Abstract
Universal varicella vaccination (UVV) in England and Wales has been hindered by its potential impact on exogenous boosting and increase in herpes zoster (HZ) incidence. We projected the impact of ten UVV strategies in England and Wales on the incidence of varicella and HZ and evaluated their cost-effectiveness over 50 years. The Maternal-Susceptible-Exposed-Infected-Recovered-Vaccinated transmission model was extended in a dynamically changing, age-structured population. Our model estimated that one- or two-dose UVV strategies significantly reduced varicella incidence (70–92%), hospitalizations (70–90%), and mortality (16–41%) over 50 years. A small rise in HZ cases was projected with UVV, peaking 22 years after introduction at 5.3–7.1% above pre-UVV rates. Subsequently, HZ incidence steadily decreased, falling 12.2–14.1% below pre-UVV rates after 50 years. At a willingness-to-pay threshold of 20,000 GBP/QALY, each UVV strategy was cost-effective versus no UVV. Frontier analysis showed that one-dose UVV with MMRV-MSD administered at 18 months is the only cost-effective strategy compared to other strategies. HZ incidence varied under alternative exogenous boosting assumptions, but most UVV strategies remained cost-effective. HZ vaccination decreased HZ incidence with minimal impact on the cost-effectiveness. Introducing a UVV program would significantly reduce the clinical burden of varicella and be cost-effective versus no UVV after accounting for the impact on HZ incidence.
1. Introduction
Varicella, or chickenpox, is an acute and highly contagious disease caused by the varicella-zoster virus (VZV). While usually mild and self-limiting, varicella can lead to severe complications, hospitalizations, and death in rare cases [1,2]. The most common complications are bacterial infections, decreased platelets, arthritis, hepatitis, pneumonia, and encephalitis. They can occur in all age groups but are more likely to occur in neonates, immunocompromised persons, and adults [3]. Globally, varicella is one of the most common childhood infectious diseases, with incidence estimates ranging from 2 to 16 cases per 1000 persons [1,4,5]. It poses a significant economic and caregiver burden due to its high incidence and potential complications [6]. VZV has a second clinical manifestation, herpes zoster (HZ), a painful vesicular rash occurring later in adulthood upon reactivation of the latent form of the virus in nerve ganglions [1].
Live-attenuated VZV vaccines are active immunizing agents that offer protection against VZV infection. Randomized clinical trials (RCTs) and observational studies have established the safety and efficacy of these vaccines for the prevention of varicella [7,8,9] and have been licensed and added to routine childhood vaccination schedules in many countries worldwide [10]. Universal varicella vaccination (UVV) programs have led to significant reductions in disease incidence [11,12]. For example, in the United States (US), where it was introduced in 1995, varicella incidence declined by 98% from 1990 to 2016 [11]. In addition, varicella hospitalizations and deaths declined by 99% from 2012 to 2016 compared to the pre-vaccination period from 1990 to 1994 [11].
The World Health Organization (WHO) recommends UVV in countries that can maintain vaccination coverage rates of ≥80% [13]. One dose is recommended to reduce mortality and severe morbidity from varicella but is insufficient to limit virus circulation and prevent outbreaks [13]. A two-dose schedule has higher effectiveness and is therefore recommended in countries where the programmatic goal is to further reduce the number of cases and outbreaks.
In the United Kingdom (UK), varicella vaccination is not part of the routine childhood immunization program and is only offered to those in close contact with individuals at high risk of varicella and its complications [14]. Consequences of this policy impact different age groups. General practitioner (GP) consultation rates for varicella are highest among children between 1 and 3 years of age (39.7 per 1000 person-years in 2014) [15]. Most hospital admissions for varicella were in children under 10 years of age (79.4% during 2004–2013) [16]. In contrast, most HZ admissions (71.5%) over the same timeframe occurred in adults 60 years of age or older [16].
In 2010, the Joint Committee on Vaccination and Immunization (JCVI) in England recommended against a UVV program in children or a combined childhood varicella and adult HZ vaccination program due to its potential impact on exogenous boosting and HZ incidence [17]. It has been hypothesized that UVV, by reducing circulating wild-type VZV, also reduces exogenous boosting, the phenomenon by which re-exposure to VZV boosts protective cell-mediated immunity and delays reactivation of latent VZV. A decrease in exogenous boosting could potentially cause an increase in the incidence of HZ or shift the incidence of natural varicella to older age groups who are more likely than younger individuals to experience severe illness, leading to hospitalization and higher costs [17]. Prior modeling studies that evaluated the impact of UVV on HZ incidence with different assumptions for exogenous boosting found that UVV implementation may not be cost-effective when evaluated over a short time horizon in the UK [16,18].
Since the JCVI’s recommendation, several real-world studies have advanced our understanding of the impact of UVV and exogenous boosting. A recently published self-controlled case series study of 9604 UK adults with both household exposure to varicella and an episode of HZ showed that exposure to varicella is associated with a 33% reduction in risk of HZ over 20 years, suggesting that the impact of exogenous boosting may be lower than predicted in previous models [19]. Another database study examining 20 years of experience with UVV in the US found that the transient increase in the incidence of HZ predicted by the models was not observed [20]. The annual incidence of HZ in adults increased at approximately the same rate in the years before and after implementing the UVV program. The increase in HZ incidence prior to UVV implementation may be explained by historical demographic changes that were not considered by previous models [21,22].
Models that consider the interplay between exogenous boosting and demographic changes may more accurately predict the impact of varicella vaccination programs on HZ incidence. The objective of this study was to estimate the long-term clinical and economic impact of UVV strategies in England and Wales as well as their cost-effectiveness, considering exogenous boosting scenarios based on recent real-world evidence, in a population with a dynamically changing age structure.
2. Materials and Methods
2.1. Description of the Dynamic Transmission Model
A deterministic, age-structured, continuous-time, nonlinear dynamic transmission model in the form of differential equations was developed (details provided in Section S2 of the Supplementary Materials File). The model uses the Maternal-Susceptible-Exposed-Infected-Recovered-Vaccinated structure [23], extended to include health states that represent the reactivation of latent VZV causing HZ outbreaks. The age-defined compartments were defined to capture the demographic, epidemiological, behavioral, and economic inputs as well as vaccination schedules of interest. The health states tracking vaccinated persons were further subdivided to differentiate between those who received one or two varicella doses. The model incorporated a dynamically changing population using over 50 years of historical data on time-dependent mortality, fertility, and migration rates. Thus, the impact of changing demography on disease incidence can be measured, and the model reflects the range of available historical data on the epidemiology of VZV in England and Wales, especially varicella seroprevalence.
2.2. Demographic and Epidemiological Parameters
Demographic and epidemiological parameters are provided in Table 1. The model was calibrated to age-stratified varicella seroprevalence [24,25,26], and HZ incidence [18] in England and Wales. Varicella- and HZ-related death were fitted against mortality data summarized in Brisson and Edmunds [24] through calibration as well (details in Supplementary Materials File).

Table 1.
Demographic and epidemiological parameters.
Most parameters related to varicella and HZ reactivation were taken from a modeling study by Schuette and Hethcote [23]. The model stratifies varicella-infected individuals into latent and infectious health states. Susceptible individuals may become infected at a rate governed by contact rates between age groups [31,40]. The same average latent period was used for natural and breakthrough infection [23]. However, the likelihood of a vaccinated individual infecting others following breakthrough varicella was reduced due to a shorter infectious period and lower relative infectivity of breakthrough varicella than natural varicella. The relative infectivity of breakthrough varicella was based upon a 1997–2001 population-based active surveillance study in which vaccinated cases were half as contagious as unvaccinated cases [36].
Persons previously infected with VZV can undergo HZ reactivation as immunity against HZ wanes. Individuals who were successfully vaccinated against varicella can undergo HZ reactivation as well, which results in recovery with lifelong immunity or death. The model assumes that individuals can benefit from exogenous boosting, providing temporary partial immunity throughout their lifetime with parameters derived from recent real-world evidence on the impact of contact with persons with infectious varicella on rates of HZ in the UK [19]. Using a predictive model (see Section S6 of Supplementary Materials File), the waning period following natural or breakthrough varicella and the proportion of contacts leading to exogenous boosting were calculated [19].
2.3. Vaccine Properties
Vaccine properties are described in the Supplementary Materials File, Table S2. Vaccine failure rates (defined as individuals who did not seroconvert within 42 days of vaccine administration) were drawn directly from RCTs with 10 years of follow up (4% for Varivax® [Merck Sharp & Dohme LLC, Rahway, NJ, USA, V-MSD] and 5% for Varilrix® [GlaxoSmithKline, Wavre, Belgium, V-GSK]) [8,41,42]. The first and second dose take is the rate at which a complete immunological response is induced following the first dose and the second dose if the first dose does not provide complete immunity, respectively [43]. We estimated take and duration of protection using deterministic compartmental models to simulate clinical trials of one- or two-dose varicella vaccination with V-MSD and V-GSK. Our model estimated that 90.3% (95% confidence interval (CI): 87.8–92.9%) of the cohort gained permanent protection from breakthrough varicella after the first dose of V-MSD compared to 61.7% (95% CI: 58.2–65.3%) after the first dose of V-GSK. We further estimated that a total of 97.0% (95% CI: 95.2–98.8%) and 93.8% (95% CI: 92.2–95.4%) of the cohort were permanently protected after two doses of V-MSD and V-GSK, respectively [43].
2.4. Vaccination Strategies
Ten UVV strategies are included in the model (detailed in Supplementary Materials File, Table S3). Since JCVI is considering an additional visit for measles, mumps, rubella (MMR) vaccination at 18 months [44], we included varicella vaccination strategies that aligned with the MMR schedule at 12 and 18 months. By aligning with MMR, the total number of pediatric office visits are not increased, and it also provides the opportunity to use combination MMRV vaccine in a single vaccine. In the single-dose strategies, children received a quadrivalent formulation at 18 months (ProQuad® [Merck Sharp & Dohme LLC, Rahway, NJ, USA, MMRV-MSD] or Priorix-Tetra® [GlaxoSmithKline, Wavre, Belgium, MMRV-GSK]). In the 2-dose strategies, children received a monovalent formulation at 12 months (V-MSD or V-GSK), followed by a second dose of the monovalent or the quadrivalent formulation at 18 months or 4 years of age, and with or without catch-up vaccination. The first and second catch-up dose for teenagers who missed childhood varicella vaccination was aligned with the human papillomavirus vaccine schedule. Catch-up vaccination was only offered during the first two years of the program. Vaccination coverage rates were set to 91% for the first dose and a second dose given at 18 months, 88% for a second dose at 40 months [45], and 87% for catch-up vaccination based on the tetanus, diphtheria, pertussis, polio vaccine (Tdap/IPV) [46].
2.5. Health Utilities and Cost
Age-specific utility weights for healthy individuals, individuals with natural and breakthrough varicella, as well as with HZ, are provided in Supplementary Materials File, Table S10.
The model includes vaccine-related costs, direct medical care costs, and when taking the societal perspective, the costs of work lost to varicella and HZ. All costs were adjusted for inflation and expressed in 2020 GBP. The estimated cost per dose for MMRV-MSD (GBP 46.63) and MMRV-GSK (GBP 55.13) are calculated from the average list prices in Germany [47], Spain [48], and Switzerland [49], and converted to British pounds due to lack of list pricing for MMRV in the UK. Price per dose for V-MSD (GBP 30.28) and V-GSK (GBP 27.31) were obtained from the NICE British National Formulary for Children [50]. A cost for vaccine administration (GBP 9.80) was also included [51]. Direct cost components are provided in Supplementary Materials File, Tables S6 and S11. For varicella and HZ, the costs of outpatient care included consultations with general practitioners (GP) and the cost of treatments (for cases seen in GP offices). It was assumed that every HZ outbreak resulted in treatment of some type, and as such, HZ-associated outpatient costs were applied to all cases. For varicella and HZ inpatient care, the cost of treatments was added to the cost per admission and applied to hospitalized cases. Health care utilization and associated costs were assumed to be the same for natural and breakthrough varicella and wild-type and vaccine-type HZ. Parameters for workdays lost due to VZV are provided in Supplementary Materials File, Table S12.
2.6. Model Outcomes and Cost-Effectiveness Analysis
The key model outcomes projected over time included varicella cases, HZ cases, and related outpatient visits, hospitalizations, and deaths. Outcomes were categorized as those related to natural and breakthrough varicella, and those related to HZ.
Incremental cost-effectiveness ratios (ICERs) were computed by comparing each UVV strategy to no UVV from both the payer and societal perspectives. In addition, a frontier analysis was conducted to inform decisions as to which strategy or strategies should be considered when choosing among all feasible ones. In this analysis, strategies dominated by one or several others are removed. The ICER of non-dominated strategies on the frontier is performed by determining whether the additional QALYs gained by the next strategy along the frontier is worth the incremental cost. The threshold for cost-effectiveness of GBP 20,000 per QALYs in the UK was used [52]. For all analyses, costs and QALYs were discounted at 3.5% annually [53,54]. The impact of UVV was assessed over a 50-year time horizon in the base case.
2.7. Sensitivity Analysis
To examine the impact of uncertainty in key vaccine and cost parameters on the cost-effectiveness of the UVV strategies that are on the frontier, parameters (or sets of related parameters) were varied one at a time in a deterministic sensitivity analysis (DSA). In addition, a probabilistic sensitivity analysis (PSA) using 500 variates was conducted to assess the variability in ICERs relative to the no-UVV scenario as a function of the uncertainty in key parameters relating to the calibration of HZ incidence and varicella seroprevalence, vaccine strategy, and costs.
Scenario analyses were also conducted to investigate the robustness of the cost-effectiveness results under alternative assumptions. Since the impact of UVV on HZ incidence is dependent on the assumptions used for exogenous boosting, three alternative scenarios were evaluated based upon the most recent literature and previous assumptions used in modeling studies. In scenario EB1, the waning period of HZ immunity was 24.4 years, with the effectiveness of contacts being age-dependent (75%, 71%, 57%, and 32% for those 0–59, 60–69, 70–79, and >80 years of age, respectively) [55]. In scenario EB2, the waning period of HZ immunity was 81.3 years, and 100% of contacts were effective. In scenario EB3, exogenous boosting was eliminated; the waning period of HZ immunity was 81.3 years, and no contacts were effective. In a fourth scenario, we assessed the impact of including HZ vaccination, which was introduced in the UK national immunization program (NIP) in 2013 for adults 70–79 years of age [56]. Finally, we conducted a sensitivity analysis using a 1.5% annual discount rate for long-term effects, consistent with NICE recommendations for vaccines [57].
3. Results
3.1. Calibration
Detailed results of the model calibration are provided in Section S3 of the Supplementary Materials File. The model reproduced the dynamically changing population age structure compared to historical data from selected years between 1971 and 2018 (Supplementary Materials File, Figure S3). With the calibrated age structure, the model aligned well with historical VZV seroprevalence data from 1978, 1992, 2004, and 2007 (Supplementary Materials File, Figure S4). For HZ incidence, the historical data from 1986 through 2006 were not stratified by year, so a year-to-year comparison was not possible. However, the assessment of the model fit using assumed demographics for 1995 showed that model-based projections are bounded by the historical HZ data and followed the general pattern of increasing incidence with age (Supplementary Materials File, Figure S5).
3.2. Health Outcomes
3.2.1. Varicella
All proposed UVV strategies are projected to rapidly reduce total varicella incidence after introduction. Two years after introducing a UVV program, incidence decreased from approximately 1100 cases per 100,000 person-years without UVV to around 135–210 cases per 100,000 person-years depending on UVV strategy (Figure 1a). Breakthrough varicella incidence rose after vaccine introduction and stabilized at values ranging between 10 and 247 cases per 100,000 person-years. Breakthrough cases were lower for two-dose strategies compared to one-dose strategies. The highest number of breakthrough cases was projected for Strategy B (one dose MMRV-GSK) (Figure 1b).
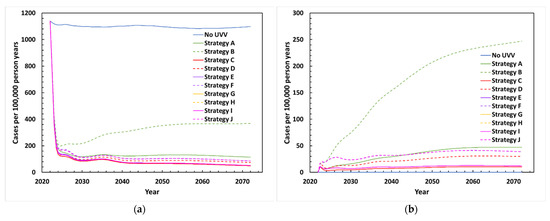
Figure 1.
Projected varicella incidence between 2022 and 2072 with various UVV strategies: (a) Total varicella cases; (b) breakthrough varicella cases. Strategy A: MMRV-MSD (18 months); Strategy B: MMRV-GSK (18 months); Strategy C: V-MSD (12 months, 18 months, catchup); Strategy D: V-GSK (12 months, 18 months, catchup); Strategy E: V-MSD (12 months) + MMRV-MSD (18 months); Strategy F: V-GSK (12 months) + MMRV-GSK (18 months); Strategy G: V-MSD (12 months) + MMRV-MSD (40 months) + V-MSD (catchup); Strategy H: V-GSK (12 months) + MMRV-GSK (40 months) + V-GSK (catchup); Strategy I: V-MSD (12 months, 40 months, catchup); Strategy J: V-GSK (12 months, 40 months, catchup).
The percentage reduction in varicella health outcomes (total varicella cases and varicella-related outpatient visits, hospitalizations, and deaths) after 50 years with each UVV strategy in comparison to no UVV is shown in Figure 2. Total varicella cases are estimated to decline by 70.0–92.1%, while the total number of varicella-related outpatient visits and hospitalizations is estimated to decline by 71.7–92.1% and by 70.5–89.8%, respectively, over a 50-year period. Varicella-related deaths are estimated to decline by 15.8–40.9% post-UVV introduction. For all health outcomes, percentage reductions were higher with two-dose strategies compared to single-dose strategies. Catch-up vaccination among adolescents 13 and 14 years of age offered during the first two years of UVV provided modest additional reductions in varicella cases. Strategies C and D, which are two-dose short-interval strategies with catch-up vaccination, resulted in the highest-percentage reductions in varicella cases (92.1% and 90.2%, respectively). The strategy with the highest-percentage reduction in varicella cases was Strategy C (two-dose short-interval strategy with V-MSD, including catch-up vaccination). There were no differences in health outcomes between Strategies G and I for MSD vaccines and between Strategies H and J for GSK vaccines, since the efficacy of monovalent varicella vaccines and the efficacy of the varicella component of quadrivalent vaccines were considered equivalent.
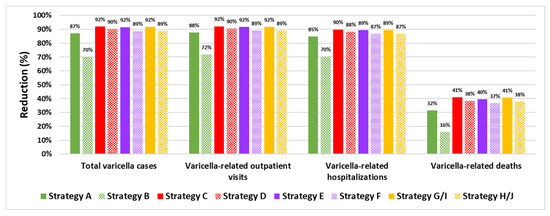
Figure 2.
Percentage reduction in varicella health outcomes with various UVV strategies in comparison to a no-UVV strategy.
3.2.2. Herpes Zoster
The projected incidence of HZ between 2022 and 2072 following UVV in the base-case scenario is depicted in Figure 3a. Without UVV, HZ incidence peaked in 40 years (2062), rising from 382 to 408 cases per 100,000 person-years (a 6.8% increase). Following UVV, HZ incidence peaked during the first 20–22 years (2042–2044) at around 402–409 cases per 100,000 person-years, a 5.3–7.1% increase compared to pre-UVV rates. HZ incidence began to decline under all UVV strategies around 2045 and fell below the pre-UVV incidence rate by 2050. By 2072, HZ incidence was estimated at around 328–335 cases per 100,000 person-years, a 12.2–14.1% decrease compared to the pre-UVV period.
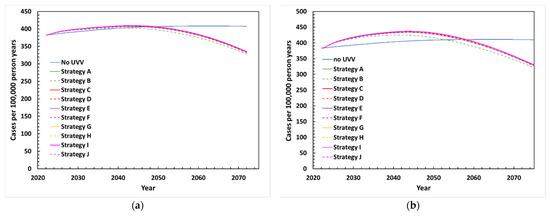
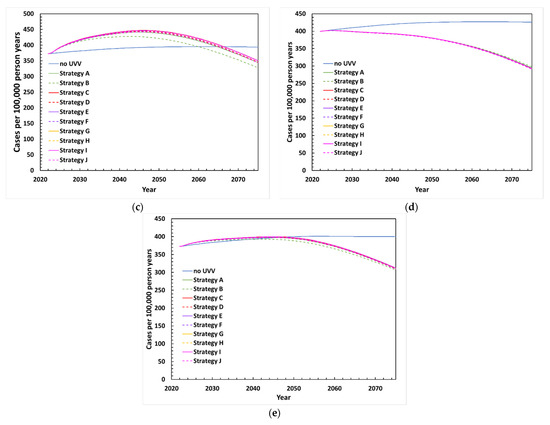
Figure 3.
Projected incidence of HZ between 2022 and 2072 with various UVV strategies under (a) base-case scenario, (b) exogenous boosting scenario EB1 with waning period of HZ immunity of 24.4 years and age dependent effective contacts (75%, 71%, 57%, and 32% for <60, 60–69, 70–79, and ≥80 years old, respectively), (c) exogenous boosting scenario EB2 with waning period of HZ immunity of 81.3 years and 100% effective contacts, (d) exogenous boosting scenario EB3 with waning period of HZ immunity of 81.3 years and 0% effective contacts, (e) HZ vaccination scenario.
3.3. Cost-Effectiveness
3.3.1. Base-Case Scenario
Results from the cost-effectiveness analysis comparing the UVV strategies over 50 years in England and Wales from a payer perspective and societal perspective are provided in Table 2. Implementation of the UVV program resulted in total direct costs ranging from GBP 2.12B to GBP 2.74B, compared to GBP 1.72B without vaccination.

Table 2.
Cost-effectiveness analysis for alternative UVV strategies from the payer and societal perspectives (2022–2072).
Each UVV strategy resulted in higher costs from the payer perspective, but also resulted in QALYs gained (fewer QALYs lost) due to the decrease in total varicella infections and HZ cases. Compared to no UVV, QALYs gained range from 54,854 (Strategy B: single-dose with MMRV-GSK) to 61,826 (Strategy C: a two-dose strategy including a catch-up with V-MSD). The ICER for each UVV strategy compared with no UVV ranged between 6809 GBP/QALY gained and 16,698 GBP/QALY gained. Thus, each strategy was cost-effective compared to no UVV when using the willingness-to-pay threshold of GBP 20,000 per QALY gained [52]. Strategies on the frontier included Strategy A (MMRV-MSD 18 months), Strategy G (V-MSD 12 months; MMRV-MSD 40 months, with catch-up), and Strategy C (V-MSD 12 and 18 months, with catch-up). All other strategies were dominated by one or more of these three strategies. If a threshold of 20,000 GBP/QALY was applied to the frontier strategies, only Strategy A would be deemed cost-effective.
From a societal perspective, Strategy A is cost-saving compared to no UVV, as QALYs were gained while reducing total costs by GBP 86M. All other strategies remained cost-effective, with each ICER at or below GBP 8003 per QALY gained vs no UVV. From the societal perspective, the same UVV strategies are on the frontier (Strategies A, G, and C). However, unlike the payer perspective, no UVV was strongly dominated by Strategy A, and thus does not lie on the frontier. Using the threshold of 20,000 GBP/QALY gained, only Strategy A would be deemed cost-effective, as the ICERs for Strategy G versus Strategy A and for Strategy C versus Strategy G exceed the threshold.
3.3.2. Deterministic Sensitivity Analysis
Figure 4 shows the robustness of the ICERs for the strategies on the frontier from the payer perspective to one-way deterministic changes in parameters on costs and vaccine properties. In each plot, the resulting upper and lower bound values of the ICER are shown, with the most influential parameters displayed at the top. For Strategy A versus no UVV (Figure 4a), the cost of the vaccine and treatment for varicella infection and HZ reactivation are the most influential parameters. The vaccine coverage, take, and waning rate also influenced the ICER, but to a much lesser degree. In each case, the ICER remained well below 10,000 GBP/QALY gained versus no UVV. For Strategy G versus Strategy A (Figure 4b) and Strategy C versus Strategy G (Figure 4c), vaccine coverage, take, and cost are influential. However, the ICERs for Strategy G and Strategy C remained above 50,000 GBP/QALY gained and 1,000,000 GBP/QALY gained, respectively.
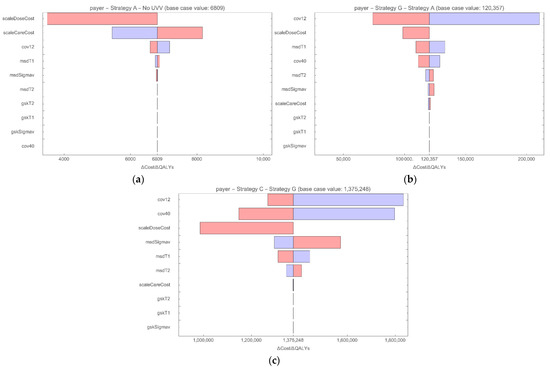
Figure 4.
Tornado diagram DSA for strategies on the frontier from the payer perspective for (a) Strategy A vs. no UVV; (b) Strategy G vs. Strategy A; (c) Strategy C vs. Strategy G). ICERs based on lower bound (red) and upper bound (blue) parameter values. cov12: coverage rate for vaccination at month 12; cov40: coverage rate for vaccination at month 40; gskSigmav: GSK vaccine, average duration of protection; gskT1: GSK vaccine, 1st dose take; gskT2: GSK vaccine, 2nd dose take; msdSigmav: MSD vaccine, average duration of protection; msdT1: MSD vaccine, 1st dose take; msdT2: MSD vaccine, 2nd dose take; scaleCareCost: multiplier for disease-related costs; scaleDoseCost: multiplier for vaccine cost per dose.
3.3.3. Probabilistic Sensitivity Analysis
The PSA was conducted on the base case to evaluate the uncertainty in the ICER resulting from variation in the calibration and uncertainty in parameters. Results of the PSA showed that the base-case results are robust. Strategies A, G, and C were compared to no UVV from a payer perspective. For Strategy A and Strategy G, all variates led to ICERs below 20,000 GBP/QALY gained. For Strategy C, 97.4% of the variates led to ICERs below 20,000 GBP/QALY gained. The spread of the points from the randomly generated parameter sets surrounding the base-case estimate of expected costs and QALYs and the likelihood of being cost-effective across a range of willingness-to-pay thresholds for the three vaccine strategies are shown in Supplementary Materials File, Figures S15 and S16, respectively.
3.3.4. Scenario Analyses
Results of the scenario analysis are presented in Table 3. Three scenarios were explored where boosting varies by duration of HZ immunity after varicella infection and by the percentage of varicella contacts that boost against HZ. In scenarios EB1 (waning period of HZ immunity is 24.4 years; age-dependent effectiveness of contacts) and EB2 (waning period of HZ immunity is 81.3 years; 100% effective contacts), peak HZ incidence for the UVV scenarios occurred around the same period (from 2042–2044 and from 2043–2046, respectively) compared to the base case (from 2042–2044). However, HZ incidence for the UVV scenarios remained higher than HZ incidence for no UVV for a longer time for EB1 (where UVV becomes lower between 2054–2059) and EB2 (between 2060–2067) compared to the base case (between 2041–2050) (Figure 3b,c). Thus, in both scenarios (and in the base case), UVV ultimately led to fewer HZ cases due to the diminishing pool of individuals at risk of HZ. The reduction in HZ cases following UVV was immediate in scenario EB3 (waning period of HZ immunity is 81.3 years, 0% effective contacts), when exogenous boosting was eliminated (Figure 3d). In scenario EB1, the incremental costs increased while the QALYs gained decreased, leading to higher ICERs compared to the base case. From the payer perspective, the ICERs ranged from 8864 GBP/QALY with Strategy A to 20,410 GBP/QALY with Strategy D. In scenario EB2, where exogenous boosting was assumed to be even higher, ICERs were higher and ranged from 12,041 GBP/QALY with Strategy A to 26,290 GBP/QALY with Strategy D. When the role of exogenous boosting was removed (EB3), ICERs decreased for all UVV strategies and ranged from 4331 GBP/QALY with Strategy A to 12,192 GBP/QALY with Strategy D. From the societal perspective, all ICERs were below GBP 20,000 for all UVV strategies and exogenous boosting scenarios. While Strategy A no longer dominated a no-UVV strategy under the EB1 and EB2 scenarios, the ICERs were only 1605 GBP/QALY and 6075 GBP/QALY, respectively. In scenario EB3, 6 of the 10 vaccination strategies dominated no UVV, with lower costs and more QALYs gained.

Table 3.
Scenario analysis for the cost-effectiveness of alternative UVV strategies by perspective (2022–2072).
We also assessed the impact of HZ vaccination, which was introduced in the UK NIP in 2013 for adults aged 70–79 years old [56]. Total costs were higher for all UVV strategies (including no UVV) as the cost of the HZ vaccination program was included. Compared to the base case without HZ vaccination, the number of varicella cases and outcomes were slightly lower (<1% reduction) for each UVV strategy. The introduction of an HZ vaccine had a slightly greater impact on reducing the incidence and burden of HZ (Figure 3e). ICERs improved slightly as HZ vaccination resulted in lower incremental costs (3% to 8% reduction) and greater incremental QALYs gained (1% to 2% higher) for all UVV strategies compared to no UVV. For example, ICERs of 6183 GBP/QALY and 6,809 GBP/QALY were obtained for Strategy A compared to no UVV in the presence and absence of an HZ vaccination program, respectively.
Finally, the results of the sensitivity analysis with a 1.5% discount rate for health outcomes are provided in Table 3. Total costs for each strategy remained the same, but incremental QALYs increased. Consequently, the ICERs for each strategy were lower than in the base case and ranged from GBP 4408 to GBP 10,778 per QALY from the payer perspective and from cost saving to GBP 5166 per QALY from the societal perspective.
4. Discussions
We adapted a dynamic transmission model to estimate the long-term health impact and cost-effectiveness of implementing a UVV program in England and Wales, considering different scenarios of exogenous boosting in a population with a dynamically changing age structure. Overall, ten vaccination strategies were considered using monovalent and quadrivalent formulations of MSD or GSK vaccines with one or two doses, short or medium intervals, and with or without catch-up vaccination.
All strategies substantially reduced the clinical and economic burden of VZV. Over 50 years, both one-dose strategies with quadrivalent MMRV formulations administered at 18 months of age with assumed coverage of 91% were projected to reduce varicella mortality by 16–32% and varicella incidence by 70–87% (with similar reductions in outpatient visits and hospitalizations). All two-dose strategies, where the first dose was administered at 12 months and the second dose at 18 or 40 months, were projected to have higher reductions in varicella mortality (37% to 41%) and incidence (89% to 92%). Breakthrough varicella cases were higher for one-dose strategies compared to two doses, and were highest for MMRV-GSK; this could be attributed to lower efficacy of one-dose MMRV-GSK [7]. Our findings are consistent with the literature as the efficacy of two doses is higher than that of one dose [7,8]. Two-dose strategies are typically recommended to reduce breakthrough cases and prevent outbreaks [13].
While the benefits of UVV on reducing the burden of varicella are well-established, the potential impact of UVV on exogenous boosting and HZ incidence has hindered the adoption of the UVV program in the UK and other European countries as this could lead to higher healthcare resource utilization. We thoroughly tested different exogenous boosting assumptions in our model. In the base case, our model used boosting assumptions based upon a 20-year real-world evidence study in UK adults conducted by Forbes et al. that showed the intensity of boosting to be lower (33%) than predicted by previous models [58]. Previously published models that included exogenous boosting generally predicted a transient increase in HZ in adults [16,18,38,59,60,61,62]. Our model also predicted a small rise in HZ incidence of between 402 and 409 cases per 100,000 person-years (a 5.3% to 7.1% increase compared to pre-UVV rates), which peaked 22 years after UVV implementation. However, there was an overall decrease in the incidence of HZ by 12.2% to 14.1% compared to the pre-UVV period after 50 years. This decrease in HZ incidence could be due to the diminishing cohort of individuals at risk for VZV reactivation due to the longer duration of the UVV program. Our model considered a dynamically changing population through inclusion of time-dependent mortality, fertility, and migration rates, using 50 years of historical data, making the model more realistic than a model with a static population. Our model showed a steady increase in HZ incidence even in the no-UVV scenario, reaching a peak of 408 cases per 100,000 person-years in 2062 (a 6.8% increase). This increase in HZ incidence in the absence of UVV could be primarily attributed to the ageing population. While this increase was comparable to the increase estimated for UVV strategies, the peak in the no-UVV scenario occurred at a later time period.
The base-case model showed that all one- and two-dose UVV programs are cost-effective compared to no UVV, at a willingness-to-pay threshold of 20,000 GBP/QALY gained, either from the payer or the societal perspective. Further, the single-dose strategy with MMRV-MSD at 18 months is cost-saving from the societal perspective. If a UVV program is to be adopted, the cost-effectiveness frontier provides information on which strategy should be selected from a cost-effectiveness standpoint. Based on both the payer and societal perspective, only the single dose of MMRV-MSD at 18 months (Strategy A) was deemed cost-effective at the willingness-to-pay-threshold of GBP 20,000 per QALY gained. Two-dose strategies with catch-up, Strategy G (V-MSD 12 months, MMRV-MSD 40 months), and Strategy C (V-MSD at 12 and 18 months) lay on the frontier but are not cost-effective because their ICERs (for Strategy G vs Strategy A and for Strategy C vs Strategy G) exceeded the willingness-to-pay threshold of 20,000 GBP/QALY. The DSA suggests that the results are robust to changes in cost of medical care and vaccine coverage, take, waning, and vaccine cost. However, beyond the cost-effectiveness of vaccination strategies, policymakers should consider several factors when implementing a UVV program, including the ability to maintain high vaccination coverage (>80%) and the flexibility of the vaccination schedule to accommodate an additional vaccination visit, as well as the programmatic goals of the country. To prevent outbreaks and reduce transmission and breakthrough varicella, two-dose strategies are recommended over one-dose strategies [13].
The results of our model are consistent with those from a similar dynamic model adapted for Turkey, Italy, Mexico, Norway, and Switzerland [22,63,64,65,66]. These studies also showed significant reductions in burden of disease after UVV; however, only the Turkey, Norway, and Switzerland adaptations accounted for the impact of exogenous boosting. The effects of UVV on HZ incidence are strongly dependent on the hypothesized boosting intensity. A range of exogenous boosting frameworks have been used in previous modeling studies, from no boosting to full permanent immunity [67]. However, the effect of exogenous boosting is still uncertain due to differences in study populations and environments and limited pre- and post-UVV data. In addition, other investigators have reported that the projected transient increases in HZ are sensitive to several parameters whose values are uncertain [38]. Recent observational studies have shown that the impact of UVV on exogenous boosting may not be as significant as previously estimated [19,22]. To understand the impact of the exogenous boosting assumption on the cost-effectiveness of the UVV strategies, robust scenario analyses were conducted. Based on our model, the higher the prevalence of varicella, the more influential the exogenous boosting assumption will be on the number of HZ cases, and thus on the costs and QALYs. Consequently, the ICERs for the UVV strategies (from the payer perspective, GBP 6,809–15,079 per QALY gained versus no UVV) increased under scenarios EB1 and EB2 where the magnitude of boosting was higher and decreased considerably under scenario EB3 without boosting (0%). Even in strategy EB2 (with 100% boosting), the ICERs from the payer perspective remained below 20,000 GBP/QALY for 6 of the 10 vaccination strategies, and below 30,000 GBP/QALY for all 10 strategies.
Brisson et al. predicted that UVV would reduce the burden of varicella in England and Wales [68]. However, these benefits would be offset by an increase in the incidence of HZ, and consequently, they reported that pediatric UVV might not be cost-effective. Another modeling study by Van Hoek et al. estimated that two-dose UVV would significantly reduce the burden of varicella with high vaccination coverage rates but with an increase in HZ incidence [18,41]. The authors evaluated the impact of a combined vaccination policy for varicella and HZ and concluded that the program would only be cost-effective over a long timeframe (80 to 100 years). A recent modeling study by Akpo et al. found two-dose UVV strategies to be cost-effective over short- and long-term time horizons [51]. The results from all these models were significantly influenced by the hypothesized impact of UVV on exogenous boosting and incidence of HZ, which were different in each model. Like our model, Melegaro et al. [61] also accounted for the impact of an ageing population and estimated a similar clinical impact on the burden of VZV [61]. They concluded that the concurrent introduction of routine HZ vaccination at 65 years of age with pediatric varicella vaccination is expected to mitigate the increase in HZ incidence and be a cost-effective policy in Italy [61]. We conducted a scenario analysis in which a HZ vaccination program (with Zostavax® (Merck Sharp & Dohme LLC, Rahway, NJ, USA) was initiated in 2013 within the model. We observed a modest impact of HZ vaccination on HZ incidence and mortality with increased costs compared to no HZ vaccination. This could be due to the narrow age range (adults 70–80 years) that is recommended to receive HZ vaccination in the current program. Nevertheless, the ICERs for each UVV strategy observed in the base case without HZ vaccination were lower than the ICERs in the HZ vaccination scenarios. The HZ vaccination program could be more effective if it was offered to a broader age group of adults who are at a high risk of HZ infection, similar to recommendations in other countries such as the US [62].
Limitations
This study has several limitations. Data specific to England and Wales for some parameters such as workdays lost and QALYs lost for HZ and PHN were not available; hence, we used published estimates from other countries in line with other studies. There is currently no pediatric vaccination visit at 18 months in England and Wales, which was the modeled timepoint for the one-dose MMRV strategies and two-dose short-interval strategies. The model did not include any costs associated with implementing (e.g., training staff) a new vaccination visit. Additionally, the list prices for MMRV formulations are not available for England and Wales; we used international reference pricing, which may differ from the actual list prices. Therefore, this is a conservative estimate of ICERs as the analysis utilizes list prices and not tender prices. There are limited data available comparing V-MSD/MMRV-MSD to V-GSK/MMRV-GSK; however, V-MSD and MMRV-MSD are generally considered immunologically equivalent to each other, as are V-GSK and MMRV-GSK. Finally, we used the temporary immunity model to estimate exogenous boosting and its duration. There is ongoing research on alternative modeling of the exogenous boosting mechanism, such as progressive immunity [67]. Different modeling approaches have led to different epidemiological results and would impact the cost-effectiveness of a UVV program.
5. Conclusions
We estimated the long-term effectiveness and cost-effectiveness of UVV in England and Wales while considering the impact on the burden of HZ using recent real-world evidence on exogenous boosting. We also used a dynamic population in our transmission model to better understand the role of an aging population on HZ incidence.
Our model estimated that all UVV strategies, including one or two doses, short or medium intervals, and monovalent or quadrivalent formulations, substantially reduced the clinical burden of varicella in terms of incidence, outpatient visits, hospitalizations, and mortality compared to no UVV. A UVV program was projected to reduce the incidence of HZ after 50 years compared to a no-UVV scenario following an initial increase in the first 20 years of its introduction. Our model also showed a similar increase in the incidence of HZ in the absence of UVV because of the ageing population. All UVV strategies were cost-effective compared to no UVV over 50 years. A one-dose UVV strategy with MMRV-MSD administered at 18 months of age was the most dominant strategy from the payer perspective and was cost-saving from the societal perspective. Our model suggests that policymakers should consider UVV to reduce the clinical and economic burden of VZV in England and Wales.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines10091416/s1, demographic model (Section S1); model for varicella and HZ natural history and vaccine effects (Section S2); calibration (Section S3); vaccine properties (Section S4); vaccination strategies (Section S5); derivation of model parameter inputs (Section S6) and sensitivity analysis (Section S7). Vaccine properties (Table S2), Summary of vaccination strategies (Table S3); Varicella, HZ and PHN health care resource utilization related parameters (Table S6); Health utility weights (Table S10); Health care resource utilization unit costs (Table S11); Parameters for indirect costs associated with varicella and HZ (Table S12). Observed and calibrated population age structure for selected years (Figure S3); Observed and fitted VZV seroprevalence data for 1978, 1992, 2004 and 2007 (Figure S4); Observed and fitted HZ incidence data (Figure S5); Incremental cost-effectiveness plane from the 500 random variates for three strategies from the payer perspective (Figure S15); Cost-effectiveness acceptability curve from the 500 random variates for three strategies from the payer perspective (Figure S16). References [69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85] are cited in Supplementary Materials.
Author Contributions
O.S., M.P. (Matthew Pillsbury), E.E. and M.P. (Manjiri Pawaskar) led the study concept and design. O.S., T.P., I.M., R.N., I.X. and M.P. (Manjiri Pawaskar) supported model inputs, data analysis and model adaptations. All authors contributed to the interpretation of results and in writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., 126 E Lincoln Ave, Rahway, NJ 07065, USA.
Institutional Review Board Statement
Not Applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to acknowledge Colleen Burgess for her support in data analysis and John Cook and Martin Senecal for their support in manuscript writing.
Conflicts of Interest
Sharomi O., Petigara T., Elbasha E., and Pawaskar M. are employees and stockholders of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Pillsbury M. was an employee of Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA when the work was completed. Matthews I. is an employee of MSD (UK) Ltd., London, UK. Xausa I. and Nachbar R. are employees of Wolfram Research Inc. under contract to Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
References
- Heininger, U.; Seward, J.F. Varicella. Lancet 2006, 368, 1365–1376. [Google Scholar] [CrossRef]
- Bonanni, P.; Breuer, J.; Gershon, A.; Gershon, M.; Hryniewicz, W.; Papaevangelou, V.; Rentier, B.; Rümke, H.; Sadzot-Delvaux, C.; Senterre, J.; et al. Varicella vaccination in Europe–taking the practical approach. BMC Med. 2009, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Bairwa, M.; Chawla, S.; Prinja, S.; Rajput, M. Should the chickenpox vaccine be included in the national immunization schedule in India? Hum. Vaccines 2011, 7, 874–877. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bardach, A.; Cafferata, M.L.; Klein, K.; Cormick, G.; Gibbons, L.; Ruvinsky, S. Incidence and use of resources for chickenpox and herpes zoster in Latin America and the Caribbean—A systematic review and meta-analysis. Pediatric Infect. Dis. J. 2012, 31, 1263–1268. [Google Scholar] [CrossRef]
- Sadzot-Delvaux, C.; Rentier, B.; Wutzler, P.; Asano, Y.; Suga, S.; Yoshikawa, T.; Plotkin, S.A. Varicella vaccination in Japan, South Korea, and Europe. J. Infect. Dis. 2008, 197 (Suppl. 2), S185–S190. [Google Scholar] [CrossRef]
- Wutzler, P.; Bonanni, P.; Burgess, M.; Gershon, A.; Sáfadi, M.A.; Casabona, G. Varicella vaccination-the global experience. Expert Rev. Vaccines 2017, 16, 833–843. [Google Scholar] [CrossRef]
- Povey, M.; Henry, O.; Bergsaker, M.A.R.; Chlibek, R.; Esposito, S.; Flodmark, C.E.; Gothefors, L.; Man, S.; Silfverdal, S.A.; Štefkovičová, M.; et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine or one dose of monovalent varicella vaccine: 10-year follow-up of a phase 3 multicentre, observer-blind, randomised, controlled trial. Lancet Infect. Dis. 2019, 19, 287–297. [Google Scholar] [CrossRef]
- Kuter, B.; Matthews, H.; Shinefield, H.; Black, S.; Dennehy, P.; Watson, B.; Reisinger, K.; Kim, L.L.; Lupinacci, L.; Hartzel, J.; et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatric Infect. Dis. J. 2004, 23, 132–137. [Google Scholar] [CrossRef]
- Baxter, R.; Ray, P.; Tran, T.N.; Black, S.; Shinefield, H.R.; Coplan, P.M.; Lewis, E.; Fireman, B.; Saddier, P. Long-term effectiveness of varicella vaccine: A 14-year, prospective cohort study. Pediatrics 2013, 131, e1389–e1396. [Google Scholar] [CrossRef]
- Varela, F.H.; Pinto, L.A.; Scotta, M.C. Global impact of varicella vaccination programs. Hum. Vaccines Immunother. 2019, 15, 645–657. [Google Scholar] [CrossRef]
- CDC. Chickenpox. Available online: https://www.cdc.gov/chickenpox/hcp/index.html (accessed on 29 September 2021).
- Kwong, J.C.; Tanuseputro, P.; Zagorski, B.; Moineddin, R.; Chan, K.J. Impact of varicella vaccination on health care outcomes in Ontario, Canada: Effect of a publicly funded program? Vaccine 2008, 26, 6006–6012. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2014, 89, 265–287. [Google Scholar]
- Green Book Chapter 34. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1056198/Green_Book_Chapter_34_v3_0.pdf (accessed on 28 July 2022).
- Walker, J.; Andrews, N.; Mathur, R.; Smeeth, L.; Thomas, S. Trends in the burden of varicella in UK general practice. Epidemiol. Infect. 2017, 145, 2678–2682. [Google Scholar] [CrossRef] [PubMed]
- Brisson, M.; Edmunds, W. Varicella vaccination in England and Wales: Cost-utility analysis. Arch. Dis. Child. 2003, 88, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Vaccination and Immunization: Statement on Varicella and Herpes Zoster Vaccines. Available online: https://www.nitag-resource.org/sites/default/files/e361266bc0e478ac95c2bcf4bc698b40186f2206_2.pdf (accessed on 28 July 2022).
- Van Hoek, A.J.; Melegaro, A.; Gay, N.; Bilcke, J.; Edmunds, W.J. The cost-effectiveness of varicella and combined varicella and herpes zoster vaccination programmes in the United Kingdom. Vaccine 2012, 30, 1225–1234. [Google Scholar] [CrossRef]
- Forbes, H.; Douglas, I.; Finn, A.; Breuer, J.; Bhaskaran, K.; Smeeth, L.; Packer, S.; Langan, S.M.; Mansfield, K.E.; Marlow, R.; et al. Risk of herpes zoster after exposure to varicella to explore the exogenous boosting hypothesis: Self controlled case series study using UK electronic healthcare data. BMJ 2020, 368, I6987. [Google Scholar] [CrossRef]
- Wolfson, L.J.; Daniels, V.J.; Altland, A.; Black, W.; Huang, W.; Ou, W. The impact of varicella vaccination on the incidence of varicella and herpes zoster in the United States: Updated evidence from observational databases, 1991–2016. Clin. Infect. Dis. 2020, 70, 995–1002. [Google Scholar] [CrossRef]
- Harpaz, R.; van Hoek, A.J. Point–Counterpoint: The Hope-Simpson Hypothesis and Its Implications Regarding an Effect of Routine Varicella Vaccination on Herpes Zoster Incidence. J. Infect. Dis. 2018, 218 (Suppl. 2), S57–S62. [Google Scholar] [CrossRef]
- Wolfson, L.J.; Daniels, V.J.; Pillsbury, M.; Kurugöl, Z.; Yardimci, C.; Kyle, J.; Dinleyici, E.C. Cost-effectiveness analysis of universal varicella vaccination in Turkey using a dynamic transmission model. PLoS ONE 2019, 14, e0220921. [Google Scholar] [CrossRef]
- Schuette, M.C.; Hethcote, H.W. Modeling the effects of varicella vaccination programs on the incidence of chickenpox and shingles. Bull. Math. Biol. 1999, 61, 1031–1064. [Google Scholar] [CrossRef]
- Brisson, M.; Edmunds, W.; Gay, N. Varicella vaccination: Impact of vaccine efficacy on the epidemiology of VZV. J. Med. Virol. 2003, 70, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Bollaerts, K.; Riera-Montes, M.; Heininger, U.; Hens, N.; Souverain, A.; Verstraeten, T.; Hartwig, S. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: Deriving incidence from seroprevalence data. Epidemiol. Infect. 2017, 145, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; De Ory, F.; Carton, M.; Cohen, D.; Van Damme, P.; Davidkin, I.; Rota, M.C.; de Melker, H.; Mossong, J.; Slacikova, M.; et al. The comparative sero-epidemiology of varicella zoster virus in 11 countries in the European region. Vaccine 2007, 25, 7866–7872. [Google Scholar] [CrossRef] [PubMed]
- Office for National Statistics (ons.gov.uk). Births by Parents’ Characteristics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthsbyparentscharacteristics (accessed on 28 July 2022).
- National Population Projections—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/nationalpopulationprojections/2018based#toc (accessed on 28 July 2022).
- Office for National Statistics (ons.gov.uk). Causes of Death over 100 Years. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/causesofdeathover100years/2017-09-18 (accessed on 28 July 2022).
- Office for National Statistics (ons.gov.uk). National Population Projections, Migration Assumptions: 2018-Based. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/methodologies/nationalpopulationprojectionsmigrationassumptions2018based (accessed on 28 July 2022).
- Prem, K.; Cook, A.R.; Jit, M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput. Biol. 2017, 13, e1005697. [Google Scholar] [CrossRef]
- Gershon, A.A.; Raker, R.; Steinberg, S.; Topf-Olstein, B.; Drusin, L.M. Antibody to varicella-zoster virus in parturient women and their offspring during the first year of life. Pediatrics 1976, 58, 692–696. [Google Scholar] [CrossRef]
- Gordon, J.; Meader, F. The Period of Infectivity and Serum Prevention of Chicken-pox. J. Am. Med. Assoc. 1929, 93, 2013–2015. [Google Scholar]
- Heymann, D. Control of Communicable Diseases Manual: An Official Report of the American Public Health Association; American Public Health Association: Washington, DC, USA, 2015. [Google Scholar]
- Izurieta, H.S.; Strebel, P.M.; Blake, P.A. Postlicensure effectiveness of varicella vaccine during an outbreak in a child care center. JAMA 1997, 278, 1495–1499. [Google Scholar] [CrossRef]
- Seward, J.F.; Zhang, J.X.; Maupin, T.J.; Mascola, L.; Jumaan, A.O. Contagiousness of varicella in vaccinated cases: A household contact study. JAMA 2004, 292, 704–708. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Anderson, R.M.; Garnett, G.P. Mass vaccination to control chickenpox: The influence of zoster. Proc. Natl. Acad. Sci. USA 1996, 93, 7231–7235. [Google Scholar] [CrossRef]
- Poletti, P.; Melegaro, A.; Ajelli, M.; del Fava, E.; Guzzetta, G.; Faustini, L.; Tomba, G.S.; Lopalco, P.; Rizzo, C.; Merler, S.; et al. Perspectives on the impact of varicella immunization on herpes zoster. A model-based evaluation from three European countries. PLoS ONE 2013, 8, e60732. [Google Scholar] [CrossRef]
- Trollor, J. Herpes zoster in general practice. Aust. Fam. Physician 1987, 16, 1137–1140. [Google Scholar]
- Wallinga, J.; Teunis, P.; Kretzschmar, M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am. J. Epidemiol. 2006, 164, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.J.; Melegaro, A.; Zagheni, E.; Edmunds, W.J.; Gay, N. Modelling the impact of a combined varicella and zoster vaccination programme on the epidemiology of varicella zoster virus in England. Vaccine 2011, 29, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Ouwens, M.J.; Littlewood, K.J.; Sauboin, C.; Téhard, B.; Denis, F.; Boëlle, P.-Y.; Alain, S. The impact of 2-dose routine measles, mumps, rubella, and varicella vaccination in France on the epidemiology of varicella and zoster using a dynamic model with an empirical contact matrix. Clin. Ther. 2015, 37, 816–829.e10. [Google Scholar] [CrossRef]
- Pillsbury, M.; Carias, C.; Samant, S.; Pawaskar, M. PIN86 Modeling Performance Parametrization of Varicella Vaccines. Value Health 2020, 23, S559. [Google Scholar] [CrossRef]
- Joint Committee on Vaccination and Immunisation. Minute of the Meeting Held on 22 June 2021. Available online: https://app.box.com/s/iddfb4ppwkmtjusir2tc/file/849032554320 (accessed on 29 April 2022).
- NHS Digital. Childhood Vaccination Coverage Statistics 2019–2020: Data Tables. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/nhs-immunisation-statistics/england---2019-20 (accessed on 28 July 2022).
- Public Health England. Td/IPV School-Based Programme to 31 August 2019: Vaccine Coverage Data Tables. Available online: https://www.gov.uk/government/publications/school-leaver-booster-tdipv-vaccine-coverage-estimates (accessed on 28 July 2022).
- Lauer Fischer. Available online: https://www.lauer-fischer.de/LF/Seiten/Verwaltung/Kundencenter/1.aspx (accessed on 15 October 2021).
- Botplus. Available online: https://botplusweb.portalfarma.com/botplus.aspx (accessed on 28 July 2022).
- Federal Office of Public Health FOPH. Available online: http://www.xn--spezialittenliste-yqb.ch/ShowPreparations.aspx (accessed on 28 July 2022).
- Varicella Vaccine Zoster. Available online: https://bnfc.nice.org.uk/medicinal-forms/varicella-zoster-vaccine.html (accessed on 15 October 2021).
- Akpo, E.I.H.; Cristeau, O.; Hunjan, M.; Casabona, G. Epidemiological Impact and Cost-Effectiveness of Varicella Vaccination Strategies in the United Kingdom. Clin. Infect. Dis. 2021, 73, e3617–e3626. [Google Scholar] [CrossRef]
- Code of Practice: Joint Committee on Vaccination and Immunisation. 2013. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/224864/JCVI_Code_of_Practice_revision_2013_-_final.pdf (accessed on 1 October 2018).
- Department of Health and Social Care. Cost-effectiveness Methodology for Immunisation Programmes and Procurement (CEMIPP). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/807856/CEMIPP_Consultation_Response_1.pdf (accessed on 19 September 2021).
- National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. Available online: https://www.nice.org.uk/process/pmg9/chapter/the-reference-case#discounting (accessed on 30 September 2021).
- Brisson, M.; Melkonyan, G.; Drolet, M.; De Serres, G.; Thibeault, R.; De Wals, P. Modeling the impact of one-and two-dose varicella vaccination on the epidemiology of varicella and zoster. Vaccine 2010, 28, 3385–3397. [Google Scholar] [CrossRef]
- Public Health England. Research and Analysis: Herpes Zoster (Shingles) Immunisation Programme 2013 to 2014: Evaluation Report. Available online: https://www.gov.uk/government/publications/herpes-zoster-shingles-immunisation-programme-2013-to-2014-evaluation-report (accessed on 19 May 2022).
- Attema, A.E.; Brouwer, W.B.F.; Claxton, K. Discounting in Economic Evaluations. Pharm. Econ. 2008, 36, 745–758. [Google Scholar] [CrossRef]
- Forbes, H.J.; Thomas, S.L.; Smeeth, L.; Clayton, T.; Farmer, R.; Bhaskaran, K.; Langan, S.M. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain 2016, 157, 30. [Google Scholar] [CrossRef]
- Brisson, M.; Edmunds, W.; Gay, N.; Law, B.; De Serres, G. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiol. Infect. 2000, 125, 651–669. [Google Scholar] [CrossRef]
- Littlewood, K.J.; Ouwens, M.J.; Sauboin, C.; Tehard, B.; Alain, S.; Denis, F. Cost-effectiveness of routine varicella vaccination using the measles, mumps, rubella and varicella vaccine in France: An economic analysis based on a dynamic transmission model for varicella and herpes zoster. Clin. Ther. 2015, 37, 830–841.e7. [Google Scholar] [CrossRef] [PubMed]
- Melegaro, A.; Marziano, V.; Del Fava, E.; Poletti, P.; Tirani, M.; Rizzo, C.; Merler, S. The impact of demographic changes, exogenous boosting and new vaccination policies on varicella and herpes zoster in Italy: A modelling and cost-effectiveness study. BMC Med. 2018, 16, 117. [Google Scholar]
- Dooling, K.L.; Guo, A.; Patel, M.; Lee, G.M.; Moore, K.; Belongia, E.A.; Harpaz, R. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. Morb. Mortal. Wkly. Rep. 2018, 67, 103. [Google Scholar] [CrossRef] [PubMed]
- Azzari, C.; Baldo, V.; Giuffrida, S.; Gani, R.; O’Brien, E.; Alimenti, C.; Daniels, V.J.; Wolfson, L.J. The cost-effectiveness of universal varicella vaccination in Italy: A model-based assessment of vaccination strategies. Clin. Econ. Outcomes Res. CEOR 2020, 12, 273. [Google Scholar]
- Graham, J.; Wolfson, L.J.; Kyle, J.; Bolde-Villarreal, C.P.; Guarneros-DeRegil, D.B.; Monsanto, H.; Pillsbury, M.; Talbird, S.; Daniels, V.J. Budget impact analysis of multiple varicella vaccination strategies: A Mexico perspective. Hum. Vaccines Immunother. 2020, 16, 886–894. [Google Scholar] [CrossRef]
- Pawaskar, M.; Burgess, C.; Pillsbury, M.; Wisløff, T.; Flem, E. Clinical and economic impact of universal varicella vaccination in Norway: A modeling study. PLoS ONE 2021, 16, e0254080. [Google Scholar]
- Heininger, U.; Pillsbury, M.; Samant, S.; Lienert, F.; Guggisberg, P.; Gani, R.; O’Brien, E.; Pawaskar, M. Health Impact and Cost-effectiveness Assessment for the Introduction of Universal Varicella Vaccination in Switzerland. Pediatric Infect. Dis. J. 2021, 40, e217–e221. [Google Scholar] [CrossRef]
- Talbird, S.E.; La, E.M.; Mauskopf, J.; Altland, A.; Daniels, V.; Wolfson, L.J. Understanding the role of exogenous boosting in modeling varicella vaccination. Expert Rev. Vaccines 2018, 17, 1021–1035. [Google Scholar] [CrossRef]
- Brisson, M.; Edmunds, W. Epidemiology of varicella-zoster virus in England and Wales. J. Med. Virol. 2003, 70, S9–S14. [Google Scholar] [CrossRef]
- Hethcote, H.W. The mathematics of infectious diseases. Siam Rev. 2000, 42, 599–653. [Google Scholar]
- Iannelli, M.; Milner, F. The basic approach to age-structured population dynamics. Mod. Meth. Numer. 2017, 10, 978–994. [Google Scholar]
- Pelovska, G.; Iannelli, M. Numerical methods for the Lotka–McKendrick’s equation. J. Comput. Appl. Math. 2006, 197, 534–557. [Google Scholar]
- Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland–Office for National Statistics (ons.gov.uk) [Internet]. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed on 28 July 2022).
- Gauthier, A.; Breuer, J.; Carrington, D.; Martin, M.; Rémy, V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol. Infect. 2009, 137, 38–47. [Google Scholar] [PubMed]
- Fleming, D.; Cross, K.; Cobb, W.; Chapman, R. Gender difference in the incidence of shingles. Epidemiol. Infect. 2004, 132, 1–5. [Google Scholar]
- Edmunds, W.; Brisson, M.; Rose, J. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine. 2001, 19, 3076–3090. [Google Scholar]
- Kudesia, G.; Partridge, S.; Farrington, C.; Soltanpoor, N. Changes in age related seroprevalence of antibody to varicella zoster virus: Impact on vaccine strategy. J. Clin. Pathol. 2002, 55, 154–155. [Google Scholar] [CrossRef]
- Yawn, B.P.; Yawn, R.A.; Lydick, E. Community impact of childhood varicella infections. J. Pediatr. 1997, 130, 759–765. [Google Scholar]
- Pre-Primary School Enrollment (Kindergarten, Nursery) for Children Aged 3-5 Years from 1970 to 2016 [Internet]. StatInvestor. Available online: https://statinvestor.com/data/32099/pre-primary-school-enrollment/ (accessed on 28 July 2022).
- Hobbelen, P.H.; Stowe, J.; Amirthalingam, G.; Miller, L.; van Hoek, A.-J. The burden of hospitalisation for varicella and herpes zoster in England from 2004 to 2013. J. Infect. 2016, 73, 241–253. [Google Scholar]
- Szende, A.; Janssen, B.; Cabases, J. Self-Reported Population Health: An International Perspective based on EQ-5D; Dordrecht (NL); Springer: Berlin, Germany, 2014. [Google Scholar]
- Pellissier, J.M.; Brisson, M.; Levin, M.J. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine. 2007, 25, 8326–8337. [Google Scholar]
- Oster, G.; Harding, G.; Dukes, E.; Edelsberg, J.; Cleary, P.D. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: Results from a population-based survey. J. Pain. 2005, 6, 356–363. [Google Scholar]
- Office for National Statistics. Available online: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/averageweeklyearningsearn01 (accessed on 28 July 2022).
- Public Health England. Shingles Vaccine Coverage Report (Adults Eligible from April to December 2021 and Vaccinated to the End of March 2022): England. Available online: https://www.gov.uk/government/publications/herpes-zoster-shingles-immunisation-programme-2021-to-2022-evaluation-reports/shingles-vaccine-coverage-report-adults-eligible-from-april-to-december-2021-and-vaccinated-to-the-end-of-march-2022-england (accessed on 28 July 2022).
- Tseng, H.F.; Harpaz, R.; Luo, Y.; Hales, C.M.; Sy, L.S.; Tartof, S.Y.; Bialek, S.; Hechter, R.C.; Jacobsen, S.J. Declining effectiveness of herpes zoster vaccine in adults aged ≥ 60 years. J. Infect. Dis. 2016, 213, 1872–1875. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).