Waning of SARS-CoV-2 Seropositivity among Healthy Young Adults over Seven Months
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Recruitment and Eligibility
2.2. Sampling and Recruitment
2.3. Data Collection
2.4. Study Visit Procedures
2.5. Laboratory Methods
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
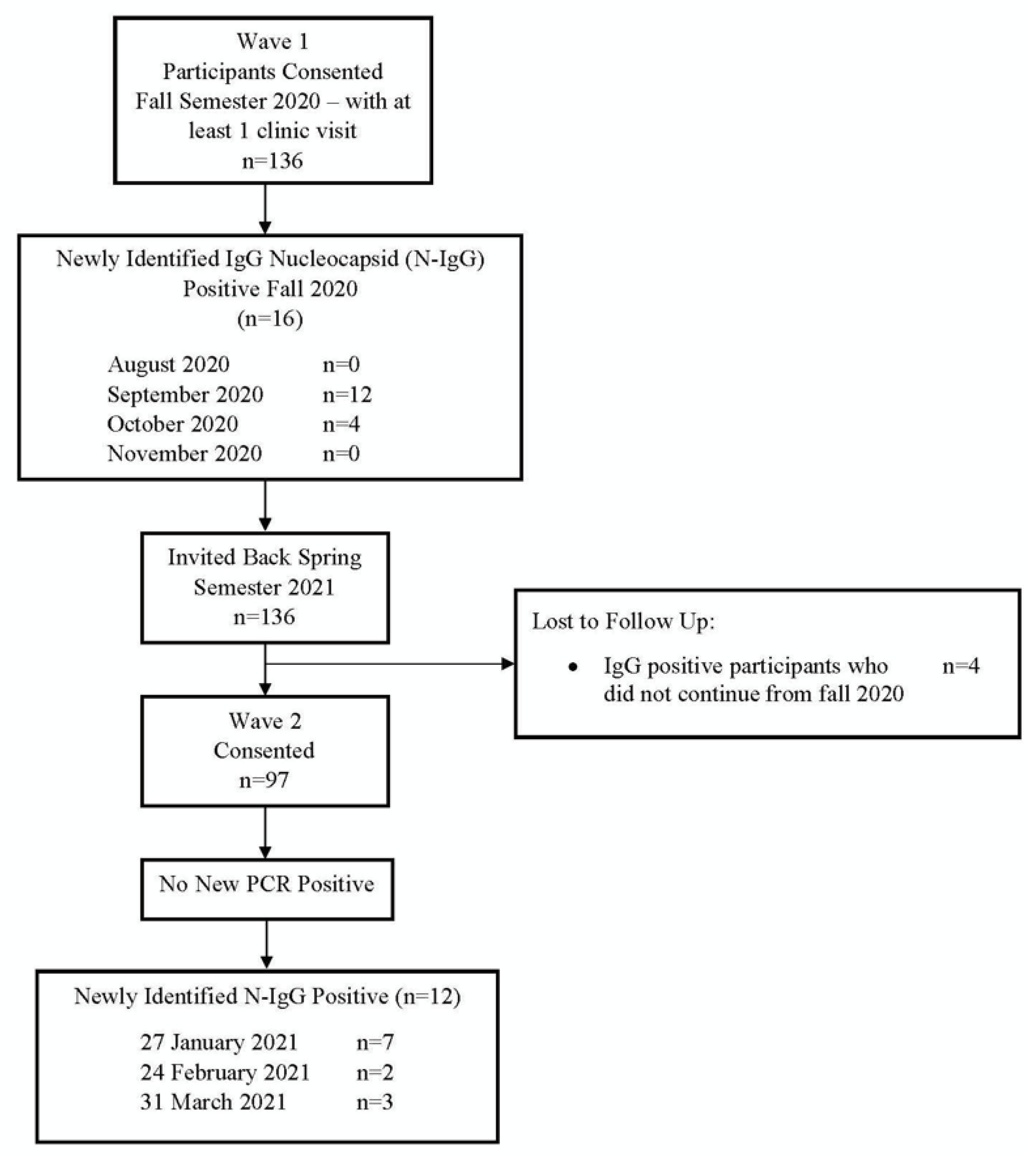
3.1. Persistence and Loss of Anti-Nucleocapsid Antibodies
3.2. Presence of Anti-Spike Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. Washington State 2019-nCoV Case Investigation Team First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Bailey, D.C.; Seamon, M.D.; Miranda, M.L. Response to a COVID-19 Outbreak on a University Campus—Indiana, August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Here’s Our List of Colleges’ Reopening Models. Chronicle of Higher Education 2020. Available online: https://www.chronicle.com/article/heres-a-list-of-colleges-plans-for-reopening-in-the-fall/ (accessed on 14 August 2022).
- Paltiel, A.D.; Zheng, A.; Walensky, R.P. Assessment of SARS-CoV-2 Screening Strategies to Permit the Safe Reopening of College Campuses in the United States. JAMA Netw. Open 2020, 3, e2016818. [Google Scholar] [CrossRef] [PubMed]
- Denny, T.N.; Andrews, L.; Bonsignori, M.; Cavanaugh, K.; Datto, M.B.; Deckard, A.; DeMarco, C.T.; DeNaeyer, N.; Epling, C.A.; Gurley, T.; et al. Implementation of a Pooled Surveillance Testing Program for Asymptomatic SARS-CoV-2 Infections on a College Campus—Duke University, Durham, North Carolina, August 2–October 11, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Sanchez, R.; Auerbach, S.; Nam, D.; Lanier, B.; Taylor, J.; Jaso, C.; Nolan, K.; Jacobs, E.A.; Hudson, F.P.; et al. COVID-19 Outbreak Among College Students After a Spring Break Trip to Mexico—Austin, Texas, March 26–April 5, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 830–835. [Google Scholar]
- Payne, D.C.; Smith-Jeffcoat, S.E.; Nowak, G.; Chukwuma, U.; Geibe, J.R.; Hawkins, R.J.; Johnson, J.A.; Thornburg, N.J.; Schiffer, J.; Weiner, Z.; et al. SARS-CoV-2 Infections and Serologic Responses from a Sample of U.S. Navy Service Members—USS Theodore Roosevelt, April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 714–721. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Zhang, X.; Wei, Z.; Zhang, L.; Xu, J.; Liang, P.; Xu, Y.; Zhang, C.; Xu, A. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16–23 years outside Wuhan and characteristics of young patients with COVID-19: A prospective contact-tracing study. J. Infect. 2020, 80, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Liu, B.; Deng, H.; Wu, G.; Deng, K.; Chen, Y.; Liao, P.; Qiu, J.; Lin, Y.; Cai, X.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Pérez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; Fernández de Larrea, N.; et al. ENE-COVID Study Group Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Kim, P.; Gordon, S.M.; Sheehan, M.M.; Rothberg, M.B. Duration of SARS-CoV-2 Natural Immunity and Protection against the Delta Variant: A Retrospective Cohort Study. Clin. Infect. Dis. 2021, 75, e185–e190. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Van Elslande, J.; Oyaert, M.; Ailliet, S.; Van Ranst, M.; Lorent, N.; Vande Weygaerde, Y.; André, E.; Lagrou, K.; Vandendriessche, S.; Vermeersch, P. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J. Clin. Virol. 2021, 136, 104765. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, T.J.; Uhrlaub, J.L.; Watanabe, M.; Wong, R.; Castaneda, Y.; Pizzato, H.A.; Thompson, M.R.; Bradshaw, C.; Weinkauf, C.C.; Bime, C.; et al. Orthogonal SARS-CoV-2 Serological Assays Enable Surveillance of Low-Prevalence Communities and Reveal Durable Humoral Immunity. Immunity 2020, 53, 925–933.e4. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild COVID-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef]
- Patel, M.M.; Thornburg, N.J.; Stubblefield, W.B.; Talbot, H.K.; Coughlin, M.M.; Feldstein, L.R.; Self, W.H. Change in Antibodies to SARS-CoV-2 over 60 Days Among Health Care Personnel in Nashville, Tennessee. JAMA 2020, 324, 1781–1782. [Google Scholar] [CrossRef]
- Anna, F.; Goyard, S.; Lalanne, A.I.; Nevo, F.; Gransagne, M.; Souque, P.; Louis, D.; Gillon, V.; Turbiez, I.; Bidard, F.C.; et al. High seroprevalence but short-lived immune response to SARS-CoV-2 infection in Paris. Eur. J. Immunol. 2021, 51, 180–190. [Google Scholar] [CrossRef]
- Tanunliong, G.; Liu, A.; Vijh, R.; Pidduck, T.; Kustra, J.; Márquez, A.C.; Choi, A.; Mclennan, M.; Hayden, A.; Kearney, C.; et al. Persistence of Anti-SARS-CoV-2 Antibodies in Long Term Care Residents over Seven Months after Two COVID-19 Outbreaks. Front. Immunol. 2022, 12, 775420. [Google Scholar] [CrossRef]
- Kellam, P.; Barclay, W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J. Gen. Virol. 2020, 101, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Mansour, M.; Leven, E.; Bouvier, N.M.; Patel, G.; Firpo-Betancourt, A.; Mendu, R.; Jhang, J.; Arinsburg, S.; Gitman, M.; et al. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: An observational study. Lancet Microbe 2020, 1, e283–e289. [Google Scholar] [CrossRef]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Murrell, I.; Forde, D.; Tyson, L.; Chichester, L.; Garratt, A.; Vineall, O.; Palmer, N.; Jones, R.; Moore, C. A longitudinal comparison of spike and nucleocapsid SARS-CoV-2 antibody responses in a tertiary hospital’s laboratory workers with validation of DBS specimen analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Bryne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Maine, G.N.; Lao, K.M.; Krishnan, S.M.; Afolayan-Oloye, O.; Fatemi, S.; Kumar, S.; VanHorn, L.; Hurand, A.; Sykes, E.; Sun, Q. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J. Clin. Virol. 2020, 133, 104663. [Google Scholar] [CrossRef]
- Murrell, I.; Forde, D.; Zelek, W.; Tyson, L.; Chichester, L.; Palmer, N.; Jones, R.; Morgan, B.P.; Moore, C. Temporal development and neutralising potential of antibodies against SARS-CoV-2 in hospitalised COVID-19 patients: An observational cohort study. PLoS ONE 2021, 16, e0245382. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Lea, C.S.; Simeonsson, K.; Kipp, A.; McNeill, C.; Wilcox, L.; Irish, W.; Morris, H.; Diaz, O.; Fallon, J.; Roper, R.L. Loss of SARS-CoV-2 Seropositivity among Healthy Young Adults over Seven Months. medRxiv 2022. [Google Scholar] [CrossRef]
- Rosadas, C.; Randell, P.; Khan, M.; McClure, M.O.; Tedder, R.S. Testing for responses to the wrong SARS-CoV-2 antigen? Lancet 2020, 396, e23. [Google Scholar] [CrossRef]
- Ng, D.L.; Goldgof, G.M.; Shy, B.R.; Levine, A.G.; Balcerek, J.; Bapat, S.P.; Prostko, J.; Rodgers, M.; Coller, K.; Pearce, S.; et al. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat. Commun. 2020, 11, 4698. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- See, R.H.; Zakhartchouk, A.N.; Petric, M.; Lawrence, D.J.; Mok, C.P.Y.; Hogan, R.J.; Rowe, T.; Zitzow, L.A.; Karunakaran, K.P.; Hitt, M.M.; et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J. Gen. Virol. 2006, 87, 641–650. [Google Scholar] [CrossRef]
- Rehm Kristina, E.; Jones Gwendolyn, J.B.; Tripp Alice, A.; Metcalf Mark, W.; Roper Rachel, L. The Poxvirus A35 Protein Is an Immunoregulator. J. Virol. 2010, 84, 418–425. [Google Scholar] [CrossRef]
- Rehm, K.E.; Roper, R.L. Deletion of the A35 gene from Modified Vaccinia Virus Ankara increases immunogenicity and isotype switching. Vaccine 2011, 29, 3276–3283. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Chen, Z.; Meyer, W.A., 3rd; Wohlgemuth, J.G. Insights from Patterns of SARS-CoV-2 Immunoglobulin G Serology Test Results from a National Clinical Laboratory, United States, March–July 2020. Popul. Health. Manag. 2021, 24, S35–S42. [Google Scholar] [CrossRef]
- Schaffner, A.; Risch, L.; Weber, M.; Thiel, S.; Jüngert, K.; Pichler, M.; Wohlwend, N.; Lung, T.; Ritzler, M.; Hillmann, D.; et al. Sustained SARS-CoV-2 nucleocapsid antibody levels in nonsevere COVID-19: A population-based study. Clin. Chem. Lab. Med. 2020, 59, e49–e51. [Google Scholar] [CrossRef]
- Matsuba, I.; Takuma, T.; Hatori, N.; Takai, M.; Watanabe, Y.; Takada, N.; Kishi, S.; Matsuzawa, Y.; Nishikawa, T.; Kunishima, T.; et al. The Proportion of Long-term Response to Anti-N Igg Antibody after 12 Months for COVID-19 Subclinical Infections and a Longitudinal Survey for COVID-19 Subclinical Infections in 2021. Intern. Med. 2022, 9628-22. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Dowlatshahi, D.P.; Dai, J.; Becker, L.M.; Hensel, J.; Snowden, L.M.; Leveille, J.M.; Brunner, M.R.; Holden, K.W.; Hopkins, N.S.; et al. Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COVID-19 immunity. JCI Insight 2020, 5, e142386. [Google Scholar] [CrossRef]

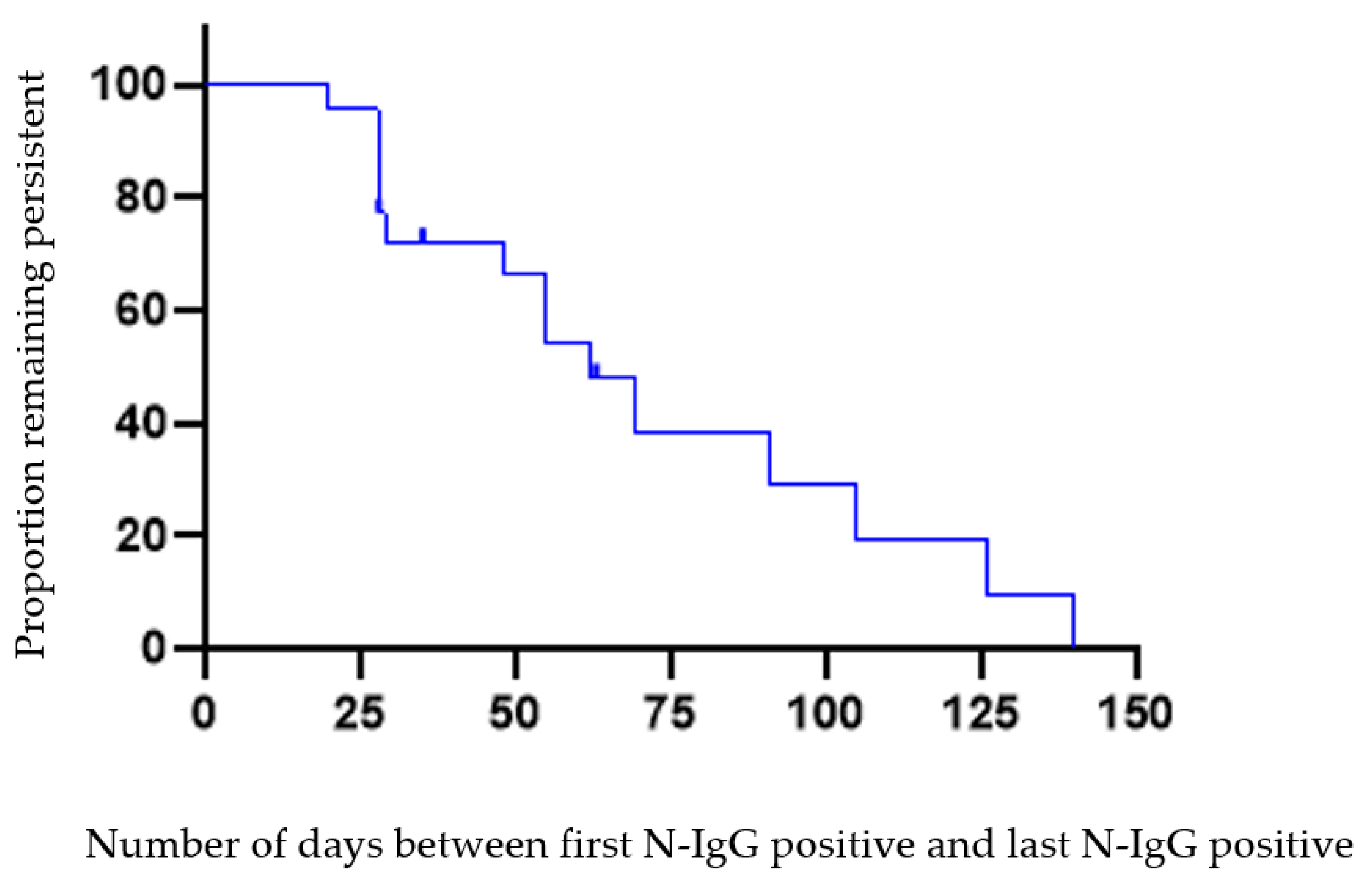

| Number of Days | PCR Positive Test to Initial N-IgG Positive (n = 19) 2 | N-IgG Persistence n = 23 4 | Loss of Persistence 5 n = 15 6 |
|---|---|---|---|
| Mean, SD | 21.21 (14.76) | 54.30 (33.51) | 82.33 (44.16) |
| Median | 19 | 48 | 63 |
| Range | 0–54 3 | 20–140 | 28–154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lea, C.S.; Simeonsson, K.; Kipp, A.M.; McNeill, C.; Wilcox, L.; Irish, W.; Morris, H.; Diaz, O.M.; Fallon, J.T.; Roper, R.L. Waning of SARS-CoV-2 Seropositivity among Healthy Young Adults over Seven Months. Vaccines 2022, 10, 1532. https://doi.org/10.3390/vaccines10091532
Lea CS, Simeonsson K, Kipp AM, McNeill C, Wilcox L, Irish W, Morris H, Diaz OM, Fallon JT, Roper RL. Waning of SARS-CoV-2 Seropositivity among Healthy Young Adults over Seven Months. Vaccines. 2022; 10(9):1532. https://doi.org/10.3390/vaccines10091532
Chicago/Turabian StyleLea, C. Suzanne, Kristina Simeonsson, Aaron M. Kipp, Charleen McNeill, Lisa Wilcox, William Irish, Hannah Morris, Omar M. Diaz, John T. Fallon, and Rachel L. Roper. 2022. "Waning of SARS-CoV-2 Seropositivity among Healthy Young Adults over Seven Months" Vaccines 10, no. 9: 1532. https://doi.org/10.3390/vaccines10091532
APA StyleLea, C. S., Simeonsson, K., Kipp, A. M., McNeill, C., Wilcox, L., Irish, W., Morris, H., Diaz, O. M., Fallon, J. T., & Roper, R. L. (2022). Waning of SARS-CoV-2 Seropositivity among Healthy Young Adults over Seven Months. Vaccines, 10(9), 1532. https://doi.org/10.3390/vaccines10091532








