Antibodies against SARS-CoV-2 Alpha, Beta, and Gamma Variants in Pregnant Women and Their Neonates under Antenatal Vaccination with Moderna (mRNA-1273) Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Collection of Samples and Variables
2.4. SRBD IgG Binding Ratio Test of SARS-CoV-2 Alpha, Beta, and Gamma Variants
2.5. Statistics
3. Results
3.1. Participant Characteristics
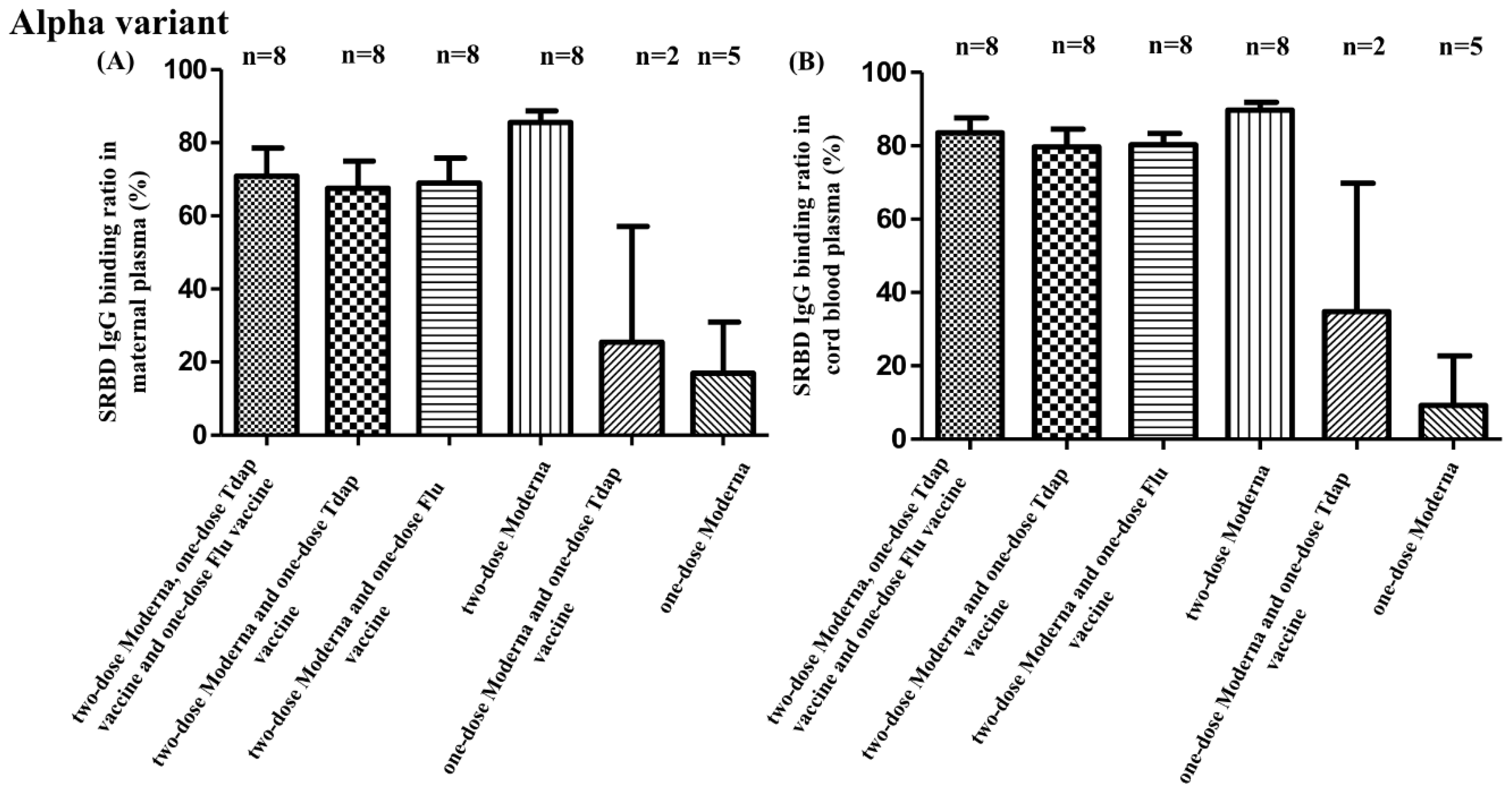
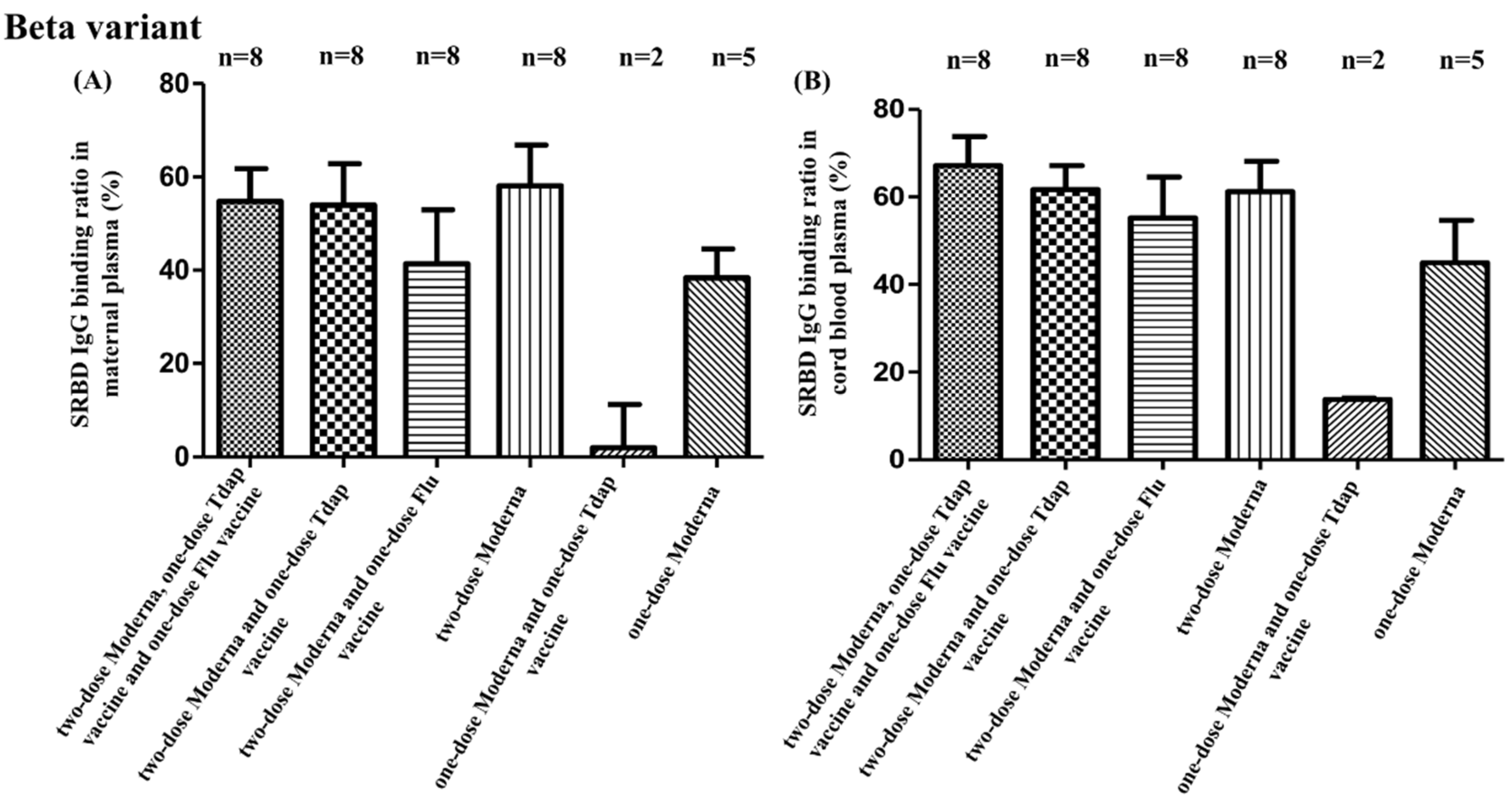
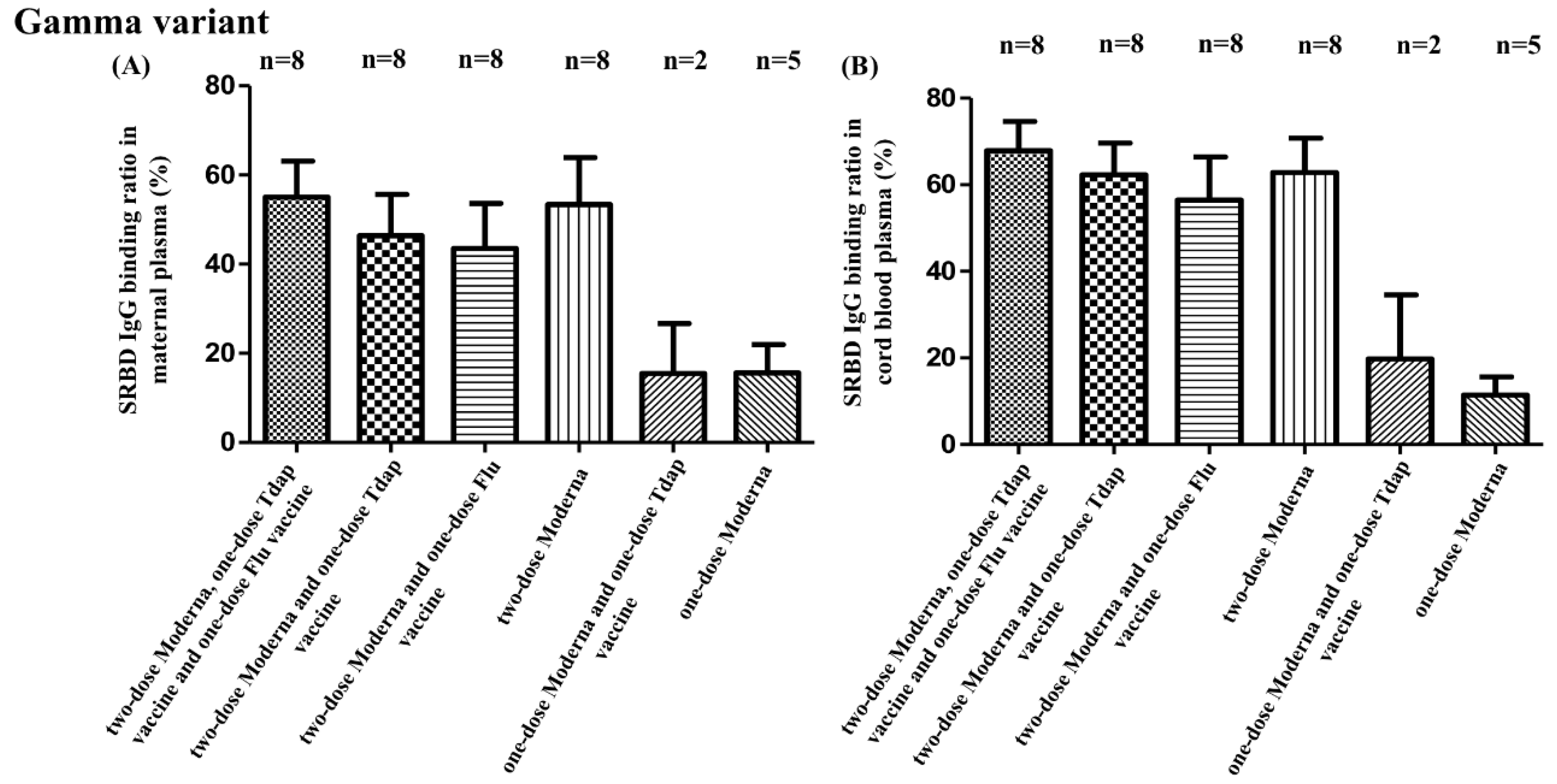
3.2. SRBD IgG Binding Ratio for SARS-CoV-2 Alpha, Beta, and Gamma Variants
3.3. SRBD IgG Binding Ratio and Other Non-COVID Vaccine Combinations
3.4. SRBD IgG Binding Ratio in Different Intervals between Two Doses of COVID-19 Vaccines
3.5. SRBD IgG Binding Ratio for Different Intervals between Second COVID-19 Vaccine and Childbirth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 8 April 2022).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Perrin, S.; Hamonic, S.; Bourget, B.; Roué, C.; Brassard, O.; Tadié, E.; Gicquel, V.; Bénézit, F.; Thibault, V.; et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: An observational study using surveillance data. Clin. Microbiol. Infect. 2021, 27, 1699.e1695–1699.e1698. [Google Scholar] [CrossRef] [PubMed]
- Pettirosso, E.; Giles, M.; Cole, S.; Rees, M. COVID-19 and pregnancy: A review of clinical characteristics, obstetric outcomes and vertical transmission. Aust. New Zealand J. Obstet. Gynaecol. 2020, 60, 640–659. [Google Scholar] [CrossRef]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ 2021, 193, E540–E548. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. COVID-19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatric Infect. Dis. J. 2020, 39, 469–477. [Google Scholar] [CrossRef]
- Gurol-Urganci, I.; Jardine, J.E.; Carroll, F.; Draycott, T.; Dunn, G.; Fremeaux, A.; Harris, T.; Hawdon, J.; Morris, E.; Muller, P.; et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: National cohort study. Am. J. Obs. Gynecol. 2021, 225, 522.e1–522.e11. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women with and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatrics 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Shen, C.J.; Fu, Y.C.; Lin, Y.P.; Shen, C.F.; Sun, D.J.; Chen, H.Y.; Cheng, C.M. Evaluation of Transplacental Antibody Transfer in SARS-CoV-2-Immunized Pregnant Women. Vaccines 2022, 10, 101. [Google Scholar] [CrossRef]
- Keller-Stanislawski, B.; Englund, J.A.; Kang, G.; Mangtani, P.; Neuzil, K.; Nohynek, H.; Pless, R.; Lambach, P.; Zuber, P. Safety of immunization during pregnancy: A review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 2014, 32, 7057–7064. [Google Scholar] [CrossRef]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Cameron, M.A.; Pannaraj, P.S.; Bline, K.E.; et al. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19-Associated Hospitalization in Infants Aged <6 Months—17 States, July 2021–January 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 264–270. [Google Scholar] [CrossRef]
- Carlsen, E.; Magnus, M.C.; Oakley, L.; Fell, D.B.; Greve-Isdahl, M.; Kinge, J.M.; Håberg, S.E. Association of COVID-19 Vaccination During Pregnancy with Incidence of SARS-CoV-2 Infection in Infants. JAMA Intern. Med. 2022, 182, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Murphy, E.A.; Sukhu, A.C.; Yee, J.; Singh, S.; Eng, D.; Zhao, Z.; Riley, L.E.; Yang, Y.J. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage into Cord Blood. Obs. Gynecol. 2021, 138, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Mithal, L.B.; Otero, S.; Shanes, E.D.; Goldstein, J.A.; Miller, E.S. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am. J. Obs. Gynecol. 2021, 225, 192–194. [Google Scholar] [CrossRef]
- Beharier, O.; Plitman Mayo, R.; Raz, T.; Nahum Sacks, K.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e150319. [Google Scholar] [CrossRef]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Mir, A.; Shinde, M.; Bender, O.; Alshammari, F.; Ansari, M.; Anwar, S. The SARS-CoV-2 mutations versus vaccine effectiveness: New opportunities to new challenges. J. Infect. Public Health 2022, 15, 228–240. [Google Scholar] [CrossRef]
- Fraley, E.; LeMaster, C.; Geanes, E.; Banerjee, D.; Khanal, S.; Grundberg, E.; Selvarangan, R.; Bradley, T. Humoral immune responses during SARS-CoV-2 mRNA vaccine administration in seropositive and seronegative individuals. BMC Med. 2021, 19, 169. [Google Scholar] [CrossRef]
- Krüttgen, A.; Lauen, M.; Klingel, H.; Imöhl, M.; Kleines, M. Two novel SARS-CoV-2 surrogate virus neutralization assays are suitable for assessing successful immunization with mRNA-1273. J. Virol. Methods 2022, 299, 114297. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kim, Y.J.; Kim, S.H.; Yoo, J.; Lee, J.; Lee, S.; Il Kim, S. Adverse reactions and production of neutralizing anti-SARS-CoV-2 antibodies after ChAdOx1 COVID-19 vaccination: A cross-sectional study in a single center. J. Infect. Public Health 2022, 15, 360–364. [Google Scholar] [CrossRef]
- Mahmoud, S.A.; Ganesan, S.; Naik, S.; Bissar, S.; Zamel, I.A.; Warren, K.N.; Zaher, W.A.; Khan, G. Serological Assays for Assessing Postvaccination SARS-CoV-2 Antibody Response. Microbiol. Spectr. 2021, 9, e0073321. [Google Scholar] [CrossRef] [PubMed]
- Kanji, J.N.; Bailey, A.; Fenton, J.; Ling, S.H.; Rivera, R.; Plitt, S.; Sligl, W.I.; Taylor, S.; Turnbull, L.; Tipples, G.; et al. Detection of SARS-CoV-2 antibodies formed in response to the BNT162b2 and mRNA-1237 mRNA vaccine by commercial antibody tests. Vaccine 2021, 39, 5563–5570. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.F.; Yen, C.L.; Fu, Y.C.; Cheng, C.M.; Shen, T.C.; Chang, P.D.; Cheng, K.H.; Liu, C.C.; Chang, Y.T.; Chen, P.L.; et al. Innate Immune Responses of Vaccinees Determine Early Neutralizing Antibody Production After ChAdOx1nCoV-19 Vaccination. Front. Immunol. 2022, 13, 807454. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obs. Gynecol 2021, 225, 303.e301–303.e317. [Google Scholar] [CrossRef] [PubMed]
- Rottenstreich, A.; Zarbiv, G.; Oiknine-Djian, E.; Vorontsov, O.; Zigron, R.; Kleinstern, G.; Wolf, D.G.; Porat, S. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 419–425. [Google Scholar] [CrossRef]
- Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 2022, 22, 277–282. [Google Scholar] [CrossRef]
- Pratama, N.R.; Wafa, I.A.; Budi, D.S.; Putra, M.; Wardhana, M.P.; Wungu, C.D.K. mRNA Covid-19 vaccines in pregnancy: A systematic review. PLoS ONE 2022, 17, e0261350. [Google Scholar] [CrossRef]
- Skoff, T.H.; Blain, A.E.; Watt, J.; Scherzinger, K.; McMahon, M.; Zansky, S.M.; Kudish, K.; Cieslak, P.R.; Lewis, M.; Shang, N.; et al. Impact of the US Maternal Tetanus, Diphtheria, and Acellular Pertussis Vaccination Program on Preventing Pertussis in Infants <2 Months of Age: A Case-Control Evaluation. Clin. Infect. Dis. 2017, 65, 1977–1983. [Google Scholar] [CrossRef]
- Wood, E.J. Molecular cloning. A laboratory manual by T Maniatis, E F Fritsch and J Sambrook. pp 545. Cold Spring Harbor Laboratory, New York. 1982. $48 ISBN 0-87969-136-0. Biochem. Educ. 1983, 11, 82. [Google Scholar] [CrossRef]
- Albrecht, M.; Arck, P.C. Vertically Transferred Immunity in Neonates: Mothers, Mechanisms and Mediators. Front. Immunol. 2020, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Committee on Obstetric Practice. ACOG Committee Opinion No. 566: Update on immunization and pregnancy: Tetanus, diphtheria, and pertussis vaccination. Obs. Gynecol. 2013, 121, 1411–1414. [Google Scholar] [CrossRef]
- Munoz, F.M. Current Challenges and Achievements in Maternal Immunization Research. Front. Immunol. 2018, 9, 436. [Google Scholar] [CrossRef]
- Eldanasory, O.A.; Rabaan, A.A.; Al-Tawfiq, J.A. Can influenza vaccine modify COVID-19 clinical course? Travel Med. Infect. Dis. 2020, 37, 101872. [Google Scholar] [CrossRef] [PubMed]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.J.; et al. Induction of trained immunity by influenza vaccination—Impact on COVID-19. PLoS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef]
- Reche, P.A. Potential Cross-Reactive Immunity to SARS-CoV-2 From Common Human Pathogens and Vaccines. Front. Immunol. 2020, 11, 586984. [Google Scholar] [CrossRef] [PubMed]
- Alkholy, U.M.; Salama, M.E.; Mahmoud, H.; Taher, A.; Elsayes, K.M. Could Bordetella pertussis vaccine protect against coronavirus COVID-19? J. Glob. Antimicrob. Resist. 2020, 22, 803–805. [Google Scholar] [CrossRef]
- Ismail, M.B.; Omari, S.A.; Rafei, R.; Dabboussi, F.; Hamze, M. COVID-19 in children: Could pertussis vaccine play the protective role? Med. Hypotheses 2020, 145, 110305. [Google Scholar] [CrossRef]
- Chilimuri, S.; Mantri, N.; Shrestha, E.; Sun, H.; Gongati, S.; Zahid, M.; Kelly, P. BNT162b2 mRNA Vaccine Interference with Co-Administration of Tdap Vaccine. Am. J. Case Rep. 2021, 22, e933003. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Vaccine Administration. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html (accessed on 8 April 2022).
- Pobre, K.; Tashani, M.; Ridda, I.; Rashid, H.; Wong, M.; Booy, R. Carrier priming or suppression: Understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines. Vaccine 2014, 32, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Tashani, M.; Jayasinghe, S.; Harboe, Z.B.; Rashid, H.; Booy, R. Potential carrier priming effect in Australian infants after 7-valent pneumococcal conjugate vaccine introduction. World J. Clin. Pediatrics 2016, 5, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Tontini, M.; Romano, M.R.; Proietti, D.; Balducci, E.; Micoli, F.; Balocchi, C.; Santini, L.; Masignani, V.; Berti, F.; Costantino, P. Preclinical studies on new proteins as carrier for glycoconjugate vaccines. Vaccine 2016, 34, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Tashani, M.; Heron, L.; Wong, M.; Rashid, H.; Booy, R. Tetanus-diphtheria-pertussis vaccine may suppress the immune response to subsequent immunization with pneumococcal CRM197-conjugate vaccine (coadministered with quadrivalent meningococcal TT-conjugate vaccine): A randomized, controlled trial. J. Travel Med. 2017, 24, tax006. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.; Baos, S.; Cappel-Porter, H.; Carson-Stevens, A.; Clout, M.; Culliford, L.; Emmett, S.R.; Garstang, J.; Gbadamoshi, L.; Hallis, B.; et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): A multicentre, randomised, controlled, phase 4 trial. Lancet 2021, 398, 2277–2287. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Gurtman, A.; Rubino, J.; Smith, W.; van Cleeff, M.; Jayawardene, D.; Giardina, P.C.; Emini, E.A.; Gruber, W.C.; Scott, D.A.; et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin. Vaccine Immunol. 2012, 19, 1296–1303. [Google Scholar] [CrossRef]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Nourbakhsh, S.; Sah, P.; Fitzpatrick, M.C.; Galvani, A.P. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLoS Biol. 2021, 19, e3001211. [Google Scholar] [CrossRef]
- Romero-Brufau, S.; Chopra, A.; Ryu, A.J.; Gel, E.; Raskar, R.; Kremers, W.; Anderson, K.S.; Subramanian, J.; Krishnamurthy, B.; Singh, A.; et al. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: Simulation agent based modeling study. BMJ 2021, 373, n1087. [Google Scholar] [CrossRef]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e5611. [Google Scholar] [CrossRef]
- Grunau, B.; Goldfarb, D.M.; Asamoah-Boaheng, M.; Golding, L.; Kirkham, T.L.; Demers, P.A.; Lavoie, P.M. Immunogenicity of Extended mRNA SARS-CoV-2 Vaccine Dosing Intervals. JAMA 2022, 327, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Ibarrondo, F.J.; Hofmann, C.; Fulcher, J.A.; Goodman-Meza, D.; Mu, W.; Hausner, M.A.; Ali, A.; Balamurugan, A.; Taus, E.; Elliott, J.; et al. Primary, Recall, and Decay Kinetics of SARS-CoV-2 Vaccine Antibody Responses. ACS Nano 2021, 15, 11180–11191. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e0034121. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health. Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Prahl, M.; Golan, Y.; Cassidy, A.G.; Matsui, Y.; Li, L.; Alvarenga, B.; Chen, H.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and early infancy. medRxiv 2021. [Google Scholar] [CrossRef]
- Kugelman, N.; Nahshon, C.; Shaked-Mishan, P.; Cohen, N.; Sher, M.L.; Gruber, M.; Marom, I.; Zolotarevsky, A.; Lavie, O.; Damti, A.; et al. Maternal and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Levels at Delivery After Receipt of the BNT162b2 Messenger RNA COVID-19 Vaccine During the Second Trimester of Pregnancy. JAMA Pediatrics 2022, 176, 290–295. [Google Scholar] [CrossRef]
| Median (IQR) | Total (N = 39) | Group A (N = 8) | Group B (N = 8) | Group C (N = 8) | Group D (N = 8) | Group E (N = 2) | Group F (N = 5) | p Value |
|---|---|---|---|---|---|---|---|---|
| Age (yrs) (IQR) | 32.1 (29.0–35.0) | 29.0 (28.25–31.0) | 32.4 (29.0–34.75) | 30.5 (26.75–33.75) | 35.0 (28.75–41.5) | 38.0 (35.0–NA) | 32.0 (26–36.5) | 0.133 |
| Parity (%) | 0.174 | |||||||
| Primiparity | 19 (48.71) | 5 (62.5) | 6 (75) | 2 (25) | 4 (50) | 1 (50) | 1 (20) | |
| Multiparity | 20 (51.29) | 3 (37.5) | 2 (25) | 6 (75) | 4 (50) | 1 (50) | 4 (80) | |
| Baby body weight (g) | 3038.8 (2782.5–3170.0) | 3032.9 (2985–3150) | 3057.5 (2810–3393.75) | 2936.9 (2723.75–3111.25) | 3153.8 (2700–3575) | 327.0 (3135–NA) | 2902.0 (2690–3082.5) | 0.664 |
| Maternal BMI | 26.8 (23.3–28.9) | 29.5 (25.2–34.6) | 26.1 (22.8–30.0) | 25.2 (23.1–27.6) | 26.3 (23.7–27.5) | 31.6 (28.0–NA) | 25.1 (22.5–27.8) | 0.125 |
| Baby gender (%) | 0.480 | |||||||
| Female | 21 (53.8) | 6 (75) | 5 (62.5) | 4 (50) | 2 (25) | 1 (50) | 3 (60) | |
| Male | 18 (46.2) | 2 (25) | 3 (37.5) | 4 (50) | 6 (75) | 1 (50) | 2 (40) | |
| Delivery GA (wks) | 38.5 (38.0–39.0) | 38.7 (38.0–40.0) | 39.0 (38.3–39.8) | 38.5 (37.3–39.0) | 38.3 (38.0 –39.8) | 38.0 (38.0–38.0) | 38.2 (36.5–39.5) | 0.756 |
| 1st COVID-19 vaccine GA (wks) | 24.9 (21.0–27.3) | 23.4 (22.0–25.0) | 23.5 (21.3–26.0) | 22.8 (21.0–24.8) | 25.0 (21.8 –27.8) | 31.5 (30.0–NA) | 30.4 (25.5–35.0) | 0.001 |
| 2nd COVID-19 vaccine GA (wks) | 28.9 (26.0–31.0) | 28.7 (26.0–31.0) | 28.3 (26.3–30.8) | 27.8 (25.3–29.8) | 31.3 (28.0 –33.0) | - | - | 0.182 |
| Interval between 2 doses of COVID-19 vaccines (wks) | 5.3 (4.0–6.0) | 5.3 (4.0–7.0) | 4.8 (4.0–5.0) | 5.0 (4.25–5.0) | 6.4 (5.0–8.0) | - | - | 0.108 |
| Interval between 2nd COVID-19 vaccine to baby delivery (wks) | 9.3 (6.5–12.0) | 10.0 (8.0–12.0) | 10.8 (8.25–13.0) | 10.8 (8.5–12.75) | 7.4 (5.0–10.0) | 6.5 (5.0–NA) | 7.2 (4.0–11.0) | 0.166 |
| Alpha Type | Beta Type | Gamma Type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | p Value | Arm 1 | Arm 2 | p Value | Arm 1 | Arm 2 | p Value | |||||
| Arm 1: ≤ 4 weeks Arm 2: > 4 weeks | N MP (%) CB (%) Ratio | 9 69.58 82.88 1.25 | 21 73.01 82.47 1.20 | 0.659 0.925 0.665 | 9 45.97 58.86 2.29 | 21 56.09 62.05 1.25 | 0.339 0.704 0.189 | 9 47.96 61.88 1.75 | 21 51.80 63.11 1.55 | 0.724 0.895 0.619 | |||
| Arm 1: ≤ 6 weeks Arm 2: > 6 weeks | N MP (%) CB (%) Ratio | 24 71.39 81.99 1.22 | 6 74.33 85.01 1.21 | 0.741 0.543 0.931 | 24 49.80 59.43 1.69 | 6 66.06 67.78 1.07 | 0.176 0.383 0.303 | 24 47.59 60.47 1.65 | 6 62.88 71.81 1.43 | 0.213 0.282 0.635 | |||
| Arm 1: ≤ 8 weeks Arm 2: > 8 weeks | N MP (%) CB (%) Ratio | 29 71.10 82.09 1.22 | 1 97.34 97.19 0.99 | 0.179 0.167 0.481 | 29 51.78 61.10 1.59 | 1 89.95 60.86 0.68 | 0.153 0.991 0.498 | 29 49.63 61.81 1.63 | 1 80.35 89.60 1.12 | 0.263 0.236 0.631 | |||
| Arm 1 | Arm 2 | Arm 3 | p Value | Arm 1 | Arm 2 | Arm 3 | p Value | Arm 1 | Arm 2 | Arm 3 | p Value | ||
| Arm 1: ≤ 4 weeks Arm 2: 4–6 weeks Arm 3: > 6 weeks | N MP (%) CB (%) Ratio | 9 69.58 82.88 1.25 | 15 72.48 81.46 1.19 | 6 74.33 85.01 1.21 | 0.892 0.795 0.911 | 9 45.97 58.86 2.29 | 15 52.10 59.76 1.33 | 6 66.06 67.78 1.07 | 0.348 0.685 0.122 | 9 47.96 61.88 1.75 | 15 47.37 59.63 1.59 | 6 62.88 71.81 1.43 | 0.467 0.552 0.839 |
| Two Doses Vaccine | Alpha Type | Beta Type | Gamma Type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Interval between Last Dose to Delivery | Arm 1 | Arm 2 | p Value | Arm 1 | Arm 2 | p Value | Arm 1 | Arm 2 | p Value | |
| Arm 1: ≤ 6 wks Arm 2: > 6 wks | N MP (%) CB (%) Ratio | 5 85.17 87.40 1.03 | 25 69.34 81.64 1.25 | 0.090 0.276 0.140 | 5 73.86 70.13 0.98 | 25 48.89 59.29 1.68 | 0.049 0.290 0.281 | 5 66.87 73.57 1.12 | 25 47.41 60.57 1.71 | 0.138 0.250 0.247 |
| Arm 1: ≤ 8 wks Arm 2: > 8 wks | N MP (%) CB (%) Ratio | 11 77.03 83.23 1.11 | 19 69.06 82.23 1.28 | 0.276 0.810 0.136 | 11 67.86 69.08 1.05 | 19 44.48 56.47 1.86 | 0.015 0.107 0.039 | 11 62.15 70.62 1.21 | 19 43.99 58.17 1.84 | 0.071 0.152 0.041 |
| Arm 1: ≤ 10 wks Arm 2: > 10 wks | N MP (%) CB (%) Ratio | 16 71.72 81.22 1.20 | 14 72.27 84.17 1.23 | 0.939 0.457 0.816 | 16 62.38 67.17 1.19 | 14 42.39 54.16 1.99 | 0.033 0.084 0.122 | 16 56.97 68.64 1.37 | 14 43.42 55.99 1.88 | 0.168 0.131 0.196 |
| Arm 1: ≤ 12 wks Arm 2: > 12 wks | N MP (%) CB (%) Ratio | 24 68.35 80.55 1.26 | 6 86.48 90.79 1.05 | 0.001 0.032 0.144 | 24 55.73 63.37 1.37 | 6 42.35 52.02 2.36 | 0.268 0.393 0.352 | 24 55.00 65.28 1.34 | 6 33.23 52.59 2.67 | 0.072 0.228 0.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-C.; Lin, Y.-P.; Cheng, C.-M.; Shen, C.-F.; Ching, A.; Chang, T.-C.; Shen, C.-J. Antibodies against SARS-CoV-2 Alpha, Beta, and Gamma Variants in Pregnant Women and Their Neonates under Antenatal Vaccination with Moderna (mRNA-1273) Vaccine. Vaccines 2022, 10, 1415. https://doi.org/10.3390/vaccines10091415
Chen W-C, Lin Y-P, Cheng C-M, Shen C-F, Ching A, Chang T-C, Shen C-J. Antibodies against SARS-CoV-2 Alpha, Beta, and Gamma Variants in Pregnant Women and Their Neonates under Antenatal Vaccination with Moderna (mRNA-1273) Vaccine. Vaccines. 2022; 10(9):1415. https://doi.org/10.3390/vaccines10091415
Chicago/Turabian StyleChen, Wei-Chun, Yen-Pin Lin, Chao-Min Cheng, Ching-Fen Shen, Alex Ching, Ting-Chang Chang, and Ching-Ju Shen. 2022. "Antibodies against SARS-CoV-2 Alpha, Beta, and Gamma Variants in Pregnant Women and Their Neonates under Antenatal Vaccination with Moderna (mRNA-1273) Vaccine" Vaccines 10, no. 9: 1415. https://doi.org/10.3390/vaccines10091415
APA StyleChen, W.-C., Lin, Y.-P., Cheng, C.-M., Shen, C.-F., Ching, A., Chang, T.-C., & Shen, C.-J. (2022). Antibodies against SARS-CoV-2 Alpha, Beta, and Gamma Variants in Pregnant Women and Their Neonates under Antenatal Vaccination with Moderna (mRNA-1273) Vaccine. Vaccines, 10(9), 1415. https://doi.org/10.3390/vaccines10091415








