Kinetic of the Antibody Response Following AddaVax-Adjuvanted Immunization with Recombinant Influenza Antigens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccination of Mice
2.2. ELISA
2.3. Hemagglutination Inhibition Assay
2.4. FluoroSpot Assay
2.5. Statistical Analysis
3. Results
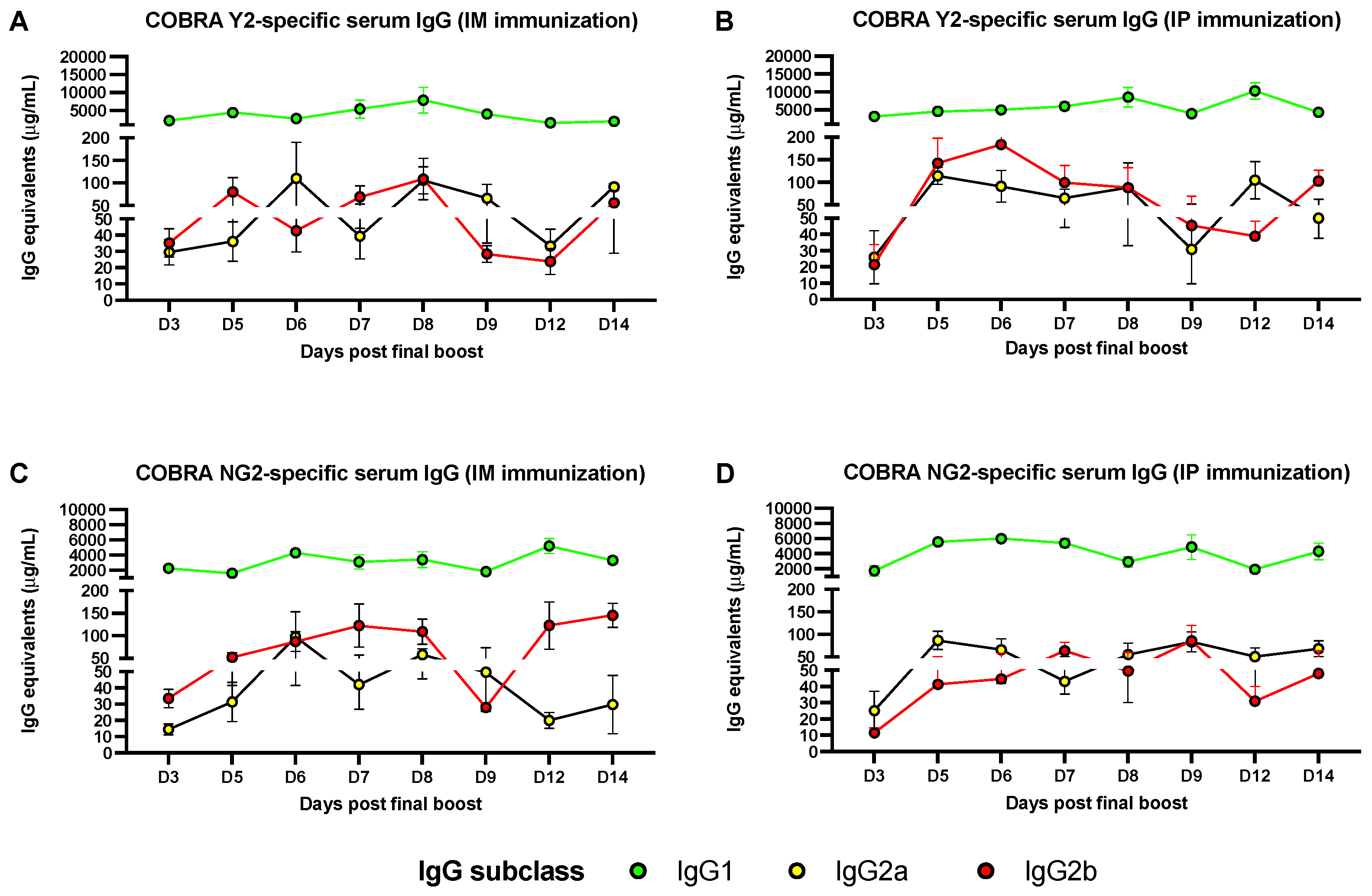
3.1. Predominant Serum IgG1 Response following IM or IP AddaVax-Adjuvanted Vaccination
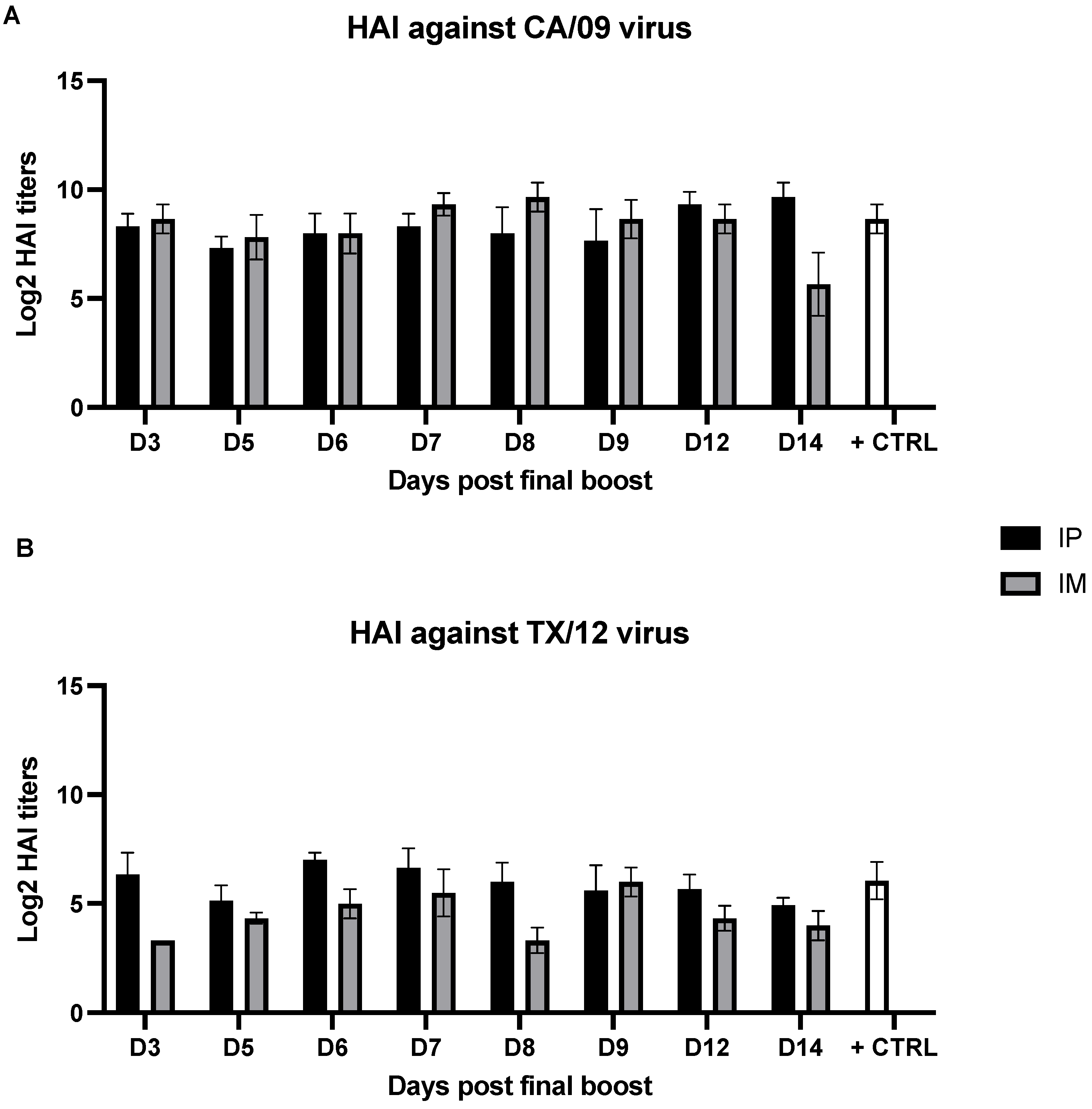
3.2. Serum Hemagglutination Inhibition Activity Is Not Affected by the Time Point of Sample Collection
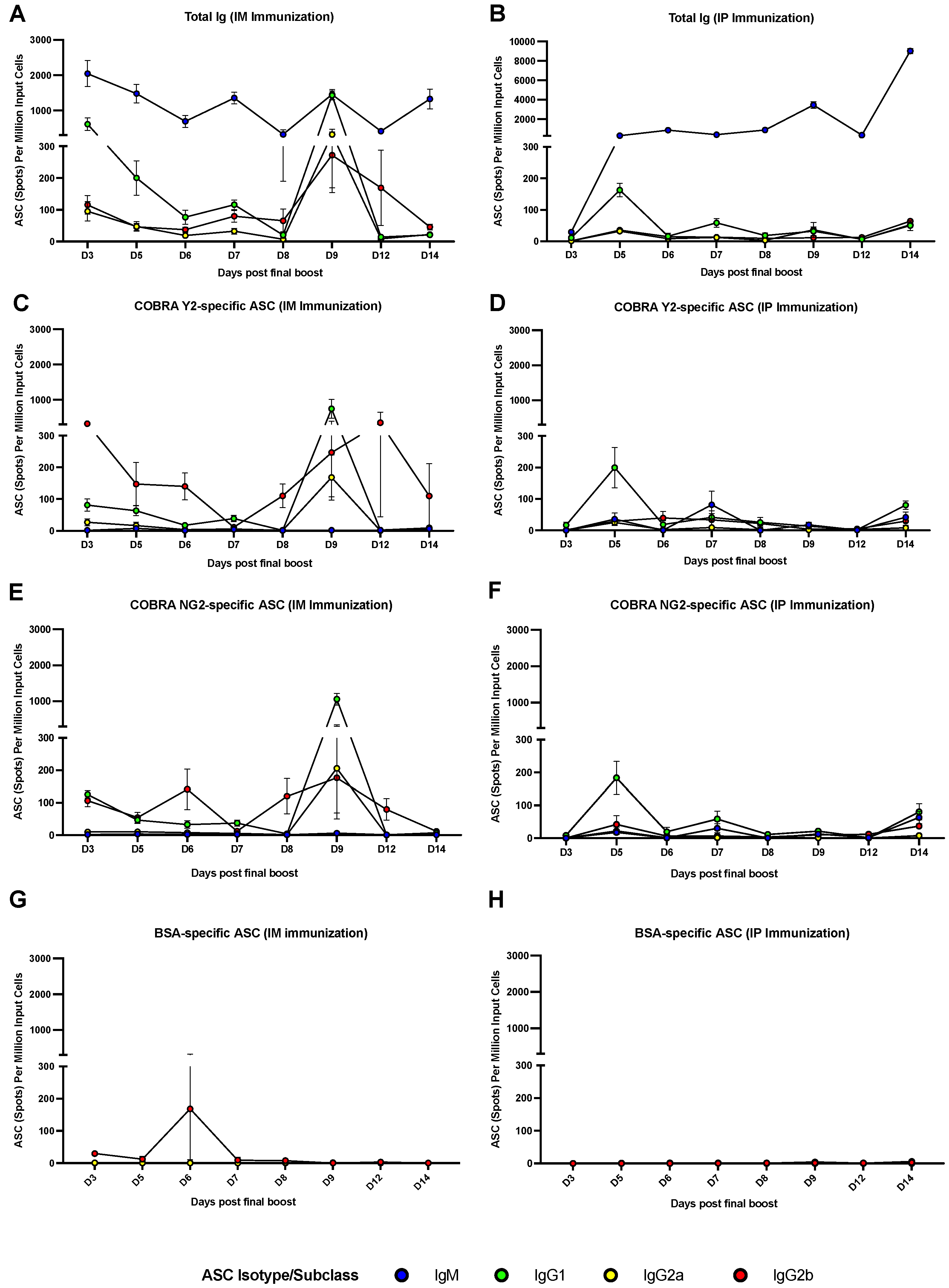
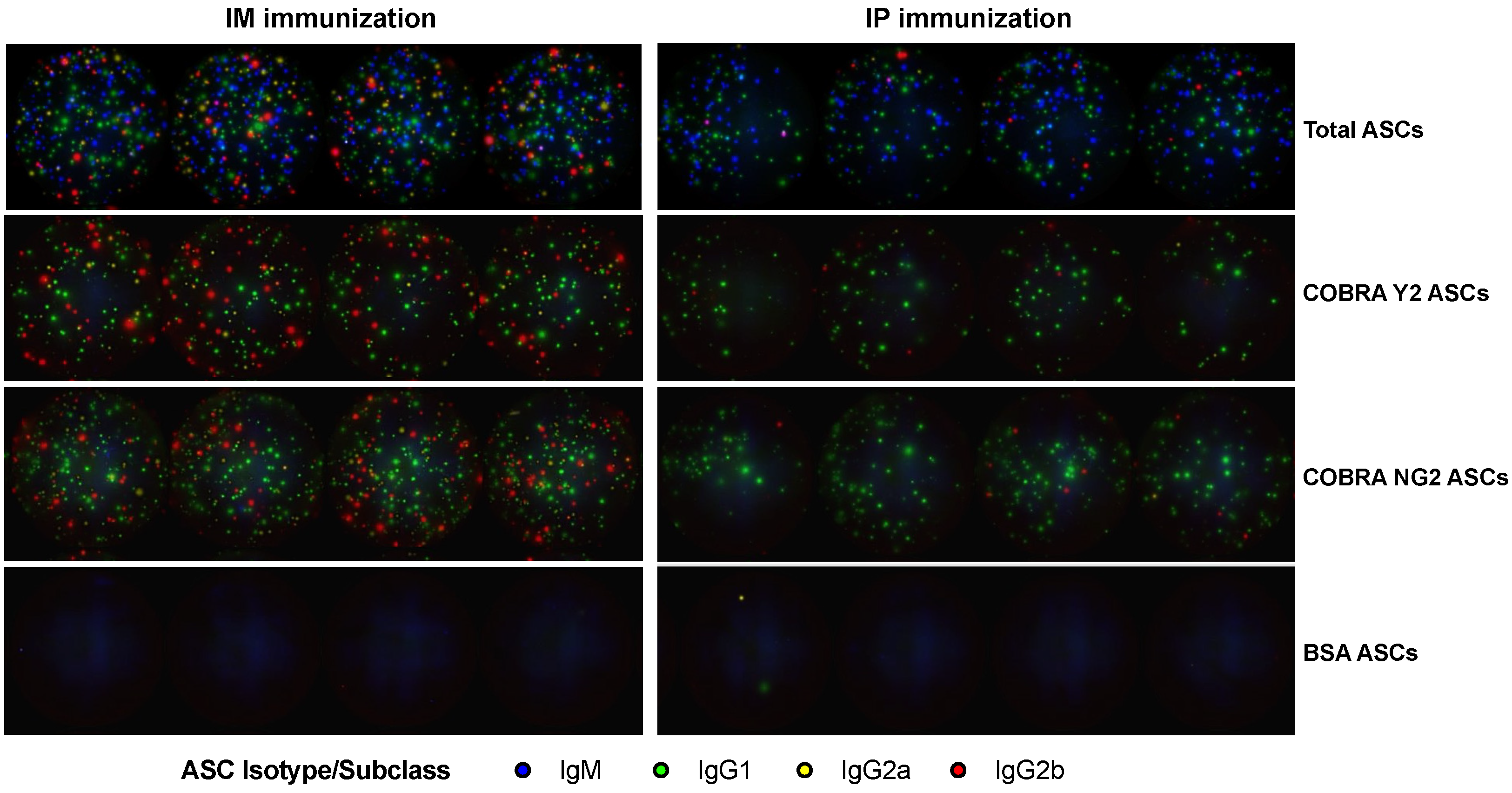
3.3. The Peak of Spleen ASCs Is Affected by the Route of Immunization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 13 August 2022).
- Guthmiller, J.J.; Utset, H.A.; Wilson, P.C. B Cell Responses against Influenza Viruses: Short-Lived Humoral Immunity against a Life-Long Threat. Viruses 2021, 13, 965. [Google Scholar] [CrossRef]
- Gordon, A.; Ortega, O.; Kuan, G.; Reingold, A.; Saborio, S.; Balmaseda, A.; Harris, E. Prevalence and seasonality of influenza-like illness in children, Nicaragua, 2005–2007. Emerg. Infect. Dis. 2009, 15, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Smetana, J.; Chlibek, R.; Shaw, J.; Splino, M.; Prymula, R. Influenza vaccination in the elderly. Hum. Vaccin. Immunother. 2018, 14, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Janoff, E.N. Influenza in immunosuppressed populations: A review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect. Dis. 2009, 9, 493–504. [Google Scholar] [CrossRef]
- Abreu, R.B.; Kirchenbaum, G.A.; Sautto, G.A.; Clutter, E.F.; Ross, T.M. Impaired memory B-cell recall responses in the elderly following recurrent influenza vaccination. PLoS ONE 2021, 16, e0254421. [Google Scholar] [CrossRef] [PubMed]
- CDC. Vaccine Effectiveness: How Well Do Flu Vaccines Work? Available online: https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm (accessed on 13 August 2022).
- CDC. Available online: https://www.cdc.gov/flu/prevent/adjuvant.htm (accessed on 13 August 2022).
- Abreu, R.B.; Clutter, E.F.; Attari, S.; Sautto, G.A.; Ross, T.M. IgA Responses Following Recurrent Influenza Virus Vaccination. Front. Immunol. 2020, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Pica, N.; Iyer, A.; Ramos, I.; Bouvier, N.M.; Fernandez-Sesma, A.; Garcia-Sastre, A.; Lowen, A.C.; Palese, P.; Steel, J. The DBA.2 mouse is susceptible to disease following infection with a broad, but limited, range of influenza A and B viruses. J. Virol. 2011, 85, 12825–12829. [Google Scholar] [CrossRef]
- Dengler, L.; May, M.; Wilk, E.; Bahgat, M.M.; Schughart, K. Immunization with live virus vaccine protects highly susceptible DBA/2J mice from lethal influenza A H1N1 infection. Virol. J. 2012, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Ross, T.M. Bivalent H1 and H3 COBRA Recombinant Hemagglutinin Vaccines Elicit Seroprotective Antibodies against H1N1 and H3N2 Influenza Viruses from 2009 to 2019. J. Virol. 2022, 96, e0165221. [Google Scholar] [CrossRef] [PubMed]
- Sautto, G.A.; Kirchenbaum, G.A.; Ecker, J.W.; Bebin-Blackwell, A.G.; Pierce, S.R.; Ross, T.M. Elicitation of Broadly Protective Antibodies following Infection with Influenza Viruses Expressing H1N1 Computationally Optimized Broadly Reactive Hemagglutinin Antigens. Immunohorizons 2018, 2, 226–237. [Google Scholar] [CrossRef]
- Sautto, G.A.; Kirchenbaum, G.A.; Abreu, R.B.; Ecker, J.W.; Pierce, S.R.; Kleanthous, H.; Ross, T.M. A Computationally Optimized Broadly Reactive Antigen Subtype-Specific Influenza Vaccine Strategy Elicits Unique Potent Broadly Neutralizing Antibodies against Hemagglutinin. J. Immunol. 2020, 204, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, D.; Silva-Moraes, V.; Sautto, G.A.; Hanley, H.B.; Gattiker, J.L.; Jefferson, A.M.; Kolhe, R.; Ross, T.M. The Effect of Waning on Antibody Levels and Memory B Cell Recall following SARS-CoV-2 Infection or Vaccination. Vaccines 2022, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Morell, A.; Terry, W.D.; Waldmann, T.A. Metabolic properties of IgG subclasses in man. J. Clin. Investig. 1970, 49, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.; Rajewsky, K. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 1988, 18, 313–316. [Google Scholar] [CrossRef]
- Wrammert, J.; Onlamoon, N.; Akondy, R.S.; Perng, G.C.; Polsrila, K.; Chandele, A.; Kwissa, M.; Pulendran, B.; Wilson, P.C.; Wittawatmongkol, O.; et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J. Virol. 2012, 86, 2911–2918. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Smith, K.; Miller, J.; Langley, W.A.; Kokko, K.; Larsen, C.; Zheng, N.Y.; Mays, I.; Garman, L.; Helms, C.; et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008, 453, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Manz, R.A.; Hauser, A.E.; Hiepe, F.; Radbruch, A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 2005, 23, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.G.; Light, A.; O’Reilly, L.A.; Ang, S.M.; Strasser, A.; Tarlinton, D. bcl-2 transgene expression inhibits apoptosis in the germinal center and reveals differences in the selection of memory B cells and bone marrow antibody-forming cells. J. Exp. Med. 2000, 191, 475–484. [Google Scholar] [CrossRef]
- Phan, T.G.; Paus, D.; Chan, T.D.; Turner, M.L.; Nutt, S.L.; Basten, A.; Brink, R. High affinity germinal center B cells are actively selected into the plasma cell compartment. J. Exp. Med. 2006, 203, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.M.; Utley, A.; Lee, K.P. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Front. Immunol. 2019, 10, 965. [Google Scholar] [CrossRef]
- Shapiro-Shelef, M.; Calame, K. Regulation of plasma-cell development. Nat. Rev. Immunol. 2005, 5, 230–242. [Google Scholar] [CrossRef]
- Davis, C.W.; Jackson, K.J.L.; McCausland, M.M.; Darce, J.; Chang, C.; Linderman, S.L.; Chennareddy, C.; Gerkin, R.; Brown, S.J.; Wrammert, J.; et al. Influenza vaccine-induced human bone marrow plasma cells decline within a year after vaccination. Science 2020, 370, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.W.; Jackson, K.J.L.; McElroy, A.K.; Halfmann, P.; Huang, J.; Chennareddy, C.; Piper, A.E.; Leung, Y.; Albarino, C.G.; Crozier, I.; et al. Longitudinal Analysis of the Human B Cell Response to Ebola Virus Infection. Cell 2019, 177, 1566–1582.e17. [Google Scholar] [CrossRef] [PubMed]
- Nivarthi, U.K.; Tu, H.A.; Delacruz, M.J.; Swanstrom, J.; Patel, B.; Durbin, A.P.; Whitehead, S.S.; Pierce, K.K.; Kirkpatrick, B.D.; Baric, R.S.; et al. Longitudinal analysis of acute and convalescent B cell responses in a human primary dengue serotype 2 infection model. eBioMedicine 2019, 41, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef]
- Huang, Y.; Franca, M.S.; Allen, J.D.; Shi, H.; Ross, T.M. Next Generation of Computationally Optimized Broadly Reactive HA Vaccines Elicited Cross-Reactive Immune Responses and Provided Protection against H1N1 Virus Infection. Vaccines 2021, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.W.; Kirchenbaum, G.A.; Pierce, S.R.; Skarlupka, A.L.; Abreu, R.B.; Cooper, R.E.; Taylor-Mulneix, D.; Ross, T.M.; Sautto, G.A. High-Yield Expression and Purification of Recombinant Influenza Virus Proteins from Stably-Transfected Mammalian Cell Lines. Vaccines 2020, 8, 462. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”—Emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines 2021, 6, 158. [Google Scholar] [CrossRef]
- Calabro, S.; Tritto, E.; Pezzotti, A.; Taccone, M.; Muzzi, A.; Bertholet, S.; De Gregorio, E.; O’Hagan, D.T.; Baudner, B.; Seubert, A. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine 2013, 31, 3363–3369. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, R.; Pezzicoli, A.; Cioncada, R.; Malzone, C.; De Gregorio, E.; D’Oro, U.; Piccioli, D. Vaccine adjuvant MF59 promotes retention of unprocessed antigen in lymph node macrophage compartments and follicular dendritic cells. J. Immunol. 2015, 194, 1717–1725. [Google Scholar] [CrossRef]
- Liang, F.; Lore, K. Local innate immune responses in the vaccine adjuvant-injected muscle. Clin. Transl. Immunol. 2016, 5, e74. [Google Scholar] [CrossRef]
- Galson, J.D.; Truck, J.; Kelly, D.F.; van der Most, R. Investigating the effect of AS03 adjuvant on the plasma cell repertoire following pH1N1 influenza vaccination. Sci. Rep. 2016, 6, 37229. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Coyle, E.M.; Manischewitz, J.; King, L.R.; Gao, J.; Germain, R.N.; Schwartzberg, P.L.; Tsang, J.S.; Golding, H.; the CHI Consortium. AS03-adjuvanted H5N1 vaccine promotes antibody diversity and affinity maturation, NAI titers, cross-clade H5N1 neutralization, but not H1N1 cross-subtype neutralization. NPJ Vaccines 2018, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Feser, J.; Naficy, A.; Bernstein, D.I.; Guptill, J.; Walter, E.B.; Berlanda-Scorza, F.; Stadlbauer, D.; Wilson, P.C.; Aydillo, T.; et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 2021, 27, 106–114. [Google Scholar] [CrossRef]
- Madsen, A.; Jul-Larsen, A.; Trieu, M.C.; Krammer, F.; Cox, R.J. Persistently high antibody responses after AS03-adjuvanted H1N1pdm09 vaccine: Dissecting the HA specific antibody response. NPJ Vaccines 2021, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Caillet, C.; Piras, F.; Bernard, M.C.; de Montfort, A.; Boudet, F.; Vogel, F.R.; Hoffenbach, A.; Moste, C.; Kusters, I. AF03-adjuvanted and non-adjuvanted pandemic influenza A (H1N1) 2009 vaccines induce strong antibody responses in seasonal influenza vaccine-primed and unprimed mice. Vaccine 2010, 28, 3076–3079. [Google Scholar] [CrossRef] [PubMed]
- Honda-Okubo, Y.; Saade, F.; Petrovsky, N. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 2012, 30, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Gorse, G.J.; Grimes, S.; Buck, H.; Mulla, H.; White, P.; Hill, H.; May, J.; Frey, S.E.; Blackburn, P. A phase 1 dose-sparing, randomized clinical trial of seasonal trivalent inactivated influenza vaccine combined with MAS-1, a novel water-in-oil adjuvant/delivery system. Vaccine 2022, 40, 1271–1281. [Google Scholar] [CrossRef]
- Kim, J.I.; Park, S.; Lee, S.; Lee, I.; Heo, J.; Hwang, M.W.; Bae, J.Y.; Kim, D.; Jang, S.I.; Park, M.S.; et al. DBA/2 mouse as an animal model for anti-influenza drug efficacy evaluation. J. Microbiol. 2013, 51, 866–871. [Google Scholar] [CrossRef]
- Carter, D.M.; Darby, C.A.; Lefoley, B.C.; Crevar, C.J.; Alefantis, T.; Oomen, R.; Anderson, S.F.; Strugnell, T.; Cortes-Garcia, G.; Vogel, T.U.; et al. Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J. Virol. 2016, 90, 4720–4734. [Google Scholar] [CrossRef] [PubMed]
- Giles, B.M.; Ross, T.M. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 2011, 29, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Giles, B.M.; Crevar, C.J.; Carter, D.M.; Bissel, S.J.; Schultz-Cherry, S.; Wiley, C.A.; Ross, T.M. A computationally optimized hemagglutinin virus-like particle vaccine elicits broadly reactive antibodies that protect nonhuman primates from H5N1 infection. J. Infect. Dis. 2012, 205, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shen, Y.; Miller, M.A.; Stabenow, J.; Williams, R.W.; Lu, L. Differing susceptibility of C57BL/6J and DBA/2J mice-parents of the murine BXD family, to severe acute respiratory syndrome coronavirus infection. Cell Biosci. 2021, 11, 137. [Google Scholar] [CrossRef]
- Haidari, G.; Cope, A.; Miller, A.; Venables, S.; Yan, C.; Ridgers, H.; Reijonen, K.; Hannaman, D.; Spentzou, A.; Hayes, P.; et al. Combined skin and muscle vaccination differentially impact the quality of effector T cell functions: The CUTHIVAC-001 randomized trial. Sci. Rep. 2017, 7, 13011. [Google Scholar] [CrossRef]
- Abreu, R.B.; Kirchenbaum, G.A.; Clutter, E.F.; Sautto, G.A.; Ross, T.M. Preexisting subtype immunodominance shapes memory B cell recall response to influenza vaccination. JCI Insight 2020, 5, e132155. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.S.; Ardito, M.; Terry, F.; Levitz, L.; Ross, T.; Moise, L.; Martin, W. Low immunogenicity predicted for emerging avian-origin H7N9: Implication for influenza vaccine design. Hum. Vaccin. Immunother. 2013, 9, 950–956. [Google Scholar] [CrossRef]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz Perez, S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef]
- Ellebedy, A.H.; Jackson, K.J.; Kissick, H.T.; Nakaya, H.I.; Davis, C.W.; Roskin, K.M.; McElroy, A.K.; Oshansky, C.M.; Elbein, R.; Thomas, S.; et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 2016, 17, 1226–1234. [Google Scholar] [CrossRef]
- Forgacs, D.; Abreu, R.B.; Sautto, G.A.; Kirchenbaum, G.A.; Drabek, E.; Williamson, K.S.; Kim, D.; Emerling, D.E.; Ross, T.M. Convergent antibody evolution and clonotype expansion following influenza virus vaccination. PLoS ONE 2021, 16, e0247253. [Google Scholar] [CrossRef]
- Irvine, D.J.; Aung, A.; Silva, M. Controlling timing and location in vaccines. Adv. Drug. Deliv. Rev. 2020, 158, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Diotti, R.A.; Caputo, V.; Sautto, G.A. Conventional and nontraditional delivery methods and routes of vaccine administration. In Practical Aspects of Vaccine Development; Academic Press, Kolhe, P., Ohtake, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–355. [Google Scholar]
- Tegenge, M.A.; Von Tungeln, L.S.; Mitkus, R.J.; Anderson, S.A.; Vanlandingham, M.M.; Forshee, R.A.; Beland, F.A. Pharmacokinetics and biodistribution of squalene-containing emulsion adjuvant following intramuscular injection of H5N1 influenza vaccine in mice. Regul. Toxicol. Pharmacol. 2016, 81, 113–119. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, T.M.; Gokanapudi, N.; Ge, P.; Shi, H.; Richardson, R.A.; Pierce, S.R.; Sanchez, P.; Ullah, S.; De Luca, E.; Sautto, G.A. Kinetic of the Antibody Response Following AddaVax-Adjuvanted Immunization with Recombinant Influenza Antigens. Vaccines 2022, 10, 1315. https://doi.org/10.3390/vaccines10081315
Ross TM, Gokanapudi N, Ge P, Shi H, Richardson RA, Pierce SR, Sanchez P, Ullah S, De Luca E, Sautto GA. Kinetic of the Antibody Response Following AddaVax-Adjuvanted Immunization with Recombinant Influenza Antigens. Vaccines. 2022; 10(8):1315. https://doi.org/10.3390/vaccines10081315
Chicago/Turabian StyleRoss, Ted. M., Naveen Gokanapudi, Pan Ge, Hua Shi, Robert A. Richardson, Spencer R. Pierce, Pedro Sanchez, Subhan Ullah, Eliana De Luca, and Giuseppe A. Sautto. 2022. "Kinetic of the Antibody Response Following AddaVax-Adjuvanted Immunization with Recombinant Influenza Antigens" Vaccines 10, no. 8: 1315. https://doi.org/10.3390/vaccines10081315
APA StyleRoss, T. M., Gokanapudi, N., Ge, P., Shi, H., Richardson, R. A., Pierce, S. R., Sanchez, P., Ullah, S., De Luca, E., & Sautto, G. A. (2022). Kinetic of the Antibody Response Following AddaVax-Adjuvanted Immunization with Recombinant Influenza Antigens. Vaccines, 10(8), 1315. https://doi.org/10.3390/vaccines10081315






