracts on the in vitro growth of Leishmania donovani promastigotes. Exlixir. Pharm. 2012, 46, 8189–8191. [Google Scholar]
- Sharma, L.; Dhiman, M.; Singh, A.; Sharma, M.M. Green Approach: ‘‘A Forwarding Step for Curing Leishmaniasis—A Neglected Tropical Disease’’. Front. Mol. Biosci. 2021, 8, 655584. [Google Scholar] [CrossRef]
- Ul Ain, Q.; Islam, A.; Nadhman, A.; Yasinzai, M. Comparative analysis of chemically and biologically synthesized iron oxide nanoparticles against Leishmania tropica. bioRxiv 2020, 829408. [Google Scholar] [CrossRef]
- Alti, D.; Rao, M.V.; Rao, D.N.; Maurya, R.; Kalangi, S.K. Gold–Silver Bimetallic Nanoparticles Reduced with Herbal Leaf Extracts Induce ROS-Mediated Death in Both Promastigote and Amastigote Stages of Leishmania donovani. ACS Omega 2020, 5, 16238–16245. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Mahmood, T.; Ahmad, R.; Kanwal, S.; Afridi, S. Plant-mediated synthesis of nickel oxide nanoparticles (NiO) via Geranium wallichianum: Characterization and different biological applications. Mater. Res. Express 2019, 6, 0850a7. [Google Scholar] [CrossRef]
- Sarker, A.; Amin, N.; Shimu, I.J.; Akhter, M.P.; Alam, M.A.; Rahman, M.M.; Sultana, H. Antimicrobial activity of methanolic extract of langra mango pulp. J. Pharmacogn. Phytochem. 2017, 6, 28–30. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int. J. Mol. Med. 2020, 47, 37–48. [Google Scholar] [CrossRef]
- Nile, S.H.; Keum, Y.S.; Nile, A.S.; Jalde, S.S.; Patel, R.V. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J. Biochem. Mol. Toxicol. 2017, 32, e22002. [Google Scholar] [CrossRef]
- Ahmed, G.; Thakur, A.K.; Pushpanjali; Snehil; Chaturvedi, S.K.; Shivam, P.; Jamal, F.; Singh, M.K.; Bimal, S.; Singh, S.K.; et al. Modulation of the immune response and infection pattern to Leishmania donovani in visceral leishmaniasis due to arsenic exposure: An in vitro study. PLoS ONE 2019, 14, e0210737. [Google Scholar] [CrossRef]
- Jamal, F.; Shivam, P.; Kumari, S.; Singh, M.K.; Sardar, A.H.; Pushpanjali; Murugesan, S.; Narayan, S.; Gupta, A.K.; Pandey, K.; et al. Identification of Leishmania donovani antigen in circulating immune complexes of visceral leishmaniasis subjects for diagnosis. PLoS ONE 2017, 12, e0182474. [Google Scholar] [CrossRef]
- Kumar, P.; Shivam, P.; Mandal, S.; Prasanna, P.; Kumar, S.; Prasad, S.R.; Kumar, A.; Das, P.; Ali, V.; Singh, S.K.; et al. Synthesis, characterization, and mechanistic studies of a gold nanoparticle–amphotericin B covalent conjugate with enhanced antileishmanial efficacy and reduced cytotoxicity. Int. J. Nanomed. 2019, 14, 6073–6101. [Google Scholar] [CrossRef]
- Chauhan, A.; Zubair, S.; Zia, Q.; Tufail, S.; Sherwani, A.; Sajid, M.; Owais, M. Biomimetic assemblage of nucleobase 5-fluorouracil into nano-size three-dimensional particles. Nat. Preéceéd. 2011. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef]
- Pushpanjali; Ahmed, G.; Thakur, A.K.; Snehil; Jamal, F.; Singh, M.K.; Kumar, A.; Singh, S.K.; Bimal, S.; Das, P.; et al. Exploring new immunological insight on SP15 (∼14 kDa) family protein in saliva of Indian sand-fly (Phlebotomus argentipes) in experimental visceral leishmaniasis. Cell. Immunol. 2018, 332, 51–57. [Google Scholar] [CrossRef]
- Afrin, F.; Rajesh, R.; Anam, K.; Gopinath, M.; Pal, S.; Ali, N. Characterization of Leishmania donovani Antigens Encapsulated in Liposomes That Induce Protective Immunity in BALB/c Mice. Infect. Immun. 2002, 70, 6697–6706. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Leon, R.; Vera-Ku, M.; Peraza-Sanchez, S.R.; Ku-Chulim, C.; Horta-Baas, A.; Rosado-Vallado, M. Antileishmanial activity of a mixture of Tridax procumbens and Allium sativum in mice. Parasite 2014, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Jamal, F.; Dubey, A.K.; Shivam, P.; Kumari, S.; Pushpanjali; Ahmed, G.; Dikhit, M.R.; Narayan, S.; DAS, V.N.R.; et al. Co-factor-independent phosphoglycerate mutase of Leishmania donovani modulates macrophage signalling and promotes T-cell repertoires bearing epitopes for both MHC-I and MHC-II. Parasitology 2017, 145, 292–306. [Google Scholar] [CrossRef]
- Schwartzman, G.; Asher, I.; Folen, V.; Brannon, W.; Taylor, J. Ambiguities in IR and X-Ray Characterization of Amphotericin B. J. Pharm. Sci. 1978, 67, 398–400. [Google Scholar] [CrossRef]
- Gagoś, M.; Arczewska, M.; Gruszecki, W.I. Raman Spectroscopic Study of Aggregation Process of Antibiotic Amphotericin B Induced by H+, Na+, and K+ Ions. J. Phys. Chem. B 2011, 115, 5032–5036. [Google Scholar] [CrossRef]
- Ehrenfreund-Kleinman, T.; Azzam, T.; Falk, R.; Polacheck, I.; Golenser, J.; Domb, A. Synthesis and characterization of novel water soluble amphotericin B–arabinogalactan conjugates. Biomaterials 2002, 23, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Carstens-Kass, J.; Paulini, K.; Lypaczewski, P.; Matlashewski, G. A review of the leishmanin skin test: A neglected test for a neglected disease. PLOS Negl. Trop. Dis. 2021, 15, e0009531. [Google Scholar] [CrossRef]
- Banerjee, A.; De, M.; Ali, N. Complete Cure of Experimental Visceral Leishmaniasis with Amphotericin B in Stearylamine-Bearing Cationic Liposomes Involves Down-Regulation of IL-10 and Favorable T Cell Responses. J. Immunol. 2008, 181, 1386–1398. [Google Scholar] [CrossRef]
- Afrin, F.; Chouhan, G.; Islamuddin, M.; Want, M.Y.; Ozbak, H.A.; Hemeg, H.A. Cinnamomum cassia exhibits antileishmanial activity against Leishmania donovani infection in vitro and in vivo. PLOS Negl. Trop. Dis. 2019, 13, e0007227. [Google Scholar] [CrossRef]
- Dikhit, M.R.; Kumar, A.; Das, S.; Dehury, B.; Rout, A.K.; Jamal, F.; Sahoo, G.C.; Topno, R.K.; Pandey, K.; Das, V.N.R.; et al. Identification of Potential MHC Class-II-Restricted Epitopes Derived from Leishmania donovani Antigens by Reverse Vaccinology and Evaluation of Their CD4+ T-Cell Responsiveness against Visceral Leishmaniasis. Front. Immunol. 2017, 8, 1763. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLOS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Liposomal amphotericin B and leishmaniasis: Dose and response. J. Glob. Infect. Dis. 2010, 2, 159–166. [Google Scholar] [CrossRef]
- Deray, G. Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 2002, 49 (Suppl. S1), 37–41. [Google Scholar] [CrossRef]
- Barratt, G.; Bretagne, S. Optimizing efficacy of Amphotericin B through nanomodification. Int. J. Nanomed. 2007, 2, 301–313. [Google Scholar]
- Bekersky, I.; Fielding, R.; Dressler, D.E.; Lee, J.W.; Buell, D.N.; Walsh, T.J. Plasma Protein Binding of Amphotericin B and Pharmacokinetics of Bound versus Unbound Amphotericin B after Administration of Intravenous Liposomal Amphotericin B (AmBisome) and Amphotericin B Deoxycholate. Antimicrob. Agents Chemother. 2002, 46, 834–840. [Google Scholar] [CrossRef]
- Iman, M.; Huang, Z.; Alavizadeh, S.H.; Szoka, F.C.; Jaafari, M.R. Biodistribution and In Vivo Antileishmanial Activity of 1,2-Distigmasterylhemisuccinoyl-sn-Glycero-3-Phosphocholine Liposome-Intercalated Amphotericin B. Antimicrob. Agents Chemother. 2017, 61, e02525-16. [Google Scholar] [CrossRef] [PubMed]
- Maintz, E.-M.; Hassan, M.; Huda, M.M.; Ghosh, D.; Hossain, S.; Alim, A.; Kroeger, A.; Arana, B.; Mondal, D. Introducing Single Dose Liposomal Amphotericin B for the Treatment of Visceral Leishmaniasis in Rural Bangladesh: Feasibility and Acceptance to Patients and Health Staff. J. Trop. Med. 2014, 2014, 676817. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Ali, N. Treatment of visceral leishmaniasis: Anomalous pricing and distribution of AmBisome and emergence of an indigenous liposomal amphotericin B, FUNGISOME. J. Parasit. Dis. 2014, 40, 1094–1095. [Google Scholar] [CrossRef][Green Version]
- De Assis, T.S.M.; Rosa, D.C.P.; Teixeira, E.D.M.; Cota, G.; Azeredo-Da-Silva, A.L.F.; Werneck, G.; Rabello, A. The direct costs of treating human visceral leishmaniasis in Brazil. Rev. Soc. Bras. Med. Trop. 2017, 50, 478–482. [Google Scholar] [CrossRef]
- Adler-Moore, J.P.; Proffitt, R.T. Development, Characterization, Efficacy and Mode of Action of Ambisome, A Unilamellar Liposomal Formulation of Amphotericin B. J. Liposome Res. 1993, 3, 429–450. [Google Scholar] [CrossRef]
- Yamazaki, H.; Kondo, T.; Aoki, K.; Yamashita, K.; Takaori-Kondo, A. Occurrence and improvement of renal dysfunction and serum potassium abnormality during administration of liposomal amphotericin B in patients with hematological disorders: A retrospective analysis. Diagn. Microbiol. Infect. Dis. 2018, 90, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Sinha, P.K.; Mahajan, R.; Sanz, M.G.; Lima, M.A.; Mitra, G.; Verma, N.; Das, P. Post Kala-Azar Dermal Leishmaniasis following Treatment with 20 mg/kg Liposomal Amphotericin B (Ambisome) for Primary Visceral Leishmaniasis in Bihar, India. PLOS Negl. Trop. Dis. 2014, 8, e2611. [Google Scholar] [CrossRef] [PubMed]
- Gaboriau, F.; Chéron, M.; Petit, C.; Bolard, J. Heat-induced superaggregation of amphotericin B reduces its in vitro toxicity: A new way to improve its therapeutic index. Antimicrob. Agents Chemother. 1997, 41, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.W.M.; van Vianen, W.; Roovers, P.; Frederik, P. Mild Heating of Amphotericin B-Desoxycholate: Effects on Ultrastructure, In Vitro Activity and Toxicity, and Therapeutic Efficacy in Severe Candidiasis in Leukopenic Mice. Antimicrob. Agents Chemother. 2000, 44, 1598–1603. [Google Scholar] [CrossRef][Green Version]
- Petit, C.; Yardley, V.; Gaboriau, F.; Bolard, J.; Croft, S.L. Activity of a Heat-Induced Reformulation of Amphotericin B Deoxycholate (Fungizone) against Leishmania donovani. Antimicrob. Agents Chemother. 1999, 43, 390–392. [Google Scholar] [CrossRef]
- Hatabu, T.; Takada, T.; Taguchi, N.; Suzuki, M.; Sato, K.; Kano, S. Potent Plasmodicidal Activity of a Heat-Induced Reformulation of Deoxycholate-Amphotericin B (Fungizone) against Plasmodium falciparum. Antimicrob. Agents Chemother. 2005, 49, 493–496. [Google Scholar] [CrossRef]
- Nema, S.; Brendel, R.J.; Chan, E.; Maa, Y.-F.; Overcashier, D.; Hsu, C.C. Excipients and Their Role in Approved Injectable Products: Current Usage and Future Directions. PDA J. Pharm. Sci. Technol. 2011, 65, 287–332. [Google Scholar] [CrossRef]
- Gaboriau, F.; Chéron, M.; Leroy, L.; Bolard, J. Physico-chemical properties of the heat-induced ‘superaggregates’ of amphotericin B. Biophys. Chem. 1997, 66, 1–12. [Google Scholar] [CrossRef]
- Brajtburg, J.; Bolard, J. Carrier effects on biological activity of amphotericin B. Clin. Microbiol. Rev. 1996, 9, 512–531. [Google Scholar] [CrossRef]
- Espada, R.; Valdespina, S.; Dea-Ayuela, M.A.; Molero, G.; Ballesteros, M.P.; Bolás, F.; Torrado, J.J. In vivo distribution and therapeutic efficacy of a novel amphotericin B poly-aggregated formulation. J. Antimicrob. Chemother. 2008, 61, 1125–1131. [Google Scholar] [CrossRef]
- Iman, M.; Huang, Z.; Szoka, F.C.; Jaafari, M.R. Characterization of the colloidal properties, in vitro antifungal activity, antileishmanial activity and toxicity in mice of a distigmasterylhemisuccinoyl-glycero-phosphocholine liposome-intercalated amphotericin B. Int. J. Pharm. 2011, 408, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, J.; Wieczór, M.; Bączek, T.; Gruszecki, M.; Czub, J. Thermodynamics and kinetics of amphotericin B self-association in aqueous solution characterized in molecular detail. Sci. Rep. 2016, 6, 19109. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sun, L.; Bevan, M.A.; Crooks, R.M. Comparison of Nanoparticle Size and Electrophoretic Mobility Measurements Using a Carbon-Nanotube-Based Coulter Counter, Dynamic Light Scattering, Transmission Electron Microscopy, and Phase Analysis Light Scattering. Langmuir 2004, 20, 6940–6945. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef]
- Asthana, S.; Jaiswal, A.K.; Gupta, P.K.; Pawar, V.K.; Dube, A.; Chourasia, M.K. Immunoadjuvant Chemotherapy of Visceral Leishmaniasis in Hamsters Using Amphotericin B-Encapsulated Nanoemulsion Template-Based Chitosan Nanocapsules. Antimicrob. Agents Chemother. 2013, 57, 1714–1722. [Google Scholar] [CrossRef]
- Corral, M.J.; González-Sánchez, E.; Cuquerella, M.; Alunda, J.M. In Vitro Synergistic Effect of Amphotericin B and Allicin on Leishmania donovani and L. infantum. Antimicrob. Agents Chemother. 2014, 58, 1596–1602. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Sulahian, A.; Garin, Y.J.; Farinotti, R.; Derouin, F. Therapy of visceral leishmaniasis due to Leishmania infantum: Experimental assessment of efficacy of AmBisome. Antimicrob. Agents Chemother. 1996, 40, 1214–1218. [Google Scholar] [CrossRef]
- Vermeersch, M.; da Luz, R.I.; Toteé, K.; Timmermans, J.-P.; Cos, P.; Maes, L. In Vitro Susceptibilities of Leishmania donovani Promastigote and Amastigote Stages to Antileishmanial Reference Drugs: Practical Relevance of Stage-Specific Differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef]
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef]
- Shadab; Jha, B.; Asad, M.; Deepthi, M.; Kamran, M.; Ali, N. Apoptosis-like cell death in Leishmania donovani treated with KalsomeTM10, a new liposomal amphotericin B. PLoS ONE 2017, 12, e0171306. [Google Scholar] [CrossRef]
- Gunjan, S.; Sharma, T.; Yadav, K.; Chauhan, B.S.; Singh, S.K.; Siddiqi, M.I.; Tripathi, R. Artemisinin Derivatives and Synthetic Trioxane Trigger Apoptotic Cell Death in Asexual Stages of Plasmodium. Front. Cell. Infect. Microbiol. 2018, 8, 256. [Google Scholar] [CrossRef]
- Alexander, J.; Satoskar, A.R.; Russell, D.G. Leishmania species: Models of intracellular parasitism. J. Cell Sci. 1999, 112 Pt 18, 2993–3002. [Google Scholar] [CrossRef]
- Asad, M.; Bhattacharya, P.; Banerjee, A.B.; Ali, N. Therapeutic and immunomodulatory activities of short-course treatment of murine visceral leishmaniasis with KALSOME™10, a new liposomal amphotericin B. BMC Infect. Dis. 2015, 15, 188. [Google Scholar] [CrossRef]
- Saha, S.; Mondal, S.; Ravindran, R.; Bhowmick, S.; Modak, D.; Mallick, S.; Rahman, M.; Kar, S.; Goswami, R.; Guha, S.K.; et al. IL-10- and TGF-β-Mediated Susceptibility in Kala-azar and Post-kala-azar Dermal Leishmaniasis: The Significance of Amphotericin B in the Control of Leishmania donovani Infection in India. J. Immunol. 2007, 179, 5592–5603. [Google Scholar] [CrossRef]
- Asad, M.D.; Ali, N. Dynamicity of Immune Regulation during Visceral Leishmaniasis. Proc. Indian Natl. Sci. Acad. 2014, 80, 247. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Ali, N. Involvement and interactions of different immune cells and their cytokines in human visceral leishmaniasis. Rev. Soc. Bras. Med. Trop. 2013, 46, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. Paromomycin in the treatment of leishmaniasis. Expert Opin. Investig. Drugs 2008, 17, 787–794. [Google Scholar] [CrossRef]



| Sample | Average Zeta Diameter (nm) | Zeta Potential (mV) | Z-Average a (nm) | PDI | Aqueous Solubility (mg AmB/mL) | Loading Capacity (%) |
|---|---|---|---|---|---|---|
| AmB-NA | 96 ± 0.8 | −34.2 ± 0.3 | 101 ± 0.7 | 0.22 ± 0.00 | 1.0 | 78 |
| DRUGS | PROMASTIGOTE | AMASTIGOTES | |||
|---|---|---|---|---|---|
| IC50 | IC90 | IC50 | IC90 | CC50–SI | |
| AMBISOME | 0.06 µM | 0.25 µM | 0.08 µM | 0.5 µM | 1.5–18.2 |
| PURE AMB | 0.08 µM | 0.3 µM | 0.16 µM | 0.9 µM | 2.0–12.5 |
| AMB-NA | 0.05 µM | 0.2 µM | 0.06 µM | 0.4 µM | 1.2–19.3 |
| FUNGIZONE | 0.08 µM | 0.3 µM | 0.16 µM | 0.8 µM | 1.8–11.3 |
| Study Groups | Healthy (n = 5) | Infected Mice (n = 13) | ||||
|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | |||||
| Balb/c (n = 5) | Balb/c (n = 3) AmB | Balb/c (n = 3) AmB | (n = 3) Ambisome | (n = 3) AmB-NA (SD) | (n = 3) AmB-NA (DD) | |
| Age (weeks) | 12–16 | 12–16 | 12–16 | 12–16 | 12–16 | 12–16 |
| Weight (g) | 25–35 | 25–35 | 25–35 | 25–35 | 25–35 | 25–35 |
| Body Temperature (in °F) | 97.5 ± 0.5 | 100.56 ± 1.89 | 97.76 ± 1.83 | 97.22 ± 0.375 | 97.56 ± 1.83 | 97.4 ± 0.35 |
| Hepatomegaly (in mm) | 0 | 4.3 ± 2.26 | 1.8 ± 1.76 | 0.9 ± 0.94 | 0.88 ± 0.72 | 0.69 ± 0.82 |
| Splenomegaly (in mm) | 0 | 7.8 ± 3.39 | 2.9 ± 2.99 | 0.8 ± 1.13 | 0.75 ± 1.12 | 0.71 ± 1.03 |
| Haemoglobins (g/dl) | 14.76 ± 0.95 | 9 ± 1.44 | 10.8 ± 1.84 | 14.11 ± 0.89 | 13.5 ± 1.64 | 14.2 ± 0.69 |
| WBC (white blood cells) (per mm2) | 6760 ± 1581.37 | 9850 ± 949.10 | 6715 ± 1045.10 | 5809 ± 486.85 | 5909 ± 386.75 | 5509 ± 687.15 |
| Lymphocytes (%) | 32.46 ± 5.73 | 46.5 ± 8.19 | 29.5 ± 6.5 | 25.6 ± 2.54 | 24.4 ± 2.28 | 24.2 ± 2.74 |
| AST (U/L) | 150.1 ± 14.35 | 235.9 ± 16.01 | 180.2 ± 13.93 | 150.6 ± 18.60 | 170.1 ± 12.60 | 155.4 ± 17.12 |
| ALT (U/L) | 90.05 ± 10.58 | 125.12 ± 13.73 | 129.96 ± 14.53 | 102.23 ± 9.28 | 98.45 ± 9.53 | 97.85 ± 7.83 |
| Blood Urea (mg/dl) | 20.5 ± 4.72 | 25.1 ± 6.84 | 22.6 ± 6.58 | 19.31 ± 5.83 | 20.31 ± 6.83 | 19.81 ± 8.72 |
| Serum Creatinine (mg/dl) | 0.41 ± 0.15 | 0.42 ± 0.15 | 0.45 ± 0.14 | 0.43 ± 0.15 | 0.45 ± 0.15 | 0.42 ± 0.14 |
| Sodium (mEq/L) | 137 ± 7.58 | 139.8 ± 3.04 | 136.3 ± 4.83 | 132.6 ± 5.25 | 133.6 ± 5.31 | 132.5 ± 3.78 |
| Potassium (mEq/L) | 4.64 ± 0.65 | 4.26 ± 0.33 | 4.4 ± 0.21 | 4.93 ± 0.45 | 4.87 ± 0.93 | 4.75 ± 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal, F.; Altaf, I.; Ahmed, G.; Asad, S.; Ahmad, H.; Zia, Q.; Azhar, A.; Farheen, S.; Shafi, T.; Karim, S.; et al. Amphotericin B Nano-Assemblies Circumvent Intrinsic Toxicity and Ensure Superior Protection in Experimental Visceral Leishmaniasis with Feeble Toxic Manifestation. Vaccines 2023, 11, 100. https://doi.org/10.3390/vaccines11010100
Jamal F, Altaf I, Ahmed G, Asad S, Ahmad H, Zia Q, Azhar A, Farheen S, Shafi T, Karim S, et al. Amphotericin B Nano-Assemblies Circumvent Intrinsic Toxicity and Ensure Superior Protection in Experimental Visceral Leishmaniasis with Feeble Toxic Manifestation. Vaccines. 2023; 11(1):100. https://doi.org/10.3390/vaccines11010100
Chicago/Turabian StyleJamal, Fauzia, Ishrat Altaf, Ghufran Ahmed, Sheikh Asad, Hira Ahmad, Qamar Zia, Asim Azhar, Saba Farheen, Taj Shafi, Shabana Karim, and et al. 2023. "Amphotericin B Nano-Assemblies Circumvent Intrinsic Toxicity and Ensure Superior Protection in Experimental Visceral Leishmaniasis with Feeble Toxic Manifestation" Vaccines 11, no. 1: 100. https://doi.org/10.3390/vaccines11010100
APA StyleJamal, F., Altaf, I., Ahmed, G., Asad, S., Ahmad, H., Zia, Q., Azhar, A., Farheen, S., Shafi, T., Karim, S., Zubair, S., & Owais, M. (2023). Amphotericin B Nano-Assemblies Circumvent Intrinsic Toxicity and Ensure Superior Protection in Experimental Visceral Leishmaniasis with Feeble Toxic Manifestation. Vaccines, 11(1), 100. https://doi.org/10.3390/vaccines11010100






