Comparison of COVID-19 Vaccine Policies in Italy, India, and South Africa
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
2.2. Policies Information
3. Results
3.1. Basic Information on COVID-19 Vaccination in the Three Countries
3.2. Core COVID-19 Vaccine Policies of the Three Countries
3.2.1. Italy
3.2.2. India
3.2.3. South Africa
3.3. The Effectiveness of COVID-19 Vaccination in These Three Countries
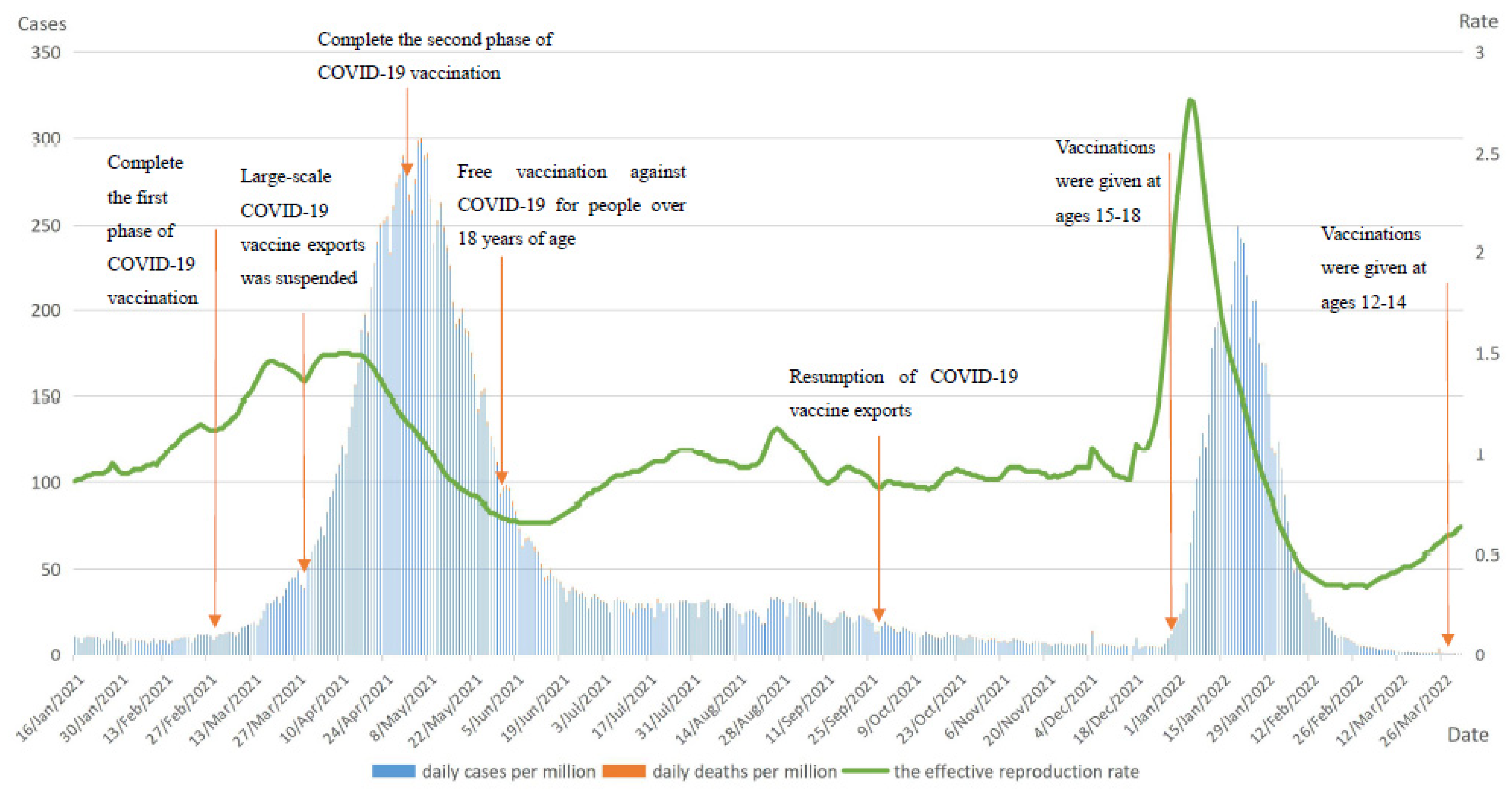
3.3.1. Italy
3.3.2. India
3.3.3. South Africa
4. Discussion
4.1. Active Promotion of COVID-19 Vaccination Is Essential for the Full Construction of the Population Immunization
4.2. Promoting Equitable Global Distribution of COVID-19 Vaccine Is Critical
4.3. Comprehensive Prevention and Control Measures Are Necessary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Weekly Trends by Country—Worldometer. Available online: https://www.worldometers.info/coronavirus/weekly-trends/ (accessed on 1 April 2022).
- WHO: Health Services Disrupted by New Crown Outbreak in 90% of Countries Worldwide. UN News. Available online: https://news.un.org/zh/story/2020/08/1065742 (accessed on 10 April 2022).
- Kashte, S.; Gulbake, A.; El-Amin, S.F., III; Gupta, A. COVID-19 vaccines: Rapid development, implications, challenges and future prospects. Hum. Cell. 2021, 34, 711–733. [Google Scholar] [CrossRef] [PubMed]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Delamater, P.L.; Street, E.J.; Leslie, T.F.; Yang, Y.T.; Jacobsen, K.H. Complexity of the Basic Reproduction Number (R0). Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Noland, R.B. Mobility and the effective reproduction rate of COVID-19. J. Transp. Health 2021, 20, 101016. [Google Scholar] [CrossRef] [PubMed]
- PMC. Acceptability of a COVID-19 Vaccine among Adults in the United States: How Many People Would Get Vaccinated? Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7440153/ (accessed on 8 April 2022).
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.L.; Clark, T.; Raman, R.; Sasisekharan, R. Insights on the mutational landscape of the SARS-CoV-2 Omicron variant receptor-binding domain. Cell Rep. Med. 2022, 3, 100527. [Google Scholar] [CrossRef] [PubMed]
- Garrett, N.; Tapley, A.; Andriesen, J.; Seocharan, I.; Fisher, L.H.; Bunts, L.; Espy, N.; Wallis, C.L.; Randhawa, A.K.; Ketter, N.; et al. High Rate of Asymptomatic Carriage Associated with Variant Strain Omicron. medRxiv 2022. [Google Scholar] [CrossRef]
- Reticencia a la Vacunación: Un Desafío Creciente para Los Programas de Inmunización. Available online: https://www.who.int/news/item/18-08-2015-vaccine-hesitancy-a-growing-challenge-for-immunization-programmes (accessed on 10 September 2022).
- Aw, J.; Seng, J.J.B.; Seah, S.S.Y.; Low, L.L. COVID-19 Vaccine Hesitancy—A Scoping Review of Literature in High-Income Countries. Vaccines 2021, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.; Montelpare, W.J. Predictors of Vaccine Hesitancy: Implications for COVID-19 Public Health Messaging. Int. J. Environ. Res. Public Health 2021, 18, 8054. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Loe, B.S.; Chadwick, A.; Vaccari, C.; Waite, F.; Rosebrock, L.; Jenner, L.; Petit, A.; Lewandowsky, S.; Vanderslott, S.; et al. COVID-19 vaccine hesitancy in the UK: The Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med. 2020, 52, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Allington, D.; McAndrew, S.; Moxham-Hall, V.; Duffy, B. Coronavirus conspiracy suspicions, general vaccine attitudes, trust and coronavirus information source as predictors of vaccine hesitancy among UK residents during the COVID-19 pandemic. Psychol. Med. 2021, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Zhou, Q.; Tan, Z.; Cao, J. The Mediating Roles of Medical Mistrust, Knowledge, Confidence and Complacency of Vaccines in the Pathways from Conspiracy Beliefs to Vaccine Hesitancy. Vaccines 2021, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Bollyky, T.J.; Gostin, L.O.; Hamburg, M.A. The Equitable Distribution of COVID-19 Therapeutics and Vaccines. JAMA 2020, 323, 2462–2463. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.; Yang, L.; Pagliusi, S. Roadmap for strengthening the vaccine supply chain in emerging countries: Manufacturers’ perspectives. Vaccine X 2020, 5, 100068. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Wang, X.; Shi, L.; Zhang, Y.; Chen, H.; Sun, G. Policy disparities in fighting COVID-19 among Japan, Italy, Singapore and China. Int. J. Equity Health 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Eubank, S.; Eckstrand, I.; Lewis, B.; Venkatramanan, S.; Marathe, M.; Barrett, C.L. Impact of Non-pharmaceutical Interventions (NPIs) to Reduce COVID-19 Mortality and Healthcare Demand. Bull. Math. Biol. 2020, 82, 52. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Mukherjee, S.; Leifels, M.; Gautam, R.; Kaushik, H.; Sharma, S.; Kumar, O. Mega festivals like MahaKumbh, a largest mass congregation, facilitated the transmission of SARS-CoV-2 to humans and endangered animals via contaminated water. Int. J. Hyg. Environ. Health 2021, 237, 113836. [Google Scholar] [CrossRef] [PubMed]



| Italy | India | South Africa | |
|---|---|---|---|
| Start date of vaccination | 27 December 2020 | 16 January 2021 | 17 February 2021 |
| Vaccines administered | Pfizer/BioNTech, Moderna, AstraZeneca, Novavax, Johnson & Johnson | Covieshield, Covaxin, Sputnik V | Pfizer/BioNTech, Johnson & Johnson |
| Total vaccinations | 136.00 million | 18.4 billion | 33.74 million |
| COVID-19 vaccination rates | 84.4% | 71.02% | 34.9% |
| Rate of fully vaccinated | 79.24% | 59.7% | 29.86% |
| Rate of booster vaccinated | 64.5% | 1.58% | 3.73% |
| Vaccine appointment | Vaccine appointments were made online or by phone and are required by age group from January to June 2021. | Vaccine appointments were made online from 28 April 2021. | No appointment necessary for vaccination. |
| Free vaccination or not | Free vaccinations by age group. | Free vaccinations for all adults in public hospitals after June 2021. Private hospitals charge about 150 rubles for vaccination. | Free vaccination for the entire population. |
| Aspects | Italy | India | South Africa | ||
|---|---|---|---|---|---|
| Countries | |||||
| Similarities | Basic vaccination plan | Phase 1 vaccination: medical staff and health workers; residents and staff of nursing homes and orphanages; elderly group over 80 years old. Phase 2 vaccination: people at high clinical risk with underlying and chronic diseases; elderly people aged 60–79 years. Phase 3 vaccination: people aged 16 years and above who are not at particular risk. Phase 4 vaccination: people other than those in the above three phases. | Phase 1 vaccination: medical workers, police officers, and other front-line personnel in epidemic prevention and public officials. Phase 2 vaccination: people aged 40–59 with underlying diseases; elderly people aged 60 and above. Phase 3 vaccination: citizens aged 45 and above. Phase 4 vaccination: people in the age group of 18–44 years old. Phase 5 vaccination: people in the age group below 18 years old. | Phase 1 vaccination: over 1 million health care workers were the first to be vaccinated. Phase 2 vaccination: older age groups over 60 years old; people with comorbidities. Phase 3 vaccination: general population in the 50–59 age group. Phase 4 vaccination: general population in the 35–49 years age group. Phase 5 vaccination: age group 18–34 years old. | |
| Vaccine development and supply |
|
|
| ||
| Vaccination of minors |
|
|
| ||
| Vaccination boosters |
| In early January 2022, India began a booster dose of COVID-19 vaccine for healthcare workers, frontline workers and people over 60 years of age with underlying medical conditions. |
| ||
| Differences | Compulsory vaccination |
| No compulsory COVID-19 vaccination. | No compulsory COVID-19 vaccination. | |
| Vaccination support policies |
| No “Green Pass” was used. | No “Green Pass” was used. | ||
| Vaccines procurement | The COVID-19 vaccines in Italy were procured centrally by the government. |
| The COVID-19 vaccines in South Africa were procured centrally by the government. | ||
| Expand and incentivize vaccination | No vouchers were given to people to incentivize vaccination. | No vouchers were given to people to incentivize vaccination. |
| ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Shi, L.; Chen, H.; Wang, X.; Jiao, J.; Liu, M.; Yang, J.; Sun, G. Comparison of COVID-19 Vaccine Policies in Italy, India, and South Africa. Vaccines 2022, 10, 1554. https://doi.org/10.3390/vaccines10091554
Yang M, Shi L, Chen H, Wang X, Jiao J, Liu M, Yang J, Sun G. Comparison of COVID-19 Vaccine Policies in Italy, India, and South Africa. Vaccines. 2022; 10(9):1554. https://doi.org/10.3390/vaccines10091554
Chicago/Turabian StyleYang, Manfei, Leiyu Shi, Haiqian Chen, Xiaohan Wang, Jun Jiao, Meiheng Liu, Junyan Yang, and Gang Sun. 2022. "Comparison of COVID-19 Vaccine Policies in Italy, India, and South Africa" Vaccines 10, no. 9: 1554. https://doi.org/10.3390/vaccines10091554
APA StyleYang, M., Shi, L., Chen, H., Wang, X., Jiao, J., Liu, M., Yang, J., & Sun, G. (2022). Comparison of COVID-19 Vaccine Policies in Italy, India, and South Africa. Vaccines, 10(9), 1554. https://doi.org/10.3390/vaccines10091554






