Humoral and Cellular Responses to a Delayed Fourth SARS-CoV-2 mRNA-Based Vaccine in Weak Responders to 3 Doses Kidney Transplant Recipients
Abstract
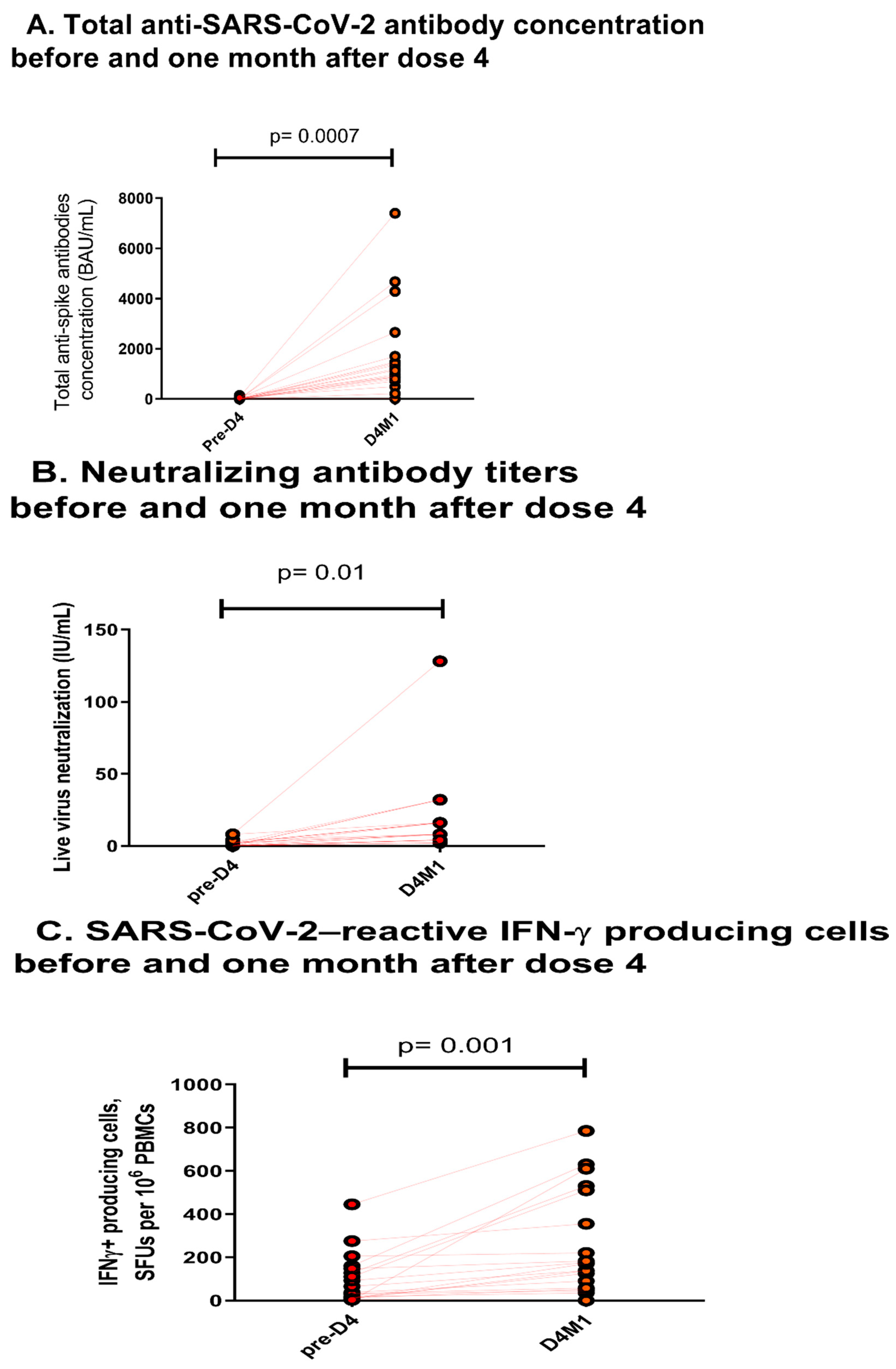
:1. Supporting Information 1: Virological Methods
1.1. Serology Assay
1.2. Neutralization Assay
1.3. EliSpot Assay
2. Supporting Information 2: Clinical Informations of Included Patients
| N = 20 | |
|---|---|
| Gender (M/F) | 11/9 |
| Age (years, mean ± SD) | 52 ± 14 |
| History of rejection in the year preceding vaccination, n | 0 |
| Time between vaccine and transplantation (months, IQR 1; 3) | 56 (22; 112) |
| No induction therapy, n (%) | 8 (40) |
| Induction therapy, n (%) | 12 (60) |
| Anti-IL2 receptor blockers | 5 (42) |
| Polyclonal antibodies | 7 (58) |
| Type of immunosuppressive regimen *, n (%) | |
| Calcineurin-inhibitors | 17 (85) |
| Tacrolimus | 16 (80) |
| Ciclosporin A | 1 (5) |
| Mycophenolic acid | 13 (65) |
| mTOR inhibitors | 7 (35) |
| Steroids | 18 (90) |
| Belatacept | 3 (15) |
| Neutrophil count before vaccination (/mm3, mean ± SD) | 5622 ± 2842 |
| Lymphocyte count before vaccination (/mm3, mean ± SD) | 1571 ± 764 |
| CD4+ T-cell count before vaccination (/mm3, mean ± SD) | 546 ± 311 |
| CD8+ T-cell count before vaccination (/mm3, mean ± SD) | 451 ± 308 |
| CD19+ T-cell count before vaccination (/mm3, mean ± SD) | 91 ± 85 |
| NK cell count before vaccination (/mm3, mean ± SD) | 253 ± 232 |
| eGFR before vaccination (mL/min/1.73 m2) | 51 ± 19 |
| Neutrophil count before 4th dose (/mm3, mean ± SD) | 6125 ± 2978 |
| Lymphocyte count before 4th dose (/mm3, mean ± SD) | 1061 ± 805 |
| eGFR before 4th dose (mL/min/1.73 m2) | 46 ± 23 |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamar, N.; Abravanel, F.; Marion, O.; Esposito, L.; Hebral, A.L.; Médrano, C.; Guitard, J.; Lavayssière, L.; Cointault, O.; Nogier, M.B.; et al. Anti-SARS-CoV-2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2 vaccine in a large cohort of solid organ transplant patients. Am. J. Transplant. 2022, 22, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Romieu-Mourez, R.; Couat, C.; del Bello, A.; Izopet, J. Assessment of 4 Doses of SARS-CoV-2 Messenger RNA-Based Vaccine in Recipients of a Solid Organ Transplant. JAMA Netw. Open 2021, 4, e2136030. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Thaunat, O.; Benotmane, I.; Masset, C.; Blancho, G. Antibody Response to a Fourth Messenger RNA COVID-19 Vaccine Dose in Kidney Transplant Recipients: A Case Series. Ann. Intern. Med. 2022, 175, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Grunau, B.; Goldfarb, D.M.; Asamoah-Boaheng, M.; Golding, L.; Kirkham, T.L.; Demers, P.A.; Lavoie, P.M. Immunogenicity of Extended mRNA SARS-CoV-2 Vaccine Dosing Intervals. JAMA-J. Am. Med. Assoc. 2022, 327, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, C.; Migueres, M.; Mansuy, J.-M.; Saivin, S.; Miedougé, M.; Chapuy-Regaud, S.; Izopet, J. Antibody titers and breakthrough infections with Omicron SARS-CoV-2. J. Infect. 2022, 84, e13–e15. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.S.; Winkler, M.S.; et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 185, 447–456.e11. [Google Scholar] [CrossRef] [PubMed]
- Bruminhent, J.; Setthaudom, C.; Chaumdee, P.; Boongird, S.; Kiertiburanakul, S.; Malathum, K.; Nongnuch, A.; Phuphuakrat, A.; Jirasiritham, S.; Janphram, C.; et al. SARS-CoV-2-specific humoral and cell-mediated immune responses after immunization with inactivated COVID-19 vaccine in kidney transplant recipients (CVIM 1 study). Am. J. Transplant. 2022, 22, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Chapuy-Regaud, S.; Miédouge, M.; Abravanel, F.; Da Silva, I.; Porcheron, M.; Fillaux, J.; Diméglio, C.; Izopet, J. Evaluation of three Quantitative Anti-SARS-CoV-2 antibody Immunoassays. Microbiol. Spectr. 2021, 9, e0137621. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Miédouge, M.; Chapuy-Regaud, S.; Mansuy, J.M.; Izopet, J. Clinical performance of a rapid test compared to microplate test to detect total anti-SARS-CoV-2 antibodies directed to the spike protein. J. Clin. Virol. 2020, 130, 104528. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Bello, A.; Kamar, N.; Marion, O.; Izopet, J.; Abravanel, F. Humoral and Cellular Responses to a Delayed Fourth SARS-CoV-2 mRNA-Based Vaccine in Weak Responders to 3 Doses Kidney Transplant Recipients. Vaccines 2022, 10, 1439. https://doi.org/10.3390/vaccines10091439
Del Bello A, Kamar N, Marion O, Izopet J, Abravanel F. Humoral and Cellular Responses to a Delayed Fourth SARS-CoV-2 mRNA-Based Vaccine in Weak Responders to 3 Doses Kidney Transplant Recipients. Vaccines. 2022; 10(9):1439. https://doi.org/10.3390/vaccines10091439
Chicago/Turabian StyleDel Bello, Arnaud, Nassim Kamar, Olivier Marion, Jacques Izopet, and Florence Abravanel. 2022. "Humoral and Cellular Responses to a Delayed Fourth SARS-CoV-2 mRNA-Based Vaccine in Weak Responders to 3 Doses Kidney Transplant Recipients" Vaccines 10, no. 9: 1439. https://doi.org/10.3390/vaccines10091439
APA StyleDel Bello, A., Kamar, N., Marion, O., Izopet, J., & Abravanel, F. (2022). Humoral and Cellular Responses to a Delayed Fourth SARS-CoV-2 mRNA-Based Vaccine in Weak Responders to 3 Doses Kidney Transplant Recipients. Vaccines, 10(9), 1439. https://doi.org/10.3390/vaccines10091439






