High Frequency of COVID-19 Vaccine Hesitancy among Canadians Immunized for Influenza: A Cross-Sectional Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Local Context during the Global Pandemic
2.2. Participants
2.3. Data Collection Procedure
2.4. Measures
- Demographics, including age, sex, race/ethnic minorities associated with increased COVID-19 infection/mortality, role (patient, health care provider, family member, other), employment status, household members, smoking status, and risk factors for severe illness from COVID-19 infection (e.g., medical co-morbidities, treatments).
- Previous seasonal influenza vaccination.
- Previous COVID-19 infection and perception of amount of COVID-19 preventative information received.
- Likelihood of receiving a future vaccine and causes of vaccine hesitancy, using questions proposed by the WHO-SAGE working group adapted to the COVID-19 vaccine [10].
- Likelihood of engaging in risk-associated behaviors based on the adult Domain-Specific Risk-Taking (DOSPERT) health and safety subscale, and medical risk domain add-on [11,12]. A risk-taking score was calculated based on the sum of scores in each question; the sum was transformed to a 0–100 scale (%) and converted to a three-level categorical variable (low, moderate, high risk-taking) by tertiles.
2.5. Data Analysis Procedure
3. Results
3.1. Study Population
3.2. Factors Associated with COVID-19 Vaccine Hesitancy
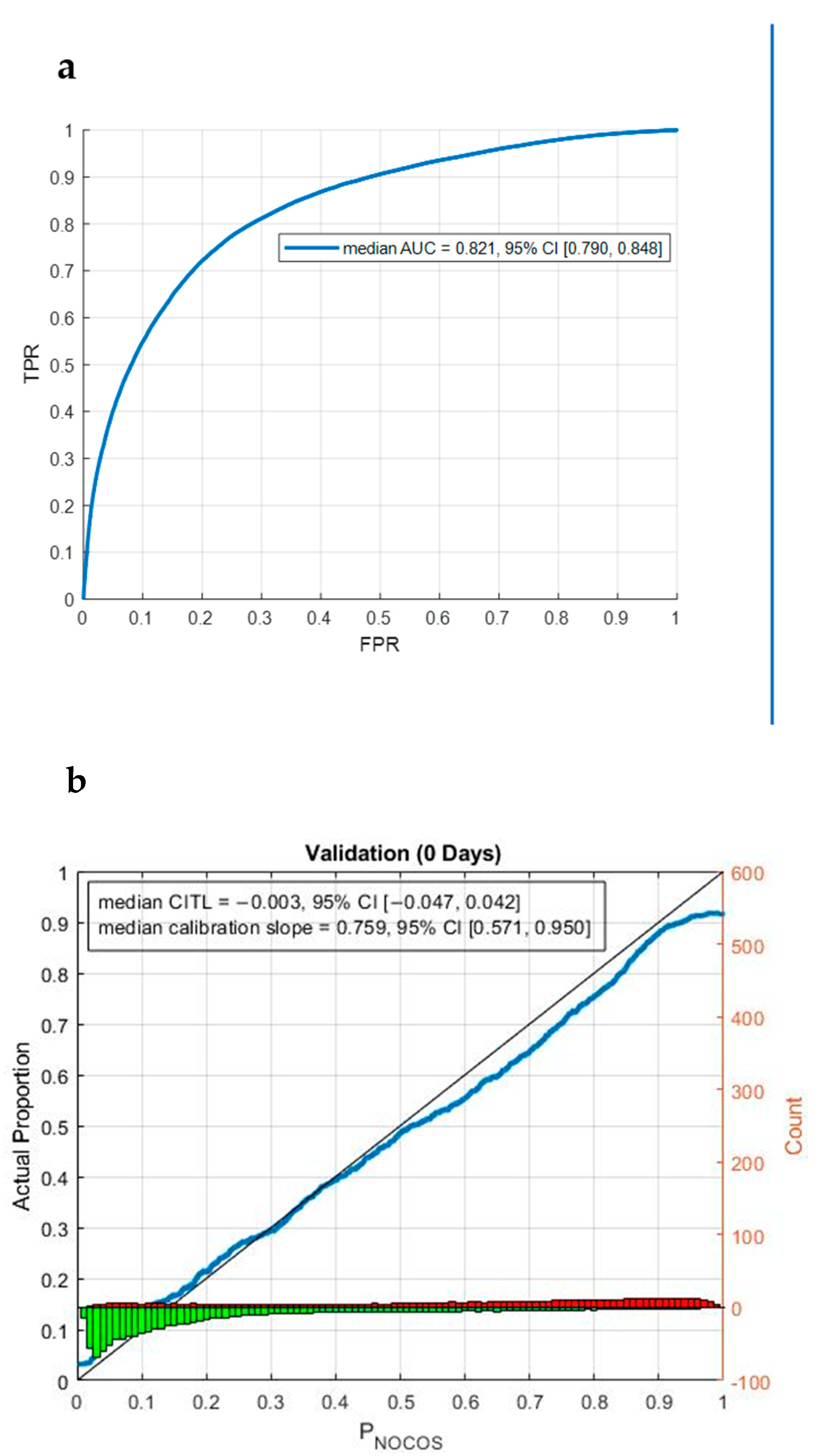
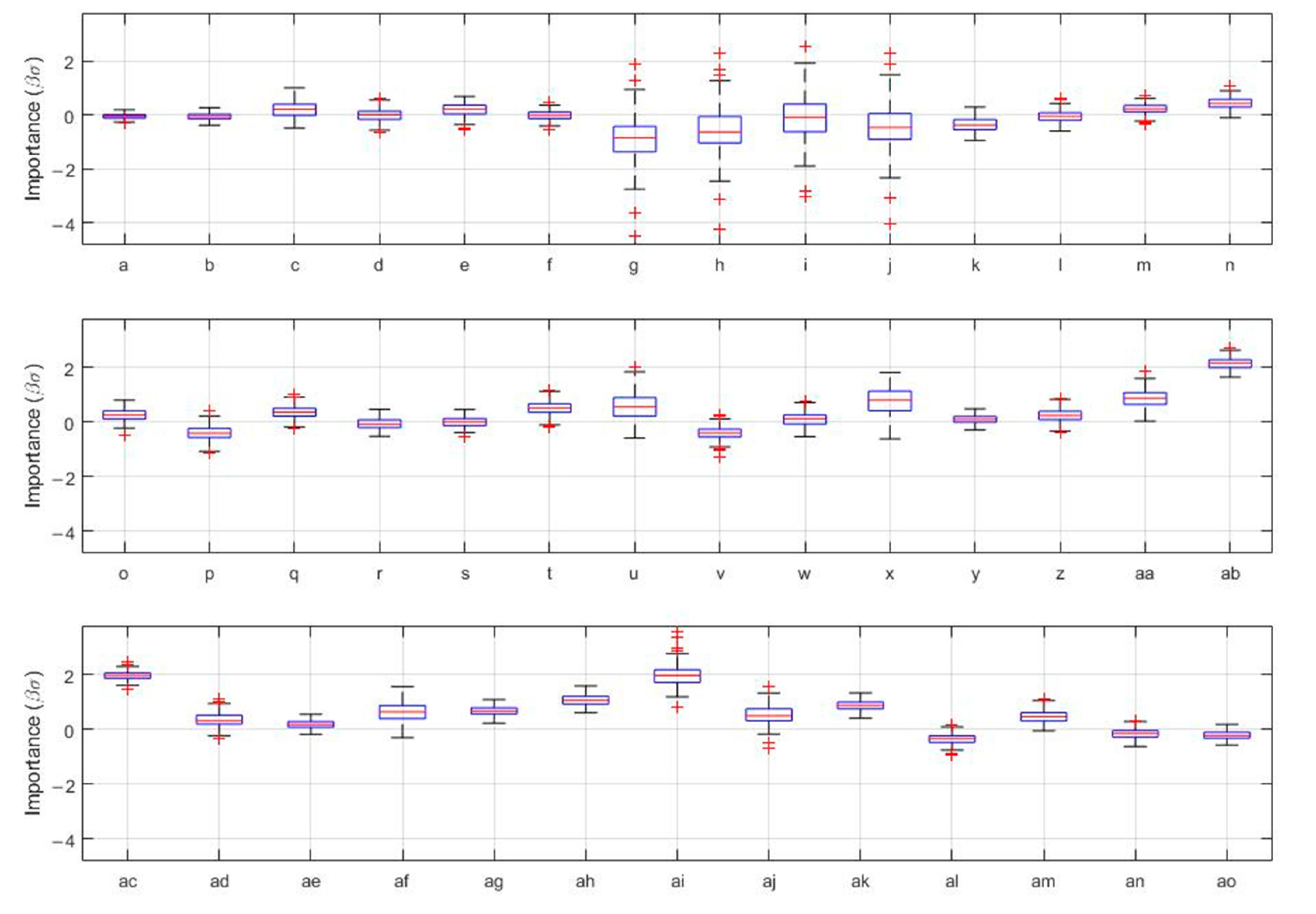
3.3. Prediction of COVID-19 Vaccine Hesitancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, I.; Maity, P. COVID-19 Outbreak: Migration, Effects on Society, Global Environment and Prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the Mrna-1273 Sars-Cov-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.D.; Wonodi, C. Oxford-Astrazeneca COVID-19 Vaccine Efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.Cov2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Government of Canada. Vaccines for COVID-19. Available online: https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/vaccines.html?utm_campaign=hc-sc-covidvaccine-22-23&utm_medium=sem&utm_source=ggl&utm_content=ad-text-en&utm_term=covid%20vaccines%20approved%20in%20canada&adv=2223-249950&id_campaign=16905919192&id_source=137027578273&id_content=593179147880&gclid=CjwKCAjwlqOXBhBqEiwA-hhitOVTnz5WMaqV9Hfw1L3zeuJMvXz0ojeVNJM5A1eeRy_J6qpa7z_AmRoC1HcQAvD_BwE&gclsrc=aw.ds (accessed on 2 August 2022).
- MacDonald, N.E.; Sage Working Group on Vaccine Hesitancy. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 22 June 2022).
- Robertson, E.; Reeve, K.S.; Niedzwiedz, C.L.; Moore, J.; Blake, M.; Green, M.; Katikireddi, S.V.; Benzeval, M.J. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav. Immun. 2021, 94, 41–50. [Google Scholar] [CrossRef]
- Sage Working Group. The Determinants of Vaccine Hesitancy: Sample Survey Questions. Available online: https://www.giant-int.org/wp-content/uploads/2020/12/4_survey_questionsRevised.pdf (accessed on 22 June 2022).
- Blais, A.-R.; Weber, E.U. A Domain Specific Risk Taking (Dospert) Scale for Adult Populations. Judgm. Decis. Mak. 2006, 1, 33–47. [Google Scholar]
- Butler, S.; Rosman, A.; Seleski, S.; Garcia, M.; Lee, S.; Barnes, J. A Medical Risk Attitude Subscale for Dospert. Judgm. Decis. Mak. 2012, 7, 189–195. [Google Scholar]
- Steyerberg, E. Choosing between Alternative Statistical Models. Clinical Prediction Models: A Practical Approach to Development, Validation and Updating; Springer: Berlin/Heidelberg, Germany, 2009; p. 107. [Google Scholar]
- Noma, H.; Shinozaki, T.; Iba, K.; Teramukai, S.; Furukawa, T.A. Confidence Intervals of Prediction Ac-curacy Measures for Multivariable Prediction Models Based on the Bootstrap-Based Optimism Correction Methods. Stat. Med. 2021, 40, 5691–5701. [Google Scholar] [CrossRef]
- Wikipedia. Dummy Variable (Statistics). Available online: https://en.wikipedia.org/wiki/dummy_variable_(statistics) (accessed on 3 September 2021).
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines 2021, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; McFadden, S.M.; Elharake, J.; Omer, S.B. Determinants of COVID-19 vaccine acceptance in the US. eClinicalMedicine 2020, 26, 100495. [Google Scholar] [CrossRef] [PubMed]
- Dror, A.A.; Eisenbach, N.; Taiber, S.; Morozov, N.G.; Mizrachi, M.; Zigron, A.; Srouji, S.; Sela, E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020, 35, 775–779. [Google Scholar] [CrossRef]
- Khubchandani, J.; Sharma, S.; Price, J.H.; Wiblishauser, M.J.; Sharma, M.; Webb, F.J. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J. Community Health 2021, 46, 270–277. [Google Scholar] [CrossRef]
- Schwarzinger, M.; Watson, V.; Arwidson, P.; Alla, F.; Luchini, S. COVID-19 Vaccine Hesitancy in a Representative Working Age Population in France: A Survey Experiment Based on Vaccine Characteristics. Lancet Public Health 2021, 6, e210. [Google Scholar] [CrossRef]
- Freeman, D.; Loe, B.S.; Chadwick, A.; Vaccari, C.; Waite, F.; Rosebrock, L.; Jenner, A.; Petit, S.; Lewandowsky, S.; Vanderslott, S.; et al. COVID-19 Vaccine Hesitancy in the UK: The Oxford Coronavirus Explanations, Attitudes, and Narratives Survey (Oceans) II. Psychol. Med. 2020, 1–15. [Google Scholar] [CrossRef]
- Office of National Statistics. Coronavirus and Vaccination Rates in People Aged 70 Years and over by Socio-Demographic Characteristic, England: 8 December 2020 to 11 March 2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthinequalities/bulletins/coronavirusandvaccinationratesinpeopleaged70yearsandoverbysociodemographiccharacteristicengland/8december2020to11march2021 (accessed on 22 July 2021).
- Razai, M.S.; Chaudhry, U.A.R.; Doerholt, K.; Bauld, L.; Majeed, A. COVID-19 Vaccination Hesitancy. BMJ 2021, 373, n1138. [Google Scholar] [CrossRef]
- Harrington, N.; Chen, Y.; O’Reilly, A.M.; Fang, C.Y. The role of trust in HPV vaccine uptake among racial and ethnic minorities in the United States: A narrative review. AIMS Public Health 2021, 8, 352–368. [Google Scholar] [CrossRef]
- Allington, D.; McAndrew, S.; Moxham-Hall, V.; Duffy, B. Coronavirus conspiracy suspicions, general vaccine attitudes, trust and coronavirus information source as predictors of vaccine hesitancy among UK residents during the COVID-19 pandemic. Psychol. Med. 2021, 1–12. [Google Scholar] [CrossRef]
- Karlsson, L.C.; Lewandowsky, S.; Antfolk, J.; Salo, P.; Lindfelt, M.; Oksanen, T.; Kivimäki, M.; Soveri, A. The association between vaccination confidence, vaccination behavior, and willingness to recommend vaccines among Finnish healthcare workers. PLoS ONE 2019, 14, e0224330. [Google Scholar] [CrossRef]
- Ozawa, S.; Stack, M.L. Public trust and vaccine acceptance international perspectives. Hum. Vaccines Immunother. 2013, 9, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, K.; Bekalu, M.; Dhawan, D.; Pinnamaneni, R.; Lang, J.; McLoud, R. Individual and Social Determinants of COVID-19 Vaccine Uptake. BMC Public Health 2021, 21, 818. [Google Scholar] [CrossRef] [PubMed]
- Lewandowsky, S.; Cook, J.; Schmid, P.; Holford, D.L.; Finn, A.; Leask, J.; Thomson, A.; Lombardi, D.; Al-Rawi, A.K.; Amazeen, M.A.; et al. The COVID-19 Vaccine Communication Handbook. A Practical Guide for Improving Vaccine Communication and Fighting Misinformation. 2021. Available online: https://www.movementdisorders.org/MDS-Files1/The_COVID-19_Vaccine_Communication_Handbook.pdf (accessed on 28 August 2022).
- Chung, Y.; Schamel, J.; Fisher, A.; Frew, P.M. Influences on Immunization Decision Making among US Parents of Young Children. Matern. Child Health J. 2017, 21, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.T.; Chapman, G.B.; Rothman, A.J.; Leask, J.; Kempe, A. Increasing Vaccination: Putting Psychological Science into Action. Psychol. Sci. Public Interest 2017, 18, 149–207. [Google Scholar] [CrossRef]
- Qendro, T.; De La Torre, M.L.; Panopalis, P.; Hazel, E.; Ward, B.J.; Colmegna, I.; Hudson, M. Suboptimal Immunization Coverage among Canadian Rheumatology Patients in Routine Clinical Care. J. Rheumatol. 2020, 47, 770–778. [Google Scholar] [CrossRef]
- Valerio, V.; Bazan, M.C.; Wang, M.; Mazer, B.D.; Pineau, C.A.; Hazel, E.M.; Bernatsky, S.; Ward, B.J.; Colmegna, I. A multimodal intervention increases influenza vaccine uptake in rheumatoid arthritis. Clin. Rheumatol. 2021, 40, 575–579. [Google Scholar] [CrossRef]
- Health Ethics & Governance, Who Headquarters. COVID-19 and Mandatory Vaccination: Ethical Considerations and Caveats. Policy Brief. April 2021. Available online: https://www.who.int/publications/i/item/who-2019-ncov-policy-brief-mandatory-vaccination-2021.1 (accessed on 28 August 2022).
- Savulescu, J. Good Reasons to Vaccinate: Mandatory or Payment for Risk? J. Med. Ethics 2021, 47, 78–85. [Google Scholar] [CrossRef]
- Shetty, P. Experts concerned about vaccination backlash. Lancet 2010, 375, 970–971. [Google Scholar] [CrossRef]
- Schwartz, J.L. Evaluating and Deploying COVID-19 Vaccines the Importance of Transparency, Scientific Integrity, and Public Trust. N. Engl. J. Med. 2020, 383, 1703–1705. [Google Scholar] [CrossRef]
- Sprengholz, P.; Betsch, C.; Böhm, R. Reactance Revisited: Consequences of Mandatory and Scarce Vaccination in the Case of COVID-19. Appl. Psychol. Health Well Being 2021, 13, 986–995. [Google Scholar] [CrossRef] [PubMed]
| Variables | No Hesitancy (10) (n = 1124) | Mild Hesitancy (7.1–9.5 *) (n = 315) | Significant Hesitancy (0–7) (n = 354) | Total (n = 1793) |
|---|---|---|---|---|
| n (%) or mean ± SD | n (%) or mean ± SD | n (%) or mean ± SD | n (%) or mean ± SD | |
| Demographics | ||||
| Age | 53.2 ± 17.0 | 49.9 ± 16.9 | 50.8 ± 16.2 | 52.2 ± 16.9 |
| Sex | ‘Are you a:’ options: female, male | |||
| Female | 628 (55.9) | 186 (59.0) | 237 (66.9) | 1051 (58.6) |
| Male | 496 (44.1) | 129 (41.0) | 117 (33.1) | 742 (41.4) |
| Role | ‘Are you a:’ options provided as listed below. | |||
| Patient | 512 (45.6) | 133 (42.2) | 175 (49.4) | 820 (45.7) |
| Healthcare professional | 337 (30.0) | 108 (34.3) | 97 (27.4) | 542 (30.2) |
| Family member | 137 (12.2) | 42 (13.3) | 49 (13.8) | 228 (12.7) |
| Other a | 138 (12.3) | 32 (10.2) | 33 (9.3) | 203 (11.3) |
| Vulnerable population b | ‘Are you:’ options: indigenous people, Latin, black | |||
| Yes | 228 (20.3) | 75 (23.8) | 113 (31.9) | 416 (23.2) |
| No | 896 (79.7) | 240 (76.2) | 241 (68.1) | 1377 (76.8) |
| Education | ‘What is the highest degree or level of school you have completed?’ | |||
| Some high school education or less | 58 (5.2) | 19 (6.0) | 29 (8.2) | 106 (5.9) |
| High school graduate | 154 (13.7) | 41 (13.0) | 65 (18.4) | 260 (14.5) |
| Technical/vocational training | 239 (21.3) | 87 (27.6) | 93 (26.3) | 419 (23.4) |
| Bachelor’s degree or above | 673 (59.9) | 168 (53.3) | 167 (47.2) | 1008 (56.2) |
| Employment | ‘Are you currently employed?’ | |||
| No | 166 (14.8) | 52 (16.5) | 69 (19.5) | 287 (16.0) |
| Yes | 682 (60.7) | 206 (65.4) | 228 (64.4) | 1116 (62.2) |
| Retired | 276 (24.6) | 57 (18.1) | 57 (16.1) | 390 (21.8) |
| Living… | ‘With whom do you live? Please indicate all options that correspond’ | |||
| With high-risk people c | 237 (21.1) | 78 (24.8) | 109 (30.8) | 424 (23.6) |
| With susceptible people d | 231 (20.6) | 55 (17.5) | 67 (18.9) | 353 (19.7) |
| With healthy adults | 382 (34.0) | 119 (37.8) | 110 (31.1) | 611 (34.1) |
| Alone | 274 (24.4) | 63 (20.0) | 68 (19.2) | 405 (22.6) |
| Immunosuppressed | ‘Please indicate if you are diagnosed with any of the following conditions/currently taking any of these medications (options)’ | |||
| Yes (other e) | 117 (10.4) | 24 (7.6) | 28 (7.9) | 169 (9.4) |
| Yes (rheumatic disease) | 106 (9.4) | 34 (10.8) | 51 (14.4) | 191 (10.7) |
| No | 901 (80.2) | 257 (81.6) | 275 (77.7) | 1433 (79.9) |
| Cancer | ||||
| Yes | 176 (15.7) | 45 (14.3) | 49 (13.8) | 270 (15.1) |
| No | 948 (84.3) | 270 (85.7) | 305 (86.2) | 1523 (84.9) |
| Hypertension | ||||
| Yes | 231 (20.6) | 65 (20.6) | 66 (18.6) | 362 (20.2) |
| No | 893 (79.4) | 250 (79.4) | 288 (81.4) | 1431 (79.8) |
| Diabetes mellitus | ||||
| Yes | 114 (10.1) | 36 (11.4) | 43 (12.1) | 193 (10.8) |
| No | 1010 (89.9) | 279 (88.6) | 311 (87.9) | 1600 (89.2) |
| Kidney disease | ||||
| Yes | 16 (1.4) | 8 (2.5) | 5 (1.4) | 29 (1.6) |
| No | 1108 (98.6) | 307 (97.5) | 349 (98.6) | 1764 (98.4) |
| Smoking | ||||
| Yes | 94 (8.4) | 26 (8.3) | 32 (9.0) | 152 (8.5) |
| No | 1030 (91.6) | 289 (91.7) | 322 (91.0) | 1641 (91.5) |
| Language f | ||||
| English | 723 (64.3) | 204 (64.8) | 258 (72.9) | 1185 (66.1) |
| French | 401 (35.7) | 111 (35.2) | 96 (27.1) | 608 (33.9) |
| Variables | No Hesitancy (10) (n = 1124) | Mild Hesitancy (7.1–9.5 *) (n = 315) | Significant Hesitancy (0–7) (n = 354) | Total (n = 1793) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Influenza vaccine status | ||||
| Previous influenza vaccine | ‘Have you ever received a FLU vaccine before?’ | |||
| Yes | 1017 (90.5) | 280 (88.9) | 299 (84.5) | 1596 (89.0) |
| No | 107 (9.5) | 35 (11.1) | 55 (15.5) | 197 (11.0) |
| Previously rejected influenza vaccine | ‘Have you ever rejected the FLU vaccine?’ | |||
| Yes | 57 (5.1) | 21 (6.7) | 35 (9.9) | 113 (6.3) |
| No | 1067 (94.9) | 294 (93.3) | 319 (90.1) | 1680 (93.7) |
| COVID-19 disease | ||||
| Previous COVID-19 diagnosis | ‘Were you diagnosed with COVID-19 in 2020?’ | |||
| Yes | 23 (2.0) | 11 (3.5) | 10 (2.8) | 44 (2.5) |
| No | 1101 (98.0) | 304 (96.5) | 344 (97.2) | 1749 (97.5) |
| Perception that they receive enough information about COVID-19 prevention | ‘Do you feel you get enough information about COVID-19 prevention?’ | |||
| No | 36 (3.2) | 28 (8.9) | 31 (8.8) | 95 (5.3) |
| Yes | 1088 (96.8) | 287 (91.1) | 323 (91.2) | 1698 (94.7) |
| COVID-19 vaccine | ||||
| COVID-19 vaccine compulsory | ‘Do you think the COVID-19 vaccine should be compulsory?’ | |||
| No | 78 (6.9) | 40 (12.7) | 99 (28.0) | 217 (12.1) |
| Unsure | 202 (18.0) | 163 (51.7) | 200 (56.5) | 565 (31.5) |
| Yes | 844 (75.1) | 112 (35.6) | 55 (15.5) | 1011 (56.4) |
| Trust that the government is making decisions in citizens’ best interest regarding COVID-19 vaccines | ‘Do you trust that our government is making decisions in our best interest with respect to the COVID-19 vaccine?’ | |||
| No | 68 (6.0) | 22 (7.0) | 75 (21.2) | 165 (9.2) |
| Yes | 1056 (94.0) | 293 (93.0) | 279 (78.8) | 1628 (90.8) |
| Access barrier to vaccination would prevent participants from receiving a COVID-19 vaccine a | ‘Would any of the following factors prevent you from getting the COVID-19 vaccine? (please indicate all that correspond)’ a | |||
| Yes | 277 (24.6) | 106 (33.7) | 130 (36.7) | 513 (28.6) |
| No | 847 (75.4) | 209 (66.3) | 224 (63.3) | 1280 (71.4) |
| Trust pharmaceutical companies in providing safe and effective COVID-19 vaccines | ‘Do you trust pharmaceutical companies to provide safe and effective COVID-19 vaccines?’ | |||
| No | 25 (2.2) | 17 (5.4) | 47 (13.3) | 89 (5.0) |
| Unsure | 339 (30.2) | 161 (51.1) | 240 (67.8) | 740 (41.3) |
| Yes | 760 (67.6) | 137 (43.5) | 67 (18.9) | 964 (53.8) |
| Concerns that a future COVID-19 vaccine might not be safe | ‘How concerned are you that a future COVID-19 vaccine might not be safe?’ | |||
| Very concerned (to the point I will refuse to get it) | 27 (2.4) | 13 (4.1) | 83 (23.4) | 123 (6.9) |
| A little concerned | 695 (61.8) | 270 (85.7) | 241 (68.1) | 1206 (67.3) |
| Not concerned at all | 402 (35.8) | 32 (10.2) | 30 (8.5) | 464 (25.9) |
| Vaccine benefits overweigh its risks | ‘Do you think that vaccine benefits, in general, are larger than their risks?’ | |||
| No | 33 (2.9) | 2 (0.6) | 19 (5.4) | 54 (3.0) |
| Unsure | 103 (9.2) | 76 (24.1) | 174 (49.2) | 353 (19.7) |
| Yes | 988 (87.9) | 237 (75.2) | 161 (45.5) | 1386 (77.3) |
| Social pressure to receive a COVID-19 vaccine in the future | ‘Do you feel social pressure to get a COVID-19 vaccine in the future?’ | |||
| Yes | 205 (18.2) | 86 (27.3) | 75 (21.2) | 366 (20.4) |
| Unsure | 103 (9.2) | 61 (19.4) | 89 (25.1) | 253 (14.1) |
| No | 816 (72.6) | 168 (53.3) | 190 (53.7) | 1174 (65.5) |
| Risk-taking behaviour ^ | ||||
| Low (≤26.67) | 378 (33.6) | 105 (33.3) | 151 (42.7) | 634 (35.4) |
| Moderate (26.68–38.33) | 317 (28.2) | 91 (28.9) | 95 (26.8) | 503 (28.1) |
| High (≥38.34) | 429 (38.2) | 119 (37.8) | 108 (30.5) | 656 (36.6) |
| Level of Hesitancy to Receive a COVID-19 Vaccine | Significant Hesitancy (Score 0–7) | Mild Hesitancy (Scores 7.1–9.5) |
|---|---|---|
| aOR * (95% CI) (df, Wald, p-Value) | ||
| Age | 1.00 (0.99–1.02) (1, 0.14, 0.712) | 1.00 (0.98–1.01) (1, 0.75, 0.387) |
| Employment | ||
| No | 1.77 (0.96–3.25) (1, 3.39, 0.066) | 1.30 (0.76–2.21) (1, 0.92, 0.338) |
| Yes | 1.72 (1.00–2.97) (1, 3.86, 0.049) | 1.17 (0.73–1.86) (1, 0.43, 0.512) |
| Retired | Ref | Ref |
| Sex | ||
| Female | 1.29 (0.91–1.83) (1, 1.99, 0.158) | 0.95 (0.70–1.28) (1, 0.13, 0.723) |
| Male | Ref | Ref |
| Role | ||
| Patient | 1.21 (0.68–2.17) (1, 0.43, 0.514) | 1.13 (0.68–1.88) (1, 0.22, 0.640) |
| Healthcare professional | 0.75 (0.41–1.36) (1, 0.91, 0.342) | 1.15 (0.70–1.91) (1, 0.31, 0.578) |
| Family member | 1.02 (0.51–2.02) (1, 0.002, 0.966) | 1.17 (0.64–2.12) (1, 0.25, 0.614) |
| Other | Ref | Ref |
| Vulnerable Population a | ||
| Yes | 1.32 (0.91–1.92) (1, 2.07, 0.151) | 1.02 (0.72–1.44) (1, 0.01, 0.919) |
| No | Ref | Ref |
| Education | ||
| Some high-school education or less | 3.57 (1.81–7.07) (1, 13.41, <0.001) | 1.85 (0.97–3.53) (1, 3.53, 0.060) |
| High-school graduate | 1.70 (1.04–2.78) (1, 4.48, 0.034) | 1.22 (0.78–1.91) (1, 0.76, 0.384) |
| Technical/vocational training | 1.54 (1.03–2.30) (1, 4.44, 0.035) | 1.50 (1.07–2.13) (1, 5.37, 0.020) |
| Bachelor’s degree or above | Ref | Ref |
| Living… | ||
| With high-risk people b | 1.52 (0.93–2.48) (1, 2.75, 0.097) | 1.40 (0.91–2.16) (1, 2.31, 0.128) |
| With susceptible people c | 1.40 (0.83–2.35) (1, 1.58, 0.209) | 1.20 (0.75–1.90) (1, 0.58, 0.448) |
| With healthy adults | 0.99 (0.63–1.58) (1, 0.001, 0.981) | 1.36 (0.92–2.02) (1, 2.37, 0.124) |
| Alone | Ref | Ref |
| Immunosuppressed | ||
| Yes—other d | 0.64 (0.33–1.24) (1, 1.77, 0.184) | 0.67 (0.38–1.19) (1, 1.89, 0.170) |
| Yes—rheumatic disease | 1.42 (0.83–2.41) (1, 1.66, 0.197) | 1.22 (0.75–1.99) (1, 0.63, 0.429) |
| No | Ref | Ref |
| Cancer | ||
| Yes | 0.81 (0.48–1.36) (1, 0.63, 0.426) | 0.97 (0.62–1.54) (1, 0.01, 0.907) |
| No | Ref | Ref |
| Hypertension | ||
| Yes | 0.77 (0.49–1.21) (1, 1.26, 0.261) | 1.12 (0.76–1.64) (1, 0.32, 0.571) |
| No | Ref | Ref |
| Diabetes mellitus | ||
| Yes | 1.60 (0.90–2.82) (1, 2.57, 0.109) | 1.57 (0.97–2.54) (1, 3.40, 0.065) |
| No | Ref | Ref |
| Kidney disease | ||
| Yes | 1.08 (0.31–3.71) (1, 0.01, 0.908) | 2.33 (0.87–6.22) (1, 2.82, 0.093) |
| No | Ref | Ref |
| Smoking | ||
| Yes | 0.66 (0.37–1.18) (1, 1.96, 0.162) | 0.81 (0.49–1.36) (1, 0.62, 0.430) |
| No | Ref | Ref |
| Previous influenza vaccine | ||
| Yes | 0.99 (0.61–1.63) (1, 0.001, 0.982) | 1.04 (0.66–1.66) (1, 0.03, 0.857) |
| No | Ref | Ref |
| Previously rejected influenza vaccine | ||
| Yes | 1.33 (0.70–2.50) (1, 0.76, 0.383) | 1.14 (0.64–2.04) (1, 0.20, 0.659) |
| No | Ref | Ref |
| Previous COVID-19 diagnosis | ||
| Yes | 2.04 (0.76–5.50) (1, 2.01, 0.157) | 1.91 (0.82–4.44) (1, 2.26, 0.133) |
| No | Ref | Ref |
| Perception of receiving enough information about COVID-19 prevention | ||
| No | 2.20 (1.10–4.40) (1, 4.91, 0.027) | 2.82 (1.55–5.11) (1, 11.65, 0.001) |
| Yes | Ref | Ref |
| COVID-19 vaccine compulsory | ||
| No | 19.76 (11.82–33.06) (1, 129.23, <0.001) | 3.98 (2.50–6.34) (1, 33.69, <0.001) |
| Unsure | 12.14 (8.03–18.35) (1, 140.28, <0.001) | 4.91 (3.57–6.74) (1, 95.60, <0.001) |
| Yes | Ref | Ref |
| Trust that the government is making decisions in citizens’ best interest regarding COVID-19 vaccines | ||
| No | 1.67 (0.98–2.82) (1, 3.59, 0.058) | 0.82 (0.47–1.44) (1, 0.48, 0.487) |
| Yes | Ref | Ref |
| Access barrier to vaccination would prevent participants from receiving a COVID-19 vaccine e | ||
| Yes | 1.30 (0.92–1.84) (1, 2.14, 0.144) | 1.15 (0.84–1.57) (1, 0.75, 0.387) |
| No | Ref | Ref |
| Trust pharmaceutical companies to provide safe and effective COVID-19 vaccines | ||
| No | 3.75 (1.82–7.73) (1, 12.78, <0.001) | 1.86 (0.91–3.81) (1, 2.87, 0.090) |
| Unsure | 3.06 (2.10–4.45) (1, 34.28, <0.001) | 1.32 (0.97–1.79) (1, 3.09, 0.079) |
| Yes | Ref | Ref |
| Concerns that a future COVID-19 vaccine might not be safe | ||
| Very concerned | 7.02 (3.30–14.94) (1, 25.56, <0.001) | 2.59 (1.12–5.97) (1, 4.95, 0.026) |
| A little concerned | 1.93 (1.16–3.22) (1, 6.39, 0.011) | 3.15 (2.06–4.81) (1, 27.91, <0.001) |
| Not concerned at all | Ref | Ref |
| Vaccine benefits outweigh its risks | ||
| No | 2.95 (1.28–6.79) (1, 6.49, 0.011) | 0.29 (0.66–1.28) (1, 2.66, 0.103) |
| Unsure | 3.81 (2.55–5.68) (1, 42.93, <0.001) | 1.74 (1.17–2.58) (1, 7.45, 0.006) |
| Yes | Ref | Ref |
| Social pressure to receive a COVID-19 vaccine in the future | ||
| Yes | 1.16 (0.76–1.77) (1, 0.46, 0.500) | 1.76 (1.24–2.50) (1, 9.98, 0.002) |
| Unsure | 2.27 (1.45–3.56) (1, 12.78, <0.001) | 2.11 (1.39–3.19) (1, 12.47, <0.001) |
| No | Ref | Ref |
| Language | ||
| English | 1.37 (0.95–1.98) (1, 2.78, 0.096) | 1.02 (0.75–1.40) (1, 0.02, 0.879) |
| French | Ref | Ref |
| Risk-taking behavior | ||
| Low | 1.60 (1.07–2.40) (1, 5.26, 0.022) | 1.08 (0.76–1.53) (1, 0.16, 0.686) |
| Moderate | 1.16 (0.76–1.77) (1, 0.46, 0.498) | 1.03 (0.72–1.46) (1, 0.02, 0.894) |
| High | Ref | Ref |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, V.; Rampakakis, E.; Zanos, T.P.; Levy, T.J.; Shen, H.C.; McDonald, E.G.; Frenette, C.; Bernatsky, S.; Hudson, M.; Ward, B.J.; et al. High Frequency of COVID-19 Vaccine Hesitancy among Canadians Immunized for Influenza: A Cross-Sectional Survey. Vaccines 2022, 10, 1514. https://doi.org/10.3390/vaccines10091514
Valerio V, Rampakakis E, Zanos TP, Levy TJ, Shen HC, McDonald EG, Frenette C, Bernatsky S, Hudson M, Ward BJ, et al. High Frequency of COVID-19 Vaccine Hesitancy among Canadians Immunized for Influenza: A Cross-Sectional Survey. Vaccines. 2022; 10(9):1514. https://doi.org/10.3390/vaccines10091514
Chicago/Turabian StyleValerio, Valeria, Emmanouil Rampakakis, Theodoros P. Zanos, Todd J. Levy, Hao Cheng Shen, Emily G. McDonald, Charles Frenette, Sasha Bernatsky, Marie Hudson, Brian J. Ward, and et al. 2022. "High Frequency of COVID-19 Vaccine Hesitancy among Canadians Immunized for Influenza: A Cross-Sectional Survey" Vaccines 10, no. 9: 1514. https://doi.org/10.3390/vaccines10091514
APA StyleValerio, V., Rampakakis, E., Zanos, T. P., Levy, T. J., Shen, H. C., McDonald, E. G., Frenette, C., Bernatsky, S., Hudson, M., Ward, B. J., & Colmegna, I. (2022). High Frequency of COVID-19 Vaccine Hesitancy among Canadians Immunized for Influenza: A Cross-Sectional Survey. Vaccines, 10(9), 1514. https://doi.org/10.3390/vaccines10091514







