Abstract
Shigella is the leading cause of global diarrheal deaths that currently lacks a licensed vaccine. Shigellosis drives antimicrobial resistance and leads to economic impact through linear growth faltering. Today, there is a robust pipeline of vaccines in clinical development which are broadly divided into parenteral glycoconjugate vaccines, consisting of O-antigen conjugated to carrier proteins, and oral live attenuated vaccines, which incorporate targeted genetic mutations seeking to optimize the balance between reactogenicity, immunogenicity and ultimately protection. Proof of efficacy has previously been shown with both approaches but for various reasons no vaccine has been licensed to date. In this report, we outline the requirements for a Shigella vaccine and describe the current pipeline in the context of the many candidates that have previously failed or been abandoned. The report refers to papers from individual vaccine developers in this special supplement of Vaccines which is focused on Shigella vaccines. Once readouts of safety and immunogenicity from current trials of lead candidate vaccines among the target population of young children in low- and middle-income countries are available, the likely time to licensure of a first Shigella vaccine will become clearer.
Keywords:
Shigella; shigellosis; flexneri; sonnei; vaccines; glycoconjugate; live attenuated; global health; diarrhea; dysentery 1. Introduction
Shigellosis is caused by an infection of the gastrointestinal tract with Gram-negative bacteria belonging to the genus Shigella. The pathogenesis of shigellosis involves the invasion of the colonic mucosa which results in inflammation. Clinical presentation is either with bloody diarrhea, otherwise known as dysentery, or watery diarrhea. Although dysentery is the classical presentation of shigellosis, watery diarrhea is the more common presentation. While dysentery is highly suggestive of shigellosis, presentation with watery diarrhea is clinically indistinguishable from the many other etiologies of watery diarrhea. Although human-restricted, shigellosis is highly transmissible by the fecal–oral route, contaminated food and water and fomites. It is estimated that 10 s to 100 s of bacteria are sufficient to cause disease [1]. Shigella is responsible for a large global burden of disease and is the leading bacterial cause of diarrheal deaths worldwide. Children under 5 years of age in low- and middle-income countries (LMICs) are most affected, particularly in the second year of life [2,3,4,5]. The Global Burden of Disease 2019 estimated 148,000 deaths from shigellosis, 93,000 of which were among children under five years of age [6]. Shigellosis is also a problem among travelers and military personnel deployed in endemic areas [7].
There are four species of Shigella, and three of these have multiple serotypes. This large number of Shigella serotypes presents a challenge for vaccine development, since immunologic protection is largely serotype-specific. Shigella flexneri is the most important species globally, particularly in low-income countries, and consists of 15 serotypes, the most common of which is S. flexneri 2a, followed by 3a and 6. Shigella sonnei has just one serotype and is the dominant species in countries that are undergoing industrial development. Shigella dysenteriae has 15 serotypes, the most significant being Shigella dysenteriae type 1, which was responsible for epidemic outbreaks with high-case fatality rates that seem to have disappeared in the 21st century. Shigella boydii, which has 19 serotypes, is responsible for a small minority of shigellosis cases and is mainly detected in South Asia [5].
In addition to the direct morbidity and mortality due to diarrhea, Shigella is a major cause of linear growth faltering among young children in LMICs [8,9]. Current WHO diarrhea treatment guidelines are for empirical antibiotics to only be given where presentation is with dysentery. Though dysentery has high specificity for shigellosis, the disease commonly presents with watery diarrhea, which cannot be clinically distinguished from other etiologies of watery diarrhea [10]. Otherwise, treatment is largely supportive with an oral rehydration solution (ORS). This means that the large majority children in LMICs with shigellosis do not receive antibiotics. Diagnosis requires stool culture which is unavailable in most LMIC healthcare settings and usually takes two to three days. There are no affordable point of care diagnostic tests for Shigella. Finally, even when initiated, the use of antibiotics for the treatment of Shigellas is becoming increasingly compromised by the rise in antimicrobial resistance among circulating Shigella isolates [11,12,13].
All of these factors make the development of a vaccine against Shigella a pressing global public health need. Several approaches have been adopted by vaccine developers for the prevention of shigellosis over the years. These broadly divide between oral whole cell approaches, consisting mostly of live attenuated but also inactivated vaccines, and parenteral subunit approaches, mostly glycoconjugates, which are designed to target the immune response to key Shigella antigens. There is evidence for efficacy with both of these vaccine approaches. In the 1960s, a live attenuated vaccine developed by David Mel of the Yugoslav Army was shown to be efficacious both among military recruits [14] and children aged 2–8 years [15] in former Yugoslavia. More recently, efficacy was demonstrated among Israeli military recruits of a S. sonnei O-antigen glycoconjugate vaccine developed by John Robbins and colleagues at the National Institutes for Health (NIH), USA [16]. In a subsequent study among Israeli children, protection was shown to extend down to three years of age [17].
Nevertheless, despite over a 100 years of Shigella vaccine development and a large numbers of clinical trials (Figure 1), there is no licensed vaccine against shigellosis. However, this situation is set to change in the coming decade with an improved understanding of the basis for protection against Shigella and several candidates set to advance to late-stage clinical trials. In this article, we will consider the requirements for a successful vaccine against Shigella and provide an overview of the candidates in the Shigella vaccines pipeline.
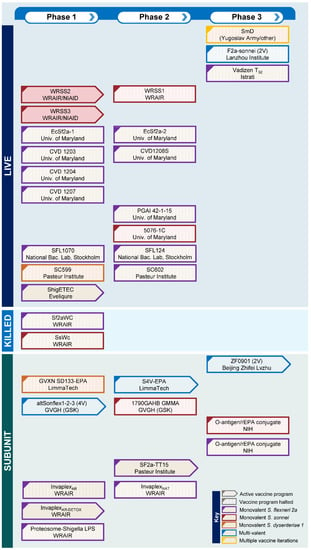
Figure 1.
Shigella vaccines pipeline indicating active and halted vaccine programs from the past 60 years by vaccine type and phase of clinical development.
2. Requirements for an Effective Shigella Vaccine, Target Antigens and Immunity to Shigella
Key attributes for Shigella vaccines have recently been published by the WHO in a Preferred Product Characteristics (PPC) document [18]. A Shigella vaccine must be safe, immunogenic and efficacious against moderate-to-severe diarrhea (MSD) due to Shigella infection among the key target population of infants and young children under 5 years in LMICs. Efficacy should be achieved through a primary immunization schedule of one to two doses delivered during the first 12 months of life with or without an additional booster dose. The PPC document stipulates 60% or more efficacy against Shigella MSD caused by vaccine serotypes and a minimum duration of protection between 24 months and 5 years. A vaccine should be safe and not immunologically interfere when coadministered with other recommended vaccines, and should be stable for two years at 2–8 °C. It should be cost-effective so that price is not a barrier to access in LMICs. The PPC document includes both oral and injectable (intramuscular, intradermal and subcutaneous) routes of administration.
Non-human primate studies [19], controlled human infection model (CHIM) studies [20] and epidemiological studies of natural infection with Shigella [21] all provide strong evidence that protection against shigellosis is largely serotype specific. These findings implicate O-antigen of lipopolysaccharide (LPS) as being the critical antigenic target for vaccine development. Shigellosis typically confers approximately 70% protection against subsequent homotypic infection but not heterotypic infection. This has implications in relation to the valency of vaccine needed for broad protection. The serotyping of Shigella isolates from the Global Enterics Multicenter Study (GEMS) [2] suggests that a vaccine consisting of Shigella flexneri 2a, 3a and 6, as well as Shigella sonnei O-antigens could provide direct coverage against 64% of global Shigella isolates [22]. Coverage could increase to 88% due to cross-protection against S. flexneri serotypes which has been observed in guinea pigs [23].
Nonetheless, there is also a body of evidence implicating surface protein antigens common to multiple serotypes in protection against shigellosis. Most prominent among these are the Ipa proteins which form the needle and extracellular complexes of the Shigella type 3 secretion system (T3SS), particular IpaB, IpaC and IpaD [24]. The T3SS is essential for the uptake of Shigella into epithelial cells. A potential advantage of whole-cell approaches to vaccine development is the inclusion of Ipa proteins along with other potentially protective protein antigens.
The relevant immunological mediators and mechanisms of protection are likely multifactorial. The most recognized is serum IgG to LPS O-antigen which forms the main immunological response to parenterally administered glycoconjugate Shigella vaccines. Both phase 3 studies with NIH S. sonnei glycoconjugate vaccines found a strong association between serum O-antigen IgG and protective efficacy [16,17]. Subsequent analyses by Cohen provide good support for this marker being a correlate of protection against shigellosis [25]. In the context of oral live attenuated Shigella vaccines and natural infection with wild-type Shigella, fecal O-antigen IgA has been closely implicated in protection. This difference is likely indicative of parenteral vaccines inducing systemic immunity, in contrast to natural infection and oral vaccines directly inducing mucosal immunity within the gastrointestinal tract.
Recent data from Shigella CHIM studies suggest that serum IgG to IpaB could also be a correlate of protection [26]. Efforts are underway to develop a standardized ELISA for antibodies to O-antigens and establish a first international standard serum for these assays [27]. As well as gauging vaccine responses in terms of absolute levels of antibodies, there have been moves to determine the functionality of these antibodies focusing on the development of a serum bactericidal assay that can be transferred between laboratories [28,29]. Several studies have focused on cellular markers of immunity leading to antibody secreting cells (ASCs), B memory cells (BM), and CD4+ and CD8+ T cells being proposed as having roles in protection against shigellosis [30,31,32].
Several animal models have been used to study shigellosis and assess the potential protective efficacy of candidate vaccines. These include the mouse pulmonary infection [33], guinea pig conjunctivitis (Sereny test) [34] and guinea pig rectocolitis models [35]. However, since Shigella is a human-restricted pathogen and even non-human primates require much higher bacterial inocula to induce shigellosis compared to humans, all of these models have limitations. For this reason, there has been a long-standing emphasis on assessing candidate Shigella vaccines in humans. Aligned with this, the establishment of the Shigella CHIM at three centers in the United States, has permitted the early assessment of vaccine efficacy in humans [36,37,38]. Consensus has been reached among these centers in relation to the overall conduct [39], clinical endpoints [40] and laboratory investigation priorities [41] for these studies.
3. Oral Whole Cell Vaccines
Oral whole cell vaccines can be divided between whole cell killed (Table 1) and live attenuated approaches (Table 2).
3.1. Whole Cell Killed Vaccines
The earliest vaccines, beginning with the first attempt at a Shigella vaccine by Kiyoshi Shiga, adopted the whole-cell killed approach [42]. Key to their development was ensuring that inactivation did not adversely affect the structure of relevant antigens. Ether, formalin and heat were all utilized as means of inactivation. In general, the early whole cell killed vaccines resulted in high levels of reactogenicity. They were tested in poorly designed studies that prevented any meaningful conclusion about efficacy [42].
Relatively recently, formalin-inactivated S. sonnei (SsWc) [43] and S. flexneri 2a (Sf2aWC) [44] monovalent whole cell vaccine candidates were developed by the Walter Reed Army Institute for Research (WRAIR) and tested in a phase 1 study in the US. The vaccines were well tolerated though immune responses were variable. While a trivalent version of the vaccine was subsequently developed [45], it has not been tested in a clinical trial. The whole cell killed approach has now largely been abandoned.

Table 1.
Shigella whole cell killed vaccines.
Table 1.
Shigella whole cell killed vaccines.
| Name | Description | Developer | Species/Serotype and Strain | Genetic Deletions | Status | Furthest Stage of Development | Reference |
|---|---|---|---|---|---|---|---|
| SsWc | S. sonnei monovalent formalin-inactivated | WRAIR | S. sonnei Moseley | None | Discontinued | Phase 1 | [43] |
| Sf2aWC | S. flexneri 2a monovalent formalin-inactivated | WRAIR | S. flexneri 2a 2457T | None | Discontinued | Phase 1 | [44] |
3.2. Live Attenuated Vaccines
3.2.1. Mel and Streptomycin-Dependent Vaccines
Studies by Mel and colleagues from the 1960s and 1970s [14,15] paved the way for the development of a large number of live attenuated vaccines over the following fifty years. The original Mel vaccines were developed by passaging Shigella strains using media containing the antibiotic streptomycin. This resulted in the strains becoming both streptomycin dependent (SmD) and attenuated, though the exact genetic mutations leading to attenuation were unknown. Although SmD Shigella vaccines were tested in subjects over multiple studies in Yugoslavia and the US, they had a number of issues. First, the primary immunization schedule consisted of four doses. Second, protection was relatively short-lived, lasting for a year. Third, some vaccine lots lost their streptomycin dependence. Finally, there were issues with manufacturing. These vaccines were never licensed and were eventually abandoned.
3.2.2. Vadizen and T32 Vaccines
Around the same time, another live attenuated vaccine, the Vadizen live vaccine, was developed in Romania by Istrati from the S. flexneri 2a T32 strain by multiple passages on nutrient agar [46]. During a period of five years, this was tested in over 36,000 subjects in 12 countries. Protection of 86.6% was reported against dysentery in children with protection lasting for 6 months [47]. Of note, the Lanzhou Institute of Biological Products developed the F2a-sonnei (FS) bivalent vaccine based on the S. flexneri 2a T32 strain containing an S. sonnei plasmid. This enabled the co-expression of O-antigens of S. flexneri 2a and S. sonnei. In field studies in China, FS was reported to give 61–65% protection against S. flexneri 2a and 57–72% S. sonnei shigellosis [48]. The current development status of this vaccine construct is unknown.
More recently, the advent of whole genome sequencing enabled the development of well-defined live attenuated Shigella vaccines with targeted genetic mutations. However, similar challenges are faced by all live attenuated vaccine candidates in balancing acceptable levels of reactogenicity with sufficient immunogenicity to confer protection. Below, we report on a selection of such vaccines that have been tested in clinical trials.
3.2.3. Center for Vaccine Development and guaBA-Deficient Vaccines
The Center for Vaccine Development at the University of Maryland has developed a number of live attenuated Shigella vaccines based on the well-characterized S. flexneri 2a 2457T strain. CVD 1203 contained mutations in the aroA and virG genes that encode proteins involved in amino acid biosynthesis and intra- and intercellular motility. Although immunogenic, the vaccine was excessively reactogenic, causing dysentery in 72% of recipients in a phase 1 study [49] and was discontinued. For CVD 1207, guaBA, virG, set and sen genes were deleted. guaBA is required for guanine biosynthesis, while set and sen encode the ShET1 and ShET2 Shigella enterotoxins, respectively. This new approach resulted in tolerability in a phase 1 study but limited immunogenicity [50].
Further iterations of CVD 1207 maintained the guaBA mutation but reverted to using wild-type virG, either with intact (CVD 1204) or deleted (CVD 1208) sen and set genes. The deletion of the Shigella enterotoxins was shown to be required since CVD1204 resulted in unacceptable reactogenicity [51]. CVD 1208S (CVD 1208 grown on animal-free media) advanced to a phase 2 study with the intent to challenge with wild-type 2457T. However, the trial was terminated following the recruitment of 20 subjects due to reactogenicity [52].
3.2.4. Pasteur Institute and virG-Deficient Vaccines
Another challenge facing development of live attenuated Shigella vaccines is the difference in reactogenicity and immunogenicity of the same vaccine among different populations. SC602 is a S. flexneri 2a candidate vaccine developed at the Pasteur Institute, Paris. It is based on the wild-type S. flexneri 2a 494 strain with deletions in virG and iuc which encodes the siderophore, aerobactin. At 104 cfu, SC602 was mildly reactogenic in US adults but immunogenic and protected against fever, dysentery and severe symptoms following challenge with S. flexneri 2a 2457T [53]. When tested in Bangladeshi adults and children, the vaccine was minimally reactogenic but induced only a limited immune response [54].
The poor immunogenicity of live attenuated vaccines has also been observed among LMIC populations with licensed vaccines against cholera, rotavirus and polio. Multiple reasons may account for this, a key one being environmental enteric dysfunction (EDD) which commonly occurs in children in LMICs [55]. EDD is an acquired enteropathy of the small intestine. It is thought to be caused by subclinical infections and is characterized by inflammation and blunting of the villi. EDD is associated with systemic inflammation and malnutrition. Reducing the time taken to test candidate vaccines in LMIC children is valuable for an early understanding of which candidate vaccines should progress.
The Pasteur Institute also developed SC599, a live attenuated Shigella vaccine candidate based on wild-type Shigella dysenteriae type 1 strain SC595 with deleted virG, ent and fep genes, the latter two genes encoding iron chelation proteins. Although well-tolerated in phase 1 trials, the vaccine was poorly immunogenic and has not progressed [56].
3.2.5. National Bacteriology Laboratory, Stockholm, and aroD-Deficient Vaccines
In the early 1990s, the National Bacteriology Laboratory in Stockholm developed live Shigella vaccine candidates attenuated through the deletion of aroD which made them auxotrophic for aromatic compounds. SFL124 was based on the moderately virulent parent strain S. flexneri SFL1. The candidate was well tolerated in phase 1 studies in adults in Sweden [57] and Vietnam [58], and in a phase 2 studies in Vietnamese children [59], but showed variable immunogenicity.
Subsequently, SLF1070 was developed with aroD deleted from the more virulent S. flexneri 2a 2457T strain. When tested in a phase 1 study in Swedish adults, a narrow safety-immunogenicity profile was demonstrated [60]. Neither candidate progressed into later clinical trials.
3.2.6. WRAIR and the WRSS Vaccines
WRAIR developed a series of live attenuated S. sonnei vaccines based on the wild-type Moseley S. sonnei strain with virG deleted. A common problem with S. sonnei strains is loss of the virulence plasmid. However, the plasmid is relatively stable in the Moseley strain. WRSS1, the first-generation vaccine of this series, was immunogenic but resulted in diarrhea and fever among US and Israeli adult volunteers in phase 1 and phase 2 studies [61,62]. WRSS1 was better tolerated when tested in a phase 1 study in Bangladeshi adults and children, but immune responses were modest, requiring multiple doses, and were of short duration [63].
Further attenuating mutations were introduced to address the reactogenicity resulting in the WRSS2 and WRSS3 candidate vaccines. While both have deleted enterotoxin genes senA and senB, WRSS3 has msbB deleted from the virulence plasmid. The removal of msbB, which encodes an acyltransferase, results in the loss of an acyl chain from the lipid A of LPS with a consequent reduction in reactogenicity [64]. Both vaccines were immunogenic and reasonably tolerated in a phase 1 trial in the US [65].
3.2.7. Typhoid Shigella Combination Vaccine
An innovative approach to Shigella vaccine development was the use of the licensed typhoid Ty21a live attenuated vaccine as a vector for the delivery of Shigella sonnei O-antigen. The 5076-1C vaccine was developed by Formal at the University of Maryland in the 1980s [66]. Though well tolerated [67], it suffered from inconsistency in production leading to variability in immunogenicity and protection afforded in CHIM studies [36,68]. Further development of the vaccine was abandoned.
3.2.8. E. coli—Shigella Combination Vaccines and ShigETEC
In order to improve the tolerability of live attenuated Shigella vaccines, in a similar approach to the Ty21a Shigella candidate 5076-1C, key Shigella antigens have been expressed in E. coli. PGAI 42-1-15 was based on E. coli O8 genetically modified to express S. flexneri 2a O-antigen [69]. Though well tolerated, the vaccine failed to protect in a S. flexneri 2a CHIM study [70]. Similar vaccines, EcSf2a-1 and ECSf2a-2, were developed using E. coli K12 that could express S. flexneri 2a O-antigen and also invade epithelial cells due to the inclusion of the invasion plasmid from S. flexneri 5, with aroD deleted from ECSf2a-2 to reduce reactogenicity. Unacceptable reactogenicity was a problem for ECSf2a-1 [71], while ECSf2a-2 failed to protect in a CHIM study [72]. This line of approach was subsequently discontinued.
An intriguing and almost opposite approach to E. coli engineered to express Shigella antigens, is the ShigETEC live attenuated vaccine being developed by Eveliqure, Vienna [73]. ShigETEC is based on wild-type S. flexneri 2a with Shigella O-antigen and Ipa antigens, the best-characterized targets of protective immunity, removed through the deletion of rfbF and ipaBC, respectively, together with the deletion of setAB to remove enterotoxins. The vaccine was engineered to express a fusion protein of LT and ST, the labile and stable toxins of enterotoxigenic E. coli (ETEC), hence the name ShigETEC. The vaccine was well tolerated and immunogenic in a recent phase 1 trial in Hungary and is set to progress into phase 2 studies [73]. A key open question is which antigens will induce protective immunity against Shigella.
Similar to the ShigaETEC approach, a new ambitious Shigella vaccine strategy at the Center for Vaccine Development is to develop a hexavalent Shigella ETEC live attenuated vaccine consisting of six Shigella strains (S. flexneri 1b, 2a, 3a and 6, S. sonnei and S. dysenteriae 1) engineered to express ETEC antigens. This is currently in preclinical development [74].

Table 2.
Shigella live attenuated vaccines.
Table 2.
Shigella live attenuated vaccines.
| Name | Description | Developer | Species/Serotype and Strain | Genetic Deletions | Status | Furthest Stage of Development | References |
|---|---|---|---|---|---|---|---|
| SmD | Streptomycin-dependent Shigella strains | Yugoslav Army | Various | Not known | Discontinued | Phase 3 | [14,15] |
| Vadizen live vaccine | S. flexneri 2a passaged on nutrient agar | Istrati | S. flexneri 2a T32 Istrati | Not known | Discontinued | Phase 3 | [46,47] |
| F2a-sonnei | S. flexneri 2a LAV expressing S. sonnei O-antigen | Lanzhou Institute | S. flexneri 2a T32 | Not known | Unknown | Phase 3 | [48] |
| CVD1203 | S. flexneri 2a monovalent LAV | University of Maryland | S. flexneri 2a 2457T | Δ aroA and virG | Discontinued | Phase 1 | [49] |
| CVD1204 | S. flexneri 2a monovalent LAV | University of Maryland | S. flexneri 2a 2457T | Δ guaBA | Discontinued | Phase 1 | [51] |
| CVD1207 | S. flexneri 2a monovalent LAV | University of Maryland | S. flexneri 2a 2457T | Δ guaBA, virG, set and sen | Discontinued | Phase 1 | [50] |
| CVD1208S | S. flexneri 2a monovalent LAV grown on animal-free media | University of Maryland | S. flexneri 2a 2457T | Δ guaBA, set and sen | Discontinued | Phase 2 | [52] |
| SC602 | S. flexneri 2a monovalent LAV | Pasteur Institute | S. flexneri 2a 494 | Δ virG and iuc | Discontinued | Phase 2 | [53,54] |
| SC599 | S. dysenteriae type 1 monovalent LAV | Pasteur Institute | S. dysenteriae type 1 SC595 | Δ virG, ent, and fep | Discontinued | Phase 1 | [56] |
| SFL124 | S. flexneri 2a monovalent LAV | National Bac. Lab, Stockholm | S. flexneri 2a SFL1 | Δ aroD | Discontinued | Phase 2 | [57,58,59] |
| SFL1070 | S. flexneri 2a monovalent LAV | National Bac. Lab, Stockholm | S. flexneri 2a 2457T | Δ aroD | Discontinued | Phase 1 | [60] |
| WRSS1 | S. sonnei monovalent LAV | WRAIR | S. Sonnei Moseley | Δ virG | Discontinued | Phase 2 | [61,62,63] |
| WRSS2 | S. sonnei monovalent LAV | WRAIR | S. Sonnei Moseley | Δ virG, senA, and senB | Active | Phase 1 | [64,65] |
| WRSS3 | S. sonnei monovalent LAV | WRAIR | S. Sonnei Moseley | Δ virG, senA, senB, and msbB | Active | Phase 1 | [64,65] |
| 5076-1C | Salmonella Typhi LAV expressing S. sonnei O-antigen | University of Maryland | Salmonella Typhi Ty21a | Δ galE | Discontinued | Phase 2 | [36,66,67,68] |
| PGAI 42-1-15 | E. coli LAV expressing S. flexneri 2a O-antigen | University of Maryland | E. coli O8 RJ 91 | Not known | Discontinued | Phase 2 | [69,70] |
| EcSf2a-1 | E. coli LAV with S. flexneri 5 invasion plasmid, expressing S. flexneri 2a O-antigen | University of Maryland | E. coli K-12 395-1 | Discontinued | Phase 1 | [71] | |
| EcSF2a-2 | E. coli LAV with S. flexneri 5 invasion plasmid, expressing S. flexneri 2a O-antigen | University of Maryland | E. coli K-12 395-1 | Δ aroD | Discontinued | Phase 2 | [71,72] |
| ShigETEC | S. flexneri 2a LAV expressing fusion protein of B subunit of ETEC heat-labile and heat-stable enterotoxins | Eveliqure | S. flexneri 2a 2457T | Δ rfbF, ipaBC, and setBA | Active | Phase 1 | [73] |
4. Subunit Vaccines
4.1. Glycoconjugate Vaccines
4.1.1. Cohen and the NIH Glyconjugate Vaccines
The glycoconjugate approach to Shigella vaccines builds on the successful use of this technology for vaccines against the encapsulated bacteria Haemophilus influenzae b, meningococcus, pneumococcus and, more recently, Salmonella typhi. With the exception of S. sonnei (which has a capsule of O-antigen [75]), Shigella bacteria lack capsules, and so the glycan exploited in these vaccines is the O-antigen of LPS. As mentioned previously, serotype-specific protection following shigellosis implicates the immune response to O-antigen (which is serotype-specific) in protection. This is the key epidemiological observation on which the Shigella glycoconjugate vaccines are based.
A large body of work saw the relatively rapid advancement of glycoconjugates developed by Robbins and colleagues at the NIH through to efficacy studies in Israel. These prototype glycoconjugate vaccines consisted of O-antigen from S. sonnei or S. flexneri 2a chemically linked to recombinant exoprotein A of Pseudomonas aeruginosa (rEPA) (Table 3). The vaccines were clinically evaluated in Israel by Cohen and colleagues who had been studying immunity to Shigella and had collected strong observational evidence for the importance of serum O-antigen IgG in protection against shigellosis [76]. The safety and immunogenicity of S. sonnei O-antigen/rEPA were demonstrated in phase 1 and phase 2 clinical trials leading to a pivotal phase 3 study which consisted of three arms with volunteers receiving S. sonnei O-antigen/rEPA, the EcSf2a-2 live attenuated S. flexneri vaccine, or placebo [16].
The finding of 74% efficacy against shigellosis due to S. sonnei in the arm receiving S. sonnei O-antigen/rEPA was remarkable considering this was after a single dose of vaccine with participants followed up for a duration of two years. The lack of cases of shigellosis due to S. flexneri 2a in the study prevented any conclusions being made on the efficacy of EcSf2a-2. Given that study findings were published 25 years ago, it is surprising that no glycoconjugate vaccine has yet been licensed. There are perhaps several reasons for this.
While protection was demonstrated in military recruits in Israel, the key target population for a global Shigella vaccine is young children in LMICs. Thirteen years later, in 2010, findings from a second efficacy study in Israel were published by Paswell and colleagues [17], this time with children receiving either S. sonnei O-antigen/rEPA or S. flexneri 2a O-antigen/rEPA. While protection was demonstrated against S. sonnei shigellosis among children aged 3–4 years, protection was not seen in children under three years of age. Loss of protection corresponded to a decrease in serum O-antigen IgG titer further supporting a role for this modality of immunity in protection against shigellosis. Again, insufficient cases of S. flexneri 2a shigellosis precluded any conclusions about the efficacy of S. flexneri 2a O-antigen/rEPA. Neither S. sonnei O-antigen/rEPA nor S. flexneri 2a O-antigen/rEPA were ever commercialized.
4.1.2. LimmaTech and Bioconjugate Vaccines
Limmatech Biologics AG, based in Zurich, developed an innovative glycoconjugate technology known as bioconjugation where E. coli bacteria are genetically engineered to synthesize and chemically couple glycans from exogenous bacteria to protein carriers, exploiting the oligosaccharide transferase PglB [77]. As proof of principle, an S. dysenteriae type 1 bioconjugate, GVXN SD133-EPA was produced with O-antigen from S. dysenteriae type 1 linked to genetically detoxified exotoxin protein A of Pseudomonas aeruginosa and was found to be safe and immunogenic in a phase 1 study [78].
Subsequently, the technology was used to develop Flexyn2a-EPA, a vaccine against S. flexneri 2a which, following promising immunogenicity in a phase 1 study in the US, was assessed for efficacy against shigellosis in a CHIM study at Johns Hopkins University. Although the vaccine failed to meet the primary endpoint of protection against all forms of shigellosis, it did protect against more severe shigellosis in a post hoc analysis [38]. This prompted a series of international expert workshops to harmonize the conduct, clinical endpoints and immunological assays of future CHIMs to facilitate comparison between vaccines in development. The resulting consensus documents were published in Clinical Infectious Diseases (2019 supplement 8). As with the NIH S. sonnei vaccine, protection was closely associated with serum IgG to S. flexneri O-antigen [38,79]. On the basis of good immunogenicity in the phase 1 study and protection against more severe shigellosis in the CHIM study, a four-valent vaccine, S4V-EPA, was developed consisting of bioconjugates against S. sonnei, flexneri 3a and flexneri 6, as well as S. flexneri 2a. This is currently completing an age-descending dose-finding study in Kenya [77].
4.1.3. Pasteur Institute and Synthetic O-Antigen Conjugate Vaccines
Separate work by Pozgay and Robbins [80,81] with different Shigella conjugate vaccine constructs demonstrated that the use of O-antigens of reduced length could result in an enhanced serum IgG response to O-antigen compared with conjugates employing native length O-antigen. This observation provided supporting justification for the synthetic approach to generate Shigella O-antigens for conjugation at the Pasteur Institute. Using an elaborate process involving multiple chemical steps, a series of S. flexneri 2a synthetic O-antigens were generated and conjugated to tetanus toxoid prior to testing in mice for immunogenicity.
Evaluation in animals led to the selection of a candidate vaccine, SF2a-TT15, with O-antigen consisting of three pentasaccharide repeating units (giving a total of 15 saccharides). This vaccine was tested by Cohen in Israeli adults and found to be highly immunogenic, even after a single dose [82]. As with the Limmatech quadrivalent vaccine, SF2a-TT15 is currently being evaluated in an age-descending dose-finding study in Kenya and an adult CHIM study at the Center for Vaccine Development in Baltimore [83].
4.1.4. Beijing Zhifei Lvzhu Biopharmaceuticals Bivalent Glycoconjugate Vaccine
Using more conventional glycoconjugate technology, Beijing Zhifei Lvzhu Biopharmaceuticals designed and developed a bivalent vaccine, ZF0901, using O-antigen from S. sonnei and S. flexneri 2a conjugated to tetanus toxoid with adipic acid dihydrazide as linker. Following a phase 1 descending age study in China to show safety [84], ZF0901 was tested in a phase 2 age-descending study in which it was found to be safe and immunogenic, and is currently being tested in a phase 3 study [85].
4.2. Other Subunit Vaccines
4.2.1. WRAIR, Invasin Complex and Proteosome Complex Vaccines
An interesting alternative subunit vaccine approach to the glycoconjugates is the invasin complex or Invaplex technology developed by WRAIR [86]. Essentially, rather than conjugating O-antigen to carrier protein, Invaplex consists of a physical mixture of Shigella LPS and Ipa proteins. The initial iteration of Invaplex, native Invaplex or InvaplexNAT, consisted of a complex of Ipa antigens (IpaB, IpaC and IpaD) and LPS extracted from wild-type S. flexneri 2a. Three doses delivered intranasally were well tolerated and immunogenic in phase 1 studies [87,88] in the US but failed to protect in a CHIM study [89].
Subsequently, a more defined version of Invaplex, artificial Invaplex or InvaplexAR, was produced using recombinant IpaB and IpaC generated in E. coli, and LPS from S. flexneri 2a which was safe but not consistently immunogenic when delivered intranasally to volunteers in a phase 1 study [86]. As a further iteration, artificial detoxified Invaplex (InvaplexAR-Detox) employs an LPS of low reactogenicity purified from S. flexneri 2a with deleted msbB genes. This has allowed the parenteral administration of InvaplexAR-Detox with good safety and immunogenicity results in a recent phase 1 study [86]. It is surprising that high antibody titers are induced by the O-antigen of LPS in the absence of conjugation. The added protective value and extent of cross-protection resulting from the inclusion of Ipa proteins in the vaccine is currently not clear, although existing data from animals and humans suggest that the immune response to IpaB and IpaC will enhance protection.
In a similar but earlier approach to Invaplex, WRAIR complexed LPS from S. flexneri 2a with proteosomes (outer membrane proteins) from meningococcus to give a Proteosome-Shigella flexneri 2a lipopolysaccharide vaccine. This vaccine was only tested in phase 1 and was administered intranasally [90]. It was well tolerated and induced a modest immune response to O-antigen.
4.2.2. GSK Vaccines Institute for Global Health and Outer Membrane Vesicle Vaccines
A different subunit approach which has similarities with whole cell killed vaccines is the use of bacterial native outer membrane vesicles (OMVs) as vaccines. The GSK Vaccines Institute for Global Health (GVGH) adopted this approach by increasing the spontaneous release of OMV (referred to as ‘GMMA’—Generalised Modules for Membrane Antigens, by GVGH), which are blebs of outer membrane from the surface of Shigella, by the deletion of tolR [91]. tolR is a gene involved in maintaining the integrity of the connection between the inner and outer membranes in Gram-negative bacteria. In addition, htrB, which, like msbB, encodes an acyl transferase, was deleted to reduce reactogenicity, and virG to prevent epithelial invasion. This technology has the advantage of being straightforward to deploy with the potential to produce vaccine at low cost. GVGH considers the vesicles to be a vehicle for the delivery of O-antigen. Another advantage of this approach is that multiple other outer membrane components are presented to the immune system, including many outer membrane proteins [92], though not Ipa proteins, likely because the T3SS is tethered to the bacterial inner membrane.
A first OMV candidate vaccine, 1790GAHB, was generated from S. sonnei 53G with the above mutations. Though well tolerated, it was poorly immunogenic compared with historic Shigella glycoconjugate vaccines whether administered intramuscularly, intradermally or intranasally in European adults [93]. This is thought to be due to low content of LPS O-antigen with only 10% of LPS molecules in 1790GAHB expressing O-antigen. Although the vaccine was able to increase pre-existing high titers of serum O-antigen IgG in Kenyan adults [94], it failed to protect in a CHIM study in Cincinnati [95]. A quadrivalent vaccine, altSonflex1-2-3, consisting of OMV from S. flexneri 1b, 2a and 3a and S. sonnei (with higher O-antigen expression than 1790GAHB) is currently being tested in a phase 1/2 clinical trial [92].

Table 3.
Shigella subunit vaccines.
Table 3.
Shigella subunit vaccines.
| Name | Description | Developer | Species/Serotype and Strain | Genetic Deletions | Status | Furthest Stage of Development | References |
|---|---|---|---|---|---|---|---|
| S. sonnei O-antigen/rEPA | S. sonnei monovalent O-antigen glycoconjugate | NIH | S. sonnei | - | Discontinued | Phase 3 | [16,76] |
| S. flexneri 2a O-antigen/rEPA | S. flexneri 2a monovalentO-antigen glycoconjugate | NIH | S. flexneri 2a | - | Discontinued | Phase 3 | [17,76] |
| S4V-EPA | Quadrivalent O-antigen bioconjugate | LimmaTech | S. flexneri 2a, 3a, 6, and S. sonnei | - | Active | Phase 2 | [38,77,79] |
| GVXN SD133-EPA | S. dysenteriae type 1 monovalent O-antigen bioconjugate | LimmaTech | S. dysenteriae type 1 | - | Discontinued | Phase 1 | [77,78] |
| SF2a-TT15 | S. flexneri 2a synthetic O-antigen conjugate | Pasteur Institute | S. flexneri 2a | - | Active | Phase 2 | [82,83] |
| ZF0901 | Bivalent O-antigen glycoconjugate | Beijing Zhifei Lvzhu Biopharmaceuticals | S. flexneri 2a and S. sonnei | - | Active | Phase 3 | [84,85] |
| InvaplexNAT | Natural S. flexneri 2a invasin complex (LPS, IpaB, IpaC, and IpaD) | WRAIR | S. flexneri 2a 2457T | - | Discontinued | Phase 2 | [86,87,88,89] |
| InvaplexAR | Artificial S. flexneri 2a invasin complex (LPS, IpaB and IpaC) | WRAIR | S. flexneri 2a 2457T | - | Discontinued | Phase 1 | [86] |
| InvaplexAR-DETOX | Artificial detoxified S. flexneri 2a invasin complex (recombinant IpaB and IpaC) | WRAIR | S. flexneri 2a 2457T | LPS from S. flexneri 2a Δ msbB | Active | Phase 1 | [86] |
| Proteosome Shigella flexneri 2a LPS | S. flexneri 2a LPS and meningococcal outer membrane proteins | WRAIR | S. flexneri 2a | - | Discontinued | Phase 1 | [90] |
| 1790GAHB | Monovalent S. sonnei native outer membrane vesicle | GVGH (GSK) | S. sonnei | Δ tolR, htrB, and virG | Discontinued | Phase 2 | [91,92,93,94,95] |
| altSonflex1-2-3 | Quadrivalent Shigella native outer membrane vesicle | GVGH (GSK) | S. flexneri 1b, 2a, 3a, and S. sonnei | Δ tolR, htrB, and virG | Active | Phase 2 | [91] |
5. Discussion
Despite 100 years of vaccine development, there is to date no licensed vaccine to protect against shigellosis, the main cause of childhood bacterial diarrheal death globally. This is despite numerous candidate vaccines having been assessed in clinical trials and clinical efficacy proven with both the early live attenuated approaches of Mel and the Yugoslav Army in the 1960s, and the glycoconjugate approach of Robbins and the NIH in the 1990s. As indicated in this report, the large majority of vaccines tested in humans to date have been live attenuated vaccines. Their lack of success consistently comes down to the difficulty of balancing reactogenicity with sufficient immunogenicity to induce protection.
Where protection was demonstrated with the Mel SmD vaccines, this required multiple doses (a primary series of four doses) and duration of protection was limited to one year. However, according to the recently published WHO Preferred Product Characteristic for Shigella vaccines [18], a Shigella vaccine should induce protective immunity for at least two years after no more than two doses. The likely need for multivalent Shigella vaccines to provide sufficient breath of coverage against global circulating Shigella strains increases the challenge faced by live attenuated candidates. To date, it has proven exceedingly difficult to achieve an acceptable balance of reactogenicity and immunogenicity with monovalent Shigella vaccines.
Glycoconjugate technology appears more promising than live attenuated approaches for a licensed Shigella vaccine. Proof of efficacy was demonstrated by Cohen with the NIH S. sonnei O-antigen-rEPA candidate 25 years ago in young Israeli adults [16] and subsequently in children down to three years of age [17]. It is both surprising and disappointing that so few Shigella conjugate vaccines have entered clinical development since 1997. This is perhaps partly a result of the emphasis on pursuing the live attenuated approach by many vaccine developers during the 1990s and 2000s at a time when limited funding was available for Shigella vaccine development.
Following the disappointing results with so many live attenuated candidates, it is reassuring to see a balance across the global portfolio of Shigella vaccines with advancing clinical development of several promising glycoconjugate vaccines, as well as live attenuated vaccines advancing. The advent of molecular determination in recent years of pathogen etiology in diarrhea cases in place of exclusive reliance on stool culture [3] has increased the global awareness of the huge public health burden caused by Shigella. Growing worldwide concern about the threat of antimicrobial resistant Shigella [96] and the value of vaccines in curbing AMR [97], as well as an appreciation that Shigella is a major cause of linear growth faltering [98], are providing added impetus for Shigella vaccine development. These factors, together with increased funding for Shigella vaccines and the strong recommendation by the WHO’s Product Development Vaccine Advisory Committee (PDVAC) of the need to develop Shigella vaccines [99], are serving to accelerate the development of promising candidates.
Although glycoconjugate candidates rarely cause issues with reactogenicity compared with live attenuated vaccines, there remains the challenge of whether current candidates will prove to be sufficiently immunogenic among young children in LMICs. The most advanced glycoconjugate vaccine is the quadrivalent bioconjugate candidate, S4V-EPA, from Limmatech. This vaccine is currently completing its phase 2 study in young children in Kenya with the expectation of a readout of interim immunogenicity this year [77]. Meanwhile, the Pasteur Institute S. flexneri 2a synthetic O-antigen conjugate vaccine, SF2a-TT15, is completing an age descending, dose-finding study in Kenya [83]. The GVGH quadrivalent OMV vaccine, altSonflex-1-2-3, is in clinical evaluation in European adults [92].
The cumulative clinical data from these trials will provide much clarity as to whether a licensed Shigella vaccine is a realistic prospect in the next few years. Should these vaccines turn out to be safe but insufficiently immunogenic, there is the possibility of enhancing immune responses using adjuvants. To date, little is known about which adjuvants may potentiate current candidate Shigella vaccines, particularly the glycoconjugates. The use of adjuvants in the clinical trials of these vaccines has so far been limited to alum and effects have been inconsistent. This represents a knowledge gap in Shigella vaccine development. The application of our understanding of new adjuvants from the COVID-19 pandemic may help address this gap but requires formulation, stability and immunogenicity studies with lead candidate vaccines to begin now.
Although there is strong evidence that serum IgG to O-antigen is a correlate of protection in adults [25], this is yet to be shown in young children in LMICs. Consequently phase 3 efficacy studies are likely to be needed for licensure and WHO prequalification of Shigella vaccines for the global pediatric market. Phase 3 studies will be lengthy and costly but ultimately, if able to demonstrate efficacy and confirm the correlate of protection status for O-antigen IgG in children, may permit the licensure of subsequent vaccines on the basis of safety and non-inferior immunogenicity. As a critical enabling activity of this, work is underway at NIBSC to develop a first Shigella International Standard Serum and harmonize Shigella ELISAs [27].
Studies are currently underway to facilitate and expedite the initiation of phase 3 trials for the most promising candidate vaccines when ready [100]. Each of the candidate vaccines described ultimately requires a manufacturer to bring them to market. The lack of commercial incentive of a Shigella vaccine for the global pediatric market is unlikely to entice one of the big five multinational vaccine companies to manufacture a Shigella vaccine. It is more likely that companies from the Developing Country Vaccine Manufacturers Network [101] will partner to produce and, if successful at phase 3, license the most promising Shigella vaccines.
6. Conclusions
Over the past 100 years, Shigella vaccine development has become a graveyard filled with numerous candidates that entered clinical trials and proved to be either too reactogenic, insufficiently immunogenic or both. Nevertheless, proof of efficacy has been attained on more than one occasion and there are currently multiple promising candidates in clinical development, with lead candidates due to read out shortly from studies in the target populations of young children in LMICs.
Author Contributions
Conceptualization, C.A.M.; writing—original draft preparation, C.A.M.; writing—review and editing, C.A.M., S.G., L.-f.M. and A.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DuPont, H.L.; Levine, M.M.; Hornick, R.B.; Formal, S.B. Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 1989, 159, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: A reanalysis of the GEMS case-control study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef]
- Platts-Mills, J.A.; Liu, J.; Rogawski, E.T.; Kabir, F.; Lertsethtakarn, P.; Siguas, M.; Khan, S.S.; Praharaj, I.; Murei, A.; Nshama, R.; et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: A reanalysis of the MAL-ED cohort study. Lancet Glob. Health 2018, 6, e1309–e1318. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K.M. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- GBD. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Lopez-Velez, R.; Lebens, M.; Bundy, L.; Barriga, J.; Steffen, R. Bacterial travellers’ diarrhoea: A narrative review of literature published over the past 10 years. Travel Med. Infect. Dis. 2022, 47, 102293. [Google Scholar] [CrossRef]
- Rogawski, E.T.; Liu, J.; Platts-Mills, J.A.; Kabir, F.; Lertsethtakarn, P.; Siguas, M.; Khan, S.S.; Praharaj, I.; Murei, A.; Nshama, R.; et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: Longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob. Health 2018, 6, e1319–e1328. [Google Scholar] [CrossRef]
- Nasrin, D.; Blackwelder, W.C.; Sommerfelt, H.; Wu, Y.; Farag, T.H.; Panchalingam, S.; Biswas, K.; Saha, D.; Hossain, M.J.; Sow, S.O.; et al. Pathogens Associated With Linear Growth Faltering in Children With Diarrhea and Impact of Antibiotic Treatment: The Global Enteric Multicenter Study. J. Infect. Dis. 2021, 224, S848–S855. [Google Scholar] [CrossRef]
- Tickell, K.D.; Brander, R.L.; Atlas, H.E.; Pernica, J.M.; Walson, J.L.; Pavlinac, P.B. Identification and management of Shigella infection in children with diarrhoea: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1235–e1248. [Google Scholar] [CrossRef]
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 19 June 2022).
- CDC. Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 19 June 2022).
- The, H.C.; Rabaa, M.A.; Thanh, D.P.; De Lappe, N.; Cormican, M.; Valcanis, M.; Howden, B.P.; Wangchuk, S.; Bodhidatta, L.; Mason, C.J.; et al. South Asia as a Reservoir for the Global Spread of Ciprofloxacin-Resistant Shigella sonnei: A Cross-Sectional Study. PLoS Med. 2016, 13, e1002055. [Google Scholar] [CrossRef]
- Mel, D.M.; Arsic, B.L.; Nikolic, B.D.; Radovanic, M.L. Studies on vaccination against bacillary dysentery. 4. Oral immunization with live monotypic and combined vaccines. Bull. World Health Organ. 1968, 39, 375–380. [Google Scholar]
- Mel, D.; Gangarosa, E.J.; Radovanovic, M.L.; Arsic, B.L.; Litvinjenko, S. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull. World Health Organ. 1971, 45, 457–464. [Google Scholar] [PubMed]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Passwell, J.H.; Ashkenazi, S.; Banet-Levi, Y.; Ramon-Saraf, R.; Farzam, N.; Lerner-Geva, L.; Even-Nir, H.; Yerushalmi, B.; Chu, C.; Shiloach, J.; et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine 2010, 28, 2231–2235. [Google Scholar] [CrossRef]
- WHO. WHO Preferred Product Characteristics for Vaccines against Shigella. Available online: https://www.who.int/publications/i/item/9789240036741 (accessed on 19 June 2022).
- Formal, S.B.; Oaks, E.V.; Olsen, R.E.; Wingfield-Eggleston, M.; Snoy, P.J.; Cogan, J.P. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 1991, 164, 533–537. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Hornick, R.B.; Snyder, M.J.; Libonati, J.P.; Formal, S.B.; Gangarosa, E.J. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J. Infect. Dis. 1972, 125, 12–16. [Google Scholar] [CrossRef]
- Cohen, D.; Bassal, R.; Goren, S.; Rouach, T.; Taran, D.; Schemberg, B.; Peled, N.; Keness, Y.; Ken-Dror, S.; Vasilev, V.; et al. Recent trends in the epidemiology of shigellosis in Israel. Epidemiol. Infect. 2014, 142, 2583–2594. [Google Scholar] [CrossRef]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef]
- Noriega, F.R.; Liao, F.M.; Maneval, D.R.; Ren, S.; Formal, S.B.; Levine, M.M. Strategy for cross-protection among Shigella flexneri serotypes. Infect. Immun. 1999, 67, 782–788. [Google Scholar] [CrossRef]
- Menard, R.; Prevost, M.C.; Gounon, P.; Sansonetti, P.; Dehio, C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc. Natl. Acad. Sci. USA 1996, 93, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Meron-Sudai, S.; Bialik, A.; Asato, V.; Goren, S.; Ariel-Cohen, O.; Reizis, A.; Hochberg, A.; Ashkenazi, S. Serum IgG antibodies to Shigella lipopolysaccharide antigens—A correlate of protection against shigellosis. Hum. Vaccines Immunother. 2019, 15, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Ndungo, E.; Randall, A.; Hazen, T.H.; Kania, D.A.; Trappl-Kimmons, K.; Liang, X.; Barry, E.M.; Kotloff, K.L.; Chakraborty, S.; Mani, S.; et al. A Novel Shigella Proteome Microarray Discriminates Targets of Human Antibody Reactivity following Oral Vaccination and Experimental Challenge. mSphere 2018, 3, e00260-18. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, C.A.; Talaat, K.R.; Kaminski, R.W.; Cohen, D.; Riddle, M.S.; Giersing, B.K. Critical needs in advancing Shigella vaccines for global health. J. Infect. Dis. 2021, 225, 1500–1503. [Google Scholar] [CrossRef]
- Nahm, M.H.; Yu, J.; Weerts, H.P.; Wenzel, H.; Tamilselvi, C.S.; Chandrasekaran, L.; Pasetti, M.F.; Mani, S.; Kaminski, R.W. Development, Interlaboratory Evaluations, and Application of a Simple, High-Throughput Shigella Serum Bactericidal Assay. mSphere 2018, 3, e00146-18. [Google Scholar] [CrossRef]
- Rossi, O.; Molesti, E.; Saul, A.; Giannelli, C.; Micoli, F.; Necchi, F. Intra-Laboratory Evaluation of Luminescence Based High-Throughput Serum Bactericidal Assay (L-SBA) to Determine Bactericidal Activity of Human Sera against Shigella. High Throughput 2020, 9, 14. [Google Scholar] [CrossRef]
- Van de Verg, L.; Herrington, D.A.; Murphy, J.R.; Wasserman, S.S.; Formal, S.B.; Levine, M.M. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect. Immun. 1990, 58, 2002–2004. [Google Scholar] [CrossRef]
- Simon, J.K.; Maciel, M., Jr.; Weld, E.D.; Wahid, R.; Pasetti, M.F.; Picking, W.L.; Kotloff, K.L.; Levine, M.M.; Sztein, M.B. Antigen-specific IgA B memory cell responses to Shigella antigens elicited in volunteers immunized with live attenuated Shigella flexneri 2a oral vaccine candidates. Clin. Immunol. 2011, 139, 185–192. [Google Scholar] [CrossRef]
- Toapanta, F.R.; Bernal, P.J.; Kotloff, K.L.; Levine, M.M.; Sztein, M.B. T cell mediated immunity induced by the live-attenuated Shigella flexneri 2a vaccine candidate CVD 1208S in humans. J. Transl. Med. 2018, 16, 61. [Google Scholar] [CrossRef]
- Mallett, C.P.; VanDeVerg, L.; Collins, H.H.; Hale, T.L. Evaluation of Shigella vaccine safety and efficacy in an intranasally challenged mouse model. Vaccine 1993, 11, 190–196. [Google Scholar] [CrossRef]
- Hartman, A.B.; Powell, C.J.; Schultz, C.L.; Oaks, E.V.; Eckels, K.H. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect. Immun. 1991, 59, 4075–4083. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.H.; Suzuki, T.; Chang, S.Y.; Park, S.M.; Sansonetti, P.J.; Sasakawa, C.; Kweon, M.N. New animal model of shigellosis in the Guinea pig: Its usefulness for protective efficacy studies. J. Immunol. 2007, 178, 2476–2482. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Levine, M.M.; Clements, M.L.; Losonsky, G.; Herrington, D.; Berman, S.; Formal, S.B. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J. Infect. Dis. 1987, 155, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Dickey, M.; Suvarnapunya, A.E.; Chandrasekaran, L.; Kaminski, R.W.; Clarkson, K.A.; McNeal, M.; Lynen, A.; Parker, S.; Hoeper, A.; et al. Establishment of a Controlled Human Infection Model with a Lyophilized Strain of Shigella sonnei 53G. mSphere 2020, 5, e00416-20. [Google Scholar] [CrossRef]
- Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.M.; Kaminski, R.W.; Porter, C.K.; Chakraborty, S.; Clarkson, K.A.; Brubaker, J.; et al. Human challenge study with a Shigella bioconjugate vaccine: Analyses of clinical efficacy and correlate of protection. eBioMedicine 2021, 66, 103310. [Google Scholar] [CrossRef]
- Talaat, K.R.; Bourgeois, A.L.; Frenck, R.W.; Chen, W.H.; MacLennan, C.A.; Riddle, M.S.; Suvarnapunya, A.E.; Brubaker, J.L.; Kotloff, K.L.; Porter, C.K. Consensus Report on Shigella Controlled Human Infection Model: Conduct of Studies. Clin. Infect. Dis. 2019, 69, S580–S590. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Riddle, M.S.; Chen, W.H.; Talaat, K.R.; Jain, V.; Bourgeois, A.L.; Frenck, R.; Kotloff, K.; Porter, C.K. Consensus Report on Shigella Controlled Human Infection Model: Clinical Endpoints. Clin. Infect. Dis. 2019, 69, S591–S595. [Google Scholar] [CrossRef]
- Kaminski, R.W.; Pasetti, M.F.; Aguilar, A.O.; Clarkson, K.A.; Rijpkema, S.; Bourgeois, A.L.; Cohen, D.; Feavers, I.; MacLennan, C.A. Consensus Report on Shigella Controlled Human Infection Model: Immunological Assays. Clin. Infect. Dis. 2019, 69, S596–S601. [Google Scholar] [CrossRef]
- Herrera, C.M.; Schmitt, J.S.; Chowdhry, E.I.; Riddle, M.S. From Kiyoshi Shiga to Present-Day Shigella Vaccines: A Historical Narrative Review. Vaccines 2022, 10, 645. [Google Scholar] [CrossRef]
- McKenzie, R.; Walker, R.I.; Nabors, G.S.; Van De Verg, L.L.; Carpenter, C.; Gomes, G.; Forbes, E.; Tian, J.H.; Yang, H.H.; Pace, J.L.; et al. Safety and immunogenicity of an oral, inactivated, whole-cell vaccine for Shigella sonnei: Preclinical studies and a Phase I trial. Vaccine 2006, 24, 3735–3745. [Google Scholar] [CrossRef]
- Chakraborty, S.; Harro, C.; DeNearing, B.; Bream, J.; Bauers, N.; Dally, L.; Flores, J.; Van de Verg, L.; Sack, D.A.; Walker, R. Evaluation of the Safety, Tolerability, and Immunogenicity of an Oral, Inactivated Whole-Cell Shigella flexneri 2a Vaccine in Healthy Adult Subjects. Clin. Vaccine Immunol. 2016, 23, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.W.; Wu, M.; Turbyfill, K.R.; Clarkson, K.; Tai, B.; Bourgeois, A.L.; Van De Verg, L.L.; Walker, R.I.; Oaks, E.V. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin. Vaccine Immunol. 2014, 21, 366–382. [Google Scholar] [CrossRef] [PubMed]
- Meitert, T.; Pencu, E.; Ciudin, L.; Tonciu, M. Vaccine strain Sh. flexneri T32-Istrati. Studies in animals and in volunteers. Antidysentery immunoprophylaxis and immunotherapy by live vaccine Vadizen (Sh. flexneri T32-Istrati). Arch. Roum. Pathol. Exp. Microbiol. 1984, 43, 251–278. [Google Scholar] [PubMed]
- Meitert, T.; Ciudin, L.; Pencu, E.; Tonciu, M.; Gheorghe, G. Efficiency of immunoprophylaxis and immunotherapy by live dysentery vaccine administration in children and adults collectivities. Arch. Roum. Pathol. Exp. Microbiol. 1982, 41, 357–369. [Google Scholar] [PubMed]
- Venkatesan, M.M.; Ranallo, R.T. Live-attenuated Shigella vaccines. Expert Rev. Vaccines 2006, 5, 669–686. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Noriega, F.; Losonsky, G.A.; Sztein, M.B.; Wasserman, S.S.; Nataro, J.P.; Levine, M.M. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect. Immun. 1996, 64, 4542–4548. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Noriega, F.R.; Samandari, T.; Sztein, M.B.; Losonsky, G.A.; Nataro, J.P.; Picking, W.D.; Barry, E.M.; Levine, M.M. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA, is highly attenuated in humans. Infect. Immun. 2000, 68, 1034–1039. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Pasetti, M.F.; Barry, E.M.; Nataro, J.P.; Wasserman, S.S.; Sztein, M.B.; Picking, W.D.; Levine, M.M. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208. J. Infect. Dis. 2004, 190, 1745–1754. [Google Scholar] [CrossRef][Green Version]
- Kotloff, K. Safety and Efficacy Study of CVD 1208S, a Live, Attenuated Oral Vaccine to Prevent Shigella Infection: Phase IIa. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00866476?cond=shigella&draw=2&rank=4&view=record (accessed on 19 June 2022).
- Coster, T.S.; Hoge, C.W.; VanDeVerg, L.L.; Hartman, A.B.; Oaks, E.V.; Venkatesan, M.M.; Cohen, D.; Robin, G.; Fontaine-Thompson, A.; Sansonetti, P.J.; et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect. Immun. 1999, 67, 3437–3443. [Google Scholar] [CrossRef]
- Rahman, K.M.; Arifeen, S.E.; Zaman, K.; Rahman, M.; Raqib, R.; Yunus, M.; Begum, N.; Islam, M.S.; Sohel, B.M.; Rahman, M.; et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine 2011, 29, 1347–1354. [Google Scholar] [CrossRef]
- Tickell, K.D.; Atlas, H.E.; Walson, J.L. Environmental enteric dysfunction: A review of potential mechanisms, consequences and management strategies. BMC Med. 2019, 17, 181. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Sadorge, C.; Jolly, N.; Poirier, B.; Bechet, S.; van der Vliet, D.; Seffer, V.; Fenner, N.; Dowling, K.; Giemza, R.; et al. Safety and immunogenicity of SC599, an oral live attenuated Shigella dysenteriae type-1 vaccine in healthy volunteers: Results of a Phase 2, randomized, double-blind placebo-controlled trial. Vaccine 2009, 27, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Pal, T.; Forsum, U.; Lindberg, A.A. Safety and immunogenicity of the live oral auxotrophic Shigella flexneri SFL124 in volunteers. Vaccine 1992, 10, 395–404. [Google Scholar] [CrossRef]
- Li, A.; Karnell, A.; Huan, P.T.; Cam, P.D.; Minh, N.B.; Tram, L.N.; Quy, N.P.; Trach, D.D.; Karlsson, K.; Lindberg, G.; et al. Safety and immunogenicity of the live oral auxotrophic Shigella flexneri SFL124 in adult Vietnamese volunteers. Vaccine 1993, 11, 180–189. [Google Scholar] [CrossRef]
- Li, A.; Cam, P.D.; Islam, D.; Minh, N.B.; Huan, P.T.; Rong, Z.C.; Karlsson, K.; Lindberg, G.; Lindberg, A.A. Immune responses in Vietnamese children after a single dose of the auxotrophic, live Shigella flexneri Y vaccine strain SFL124. J. Infect. 1994, 28, 11–23. [Google Scholar] [CrossRef]
- Karnell, A.; Li, A.; Zhao, C.R.; Karlsson, K.; Nguyen, B.M.; Lindberg, A.A. Safety and immunogenicity study of the auxotrophic Shigella flexneri 2a vaccine SFL1070 with a deleted aroD gene in adult Swedish volunteers. Vaccine 1995, 13, 88–99. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Taylor, D.N.; Sztein, M.B.; Wasserman, S.S.; Losonsky, G.A.; Nataro, J.P.; Venkatesan, M.; Hartman, A.; Picking, W.D.; Katz, D.E.; et al. Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect. Immun. 2002, 70, 2016–2021. [Google Scholar] [CrossRef]
- Orr, N.; Katz, D.E.; Atsmon, J.; Radu, P.; Yavzori, M.; Halperin, T.; Sela, T.; Kayouf, R.; Klein, Z.; Ambar, R.; et al. Community-based safety, immunogenicity, and transmissibility study of the Shigella sonnei WRSS1 vaccine in Israeli volunteers. Infect. Immun. 2005, 73, 8027–8032. [Google Scholar] [CrossRef]
- Raqib, R.; Sarker, P.; Zaman, K.; Alam, N.H.; Wierzba, T.F.; Maier, N.; Talukder, K.; Baqui, A.H.; Suvarnapunya, A.E.; Qadri, F.; et al. A phase I trial of WRSS1, a Shigella sonnei live oral vaccine in Bangladeshi adults and children. Hum. Vaccines Immunother. 2019, 15, 1326–1337. [Google Scholar] [CrossRef]
- Barnoy, S.; Jeong, K.I.; Helm, R.F.; Suvarnapunya, A.E.; Ranallo, R.T.; Tzipori, S.; Venkatesan, M.M. Characterization of WRSs2 and WRSs3, new second-generation virG(icsA)-based Shigella sonnei vaccine candidates with the potential for reduced reactogenicity. Vaccine 2010, 28, 1642–1654. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Baqar, S.; Alexander, W.; Dickey, M.; McNeal, M.; El-Khorazaty, J.; Baughman, H.; Hoeper, A.; Barnoy, S.; Suvarnapunya, A.E.; et al. A Phase I trial to evaluate the safety and immunogenicity of WRSs2 and WRSs3; two live oral candidate vaccines against Shigella sonnei. Vaccine 2018, 36, 4880–4889. [Google Scholar] [CrossRef] [PubMed]
- Formal, S.B.; Baron, L.S.; Kopecko, D.J.; Washington, O.; Powell, C.; Life, C.A. Construction of a potential bivalent vaccine strain: Introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21a typhoid vaccine strain. Infect. Immun. 1981, 34, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Tramont, E.C.; Chung, R.; Berman, S.; Keren, D.; Kapfer, C.; Formal, S.B. Safety and antigenicity of typhoid-Shigella sonnei vaccine (strain 5076-1C). J. Infect. Dis. 1984, 149, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Herrington, D.A.; Van de Verg, L.; Formal, S.B.; Hale, T.L.; Tall, B.D.; Cryz, S.J.; Tramont, E.C.; Levine, M.M. Studies in volunteers to evaluate candidate Shigella vaccines: Further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine 1990, 8, 353–357. [Google Scholar] [CrossRef]
- Levine, M.M.; Woodward, W.E.; Formal, S.B.; Gemski, P., Jr.; DuPont, H.L.; Hornick, R.B.; Snyder, M.J. Studies with a new generation of oral attenuated shigella vaccine: Escherichia coli bearing surface antigens of Shigella flexneri. J. Infect. Dis. 1977, 136, 577–582. [Google Scholar] [CrossRef]
- Levine, M.M.; Galen, J.; Barry, E.; Noriega, F.; Tacket, C.; Sztein, M.; Chatfield, S.; Dougan, G.; Losonsky, G.; Kotloff, K. Attenuated Salmonella typhi and Shigella as live oral vaccines and as live vectors. Behring Inst. Mitt. 1997, 98, 120–123. [Google Scholar]
- Kotloff, K.L.; Herrington, D.A.; Hale, T.L.; Newland, J.W.; Van De Verg, L.; Cogan, J.P.; Snoy, P.J.; Sadoff, J.C.; Formal, S.B.; Levine, M.M. Safety, immunogenicity, and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infect. Immun. 1992, 60, 2218–2224. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Losonsky, G.A.; Nataro, J.P.; Wasserman, S.S.; Hale, T.L.; Taylor, D.N.; Newland, J.W.; Sadoff, J.C.; Formal, S.B.; Levine, M.M. Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli-Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine 1995, 13, 495–502. [Google Scholar] [CrossRef]
- Girardi, P.; Harutyunyan, S.; Neuhauser, I.; Glaninger, K.; Korda, O.; Nagy, G.; Nagy, E.; Szijarto, V.; Pall, D.; Szarka, K.; et al. Evaluation of the Safety, Tolerability and Immunogenicity of ShigETEC, an Oral Live Attenuated Shigella-ETEC Vaccine in Placebo-Controlled Randomized Phase 1 Trial. Vaccines 2022, 10, 340. [Google Scholar] [CrossRef]
- Barry, E.M.; Levine, M.M. A tale of two bacterial enteropathogens and one multivalent vaccine. Cell. Microbiol. 2019, 21, e13067. [Google Scholar] [CrossRef]
- Caboni, M.; Pedron, T.; Rossi, O.; Goulding, D.; Pickard, D.; Citiulo, F.; MacLennan, C.A.; Dougan, G.; Thomson, N.R.; Saul, A.; et al. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog. 2015, 11, e1004749. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Meron-Sudai, S.; Bialik, A.; Asato, V.; Ashkenazi, S. Detoxified O-Specific Polysaccharide (O-SP)-Protein Conjugates: Emerging Approach in the Shigella Vaccine Development Scene. Vaccines 2022, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Alaimo, C. The Ongoing Journey of a Shigella Bioconjugate Vaccine. Vaccines 2022, 10, 212. [Google Scholar] [CrossRef]
- Hatz, C.F.; Bally, B.; Rohrer, S.; Steffen, R.; Kramme, S.; Siegrist, C.A.; Wacker, M.; Alaimo, C.; Fonck, V.G. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: A single blind, partially randomized Phase I study. Vaccine 2015, 33, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, K.A.; Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.; Porter, C.K.; Chakraborty, S.; Brubaker, J.; Elwood, D.; et al. Immune response characterization in a human challenge study with a Shigella flexneri 2a bioconjugate vaccine. eBioMedicine 2021, 66, 103308. [Google Scholar] [CrossRef] [PubMed]
- Pozsgay, V.; Chu, C.; Pannell, L.; Wolfe, J.; Robbins, J.B.; Schneerson, R. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc. Natl. Acad. Sci. USA 1999, 96, 5194–5197. [Google Scholar] [CrossRef]
- Robbins, J.B.; Kubler-Kielb, J.; Vinogradov, E.; Mocca, C.; Pozsgay, V.; Shiloach, J.; Schneerson, R. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei O-specific oligosaccharide-core-protein conjugates. Proc. Natl. Acad. Sci. USA 2009, 106, 7974–7978. [Google Scholar] [CrossRef]
- Cohen, D.; Atsmon, J.; Artaud, C.; Meron-Sudai, S.; Gougeon, M.L.; Bialik, A.; Goren, S.; Asato, V.; Ariel-Cohen, O.; Reizis, A.; et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: A phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 2021, 21, 546–558. [Google Scholar] [CrossRef]
- Phalipon, A.; Mulard, L.A. Toward a Multivalent Synthetic Oligosaccharide-Based Conjugate Vaccine against Shigella: State-of-the-Art for a Monovalent Prototype and Challenges. Vaccines 2022, 10, 403. [Google Scholar] [CrossRef]
- Beijing Zhifei Lvzhu Biopharmaceutical Co., Ltd. Safety Study of S.Flexneriza-S.Sonnei Bivalent Conjugate Vaccine in Healthy Volunteers Aged Above 3 Months. Available online: https://clinicaltrials.gov/ct2/show/NCT03561181 (accessed on 19 June 2022).
- Mo, Y.; Fang, W.; Li, H.; Chen, J.; Hu, X.; Wang, B.; Feng, Z.; Shi, H.; He, Y.; Huang, D.; et al. Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China. Vaccines 2021, 10, 33. [Google Scholar] [CrossRef]
- Turbyfill, K.R.; Clarkson, K.A.; Oaks, E.V.; Kaminski, R.W. From Concept to Clinical Product: A Brief History of the Novel Shigella Invaplex Vaccine’s Refinement and Evolution. Vaccines 2022, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Tribble, D.; Kaminski, R.; Cantrell, J.; Nelson, M.; Porter, C.; Baqar, S.; Williams, C.; Arora, R.; Saunders, J.; Ananthakrishnan, M.; et al. Safety and immunogenicity of a Shigella flexneri 2a Invaplex 50 intranasal vaccine in adult volunteers. Vaccine 2010, 28, 6076–6085. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.S.; Kaminski, R.W.; Williams, C.; Porter, C.; Baqar, S.; Kordis, A.; Gilliland, T.; Lapa, J.; Coughlin, M.; Soltis, C.; et al. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine 2011, 29, 7009–7019. [Google Scholar] [CrossRef] [PubMed]
- Harro, C.; Riddle, M.S.; Kaminski, R.; Turbyfill, K.R.; Gormley, R.; Porter, C.; Ranallo, R.T.; Kordis, A.; Buck, M.; Jones, A. Shigella flexneri 2a Invaplex 50 intranasal vaccine phase 2b challenge study. In Proceedings of the Vaccines for Enteric Diseases, Malaga, Spain, 9–11 September 2009. [Google Scholar]
- Fries, L.F.; Montemarano, A.D.; Mallett, C.P.; Taylor, D.N.; Hale, T.L.; Lowell, G.H. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect. Immun. 2001, 69, 4545–4553. [Google Scholar] [CrossRef]
- Micoli, F.; Nakakana, U.N.; Scorza, F.B. Towards a Four-Component GMMA-Based Vaccine against Shigella. Vaccines 2022, 10, 328. [Google Scholar] [CrossRef]
- Maggiore, L.; Yu, L.; Omasits, U.; Rossi, O.; Dougan, G.; Thomson, N.R.; Saul, A.; Choudhary, J.S.; Gerke, C. Quantitative proteomic analysis of Shigella flexneri and Shigella sonnei Generalized Modules for Membrane Antigens (GMMA) reveals highly pure preparations. Int. J. Med. Microbiol. 2016, 306, 99–108. [Google Scholar] [CrossRef]
- Launay, O.; Lewis, D.J.M.; Anemona, A.; Loulergue, P.; Leahy, J.; Scire, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety Profile and Immunologic Responses of a Novel Vaccine Against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results From Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. eBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef]
- Obiero, C.W.; Ndiaye, A.G.W.; Scire, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schutte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A.; et al. A Phase 2a Randomized Study to Evaluate the Safety and Immunogenicity of the 1790GAHB Generalized Modules for Membrane Antigen Vaccine against Shigella sonnei Administered Intramuscularly to Adults from a Shigellosis-Endemic Country. Front. Immunol. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Conti, V.; Ferruzzi, P.; Ndiaye, A.G.W.; Parker, S.; McNeal, M.M.; Dickey, M.; Granada, J.P.; Cilio, G.L.; De Ryck, I.; et al. Efficacy, safety, and immunogenicity of the Shigella sonnei 1790GAHB GMMA candidate vaccine: Results from a phase 2b randomized, placebo-controlled challenge study in adults. eClinicalMedicine 2021, 39, 101076. [Google Scholar] [CrossRef]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- WHO. Leveraging vaccines to reduce antibiotic use and prevent antimicrobial resistance: An action framework. Clin. Infect. Dis. 2021, 73, e1011–e1017. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, W.P.; Scheele, S.; Giersing, B.K. What Drives the Value of a Shigella Vaccine? Vaccines 2022, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- WHO. The 6th Annual Meeting of the WHO Product Development for Vaccines Advisory Committee (PDVAC). Available online: https://cdn.who.int/media/docs/default-source/immunization/pdvac/pdvac-2019/pdvac_2019_exec_summary.pdf?sfvrsn=9df96d4b_5&download=true (accessed on 19 June 2022).
- Pavlinac, P.B.; Rogawski McQuade, E.T.; Platts-Mills, J.A.; Kotloff, K.L.; Deal, C.; Giersing, B.K.; Isbrucker, R.A.; Kang, G.; Ma, L.F.; MacLennan, C.A.; et al. Pivotal Shigella Vaccine Efficacy Trials-Study Design Considerations from a Shigella Vaccine Trial Design Working Group. Vaccines 2022, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- DCVMN. DCVMN Developing Countries Vaccine Manufacturers Network. Available online: http://www.dcvmn.org/ (accessed on 19 June 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).