Solid Organ Rejection following SARS-CoV-2 Vaccination or COVID-19 Infection: A Systematic Review and Meta-Analysis
Abstract
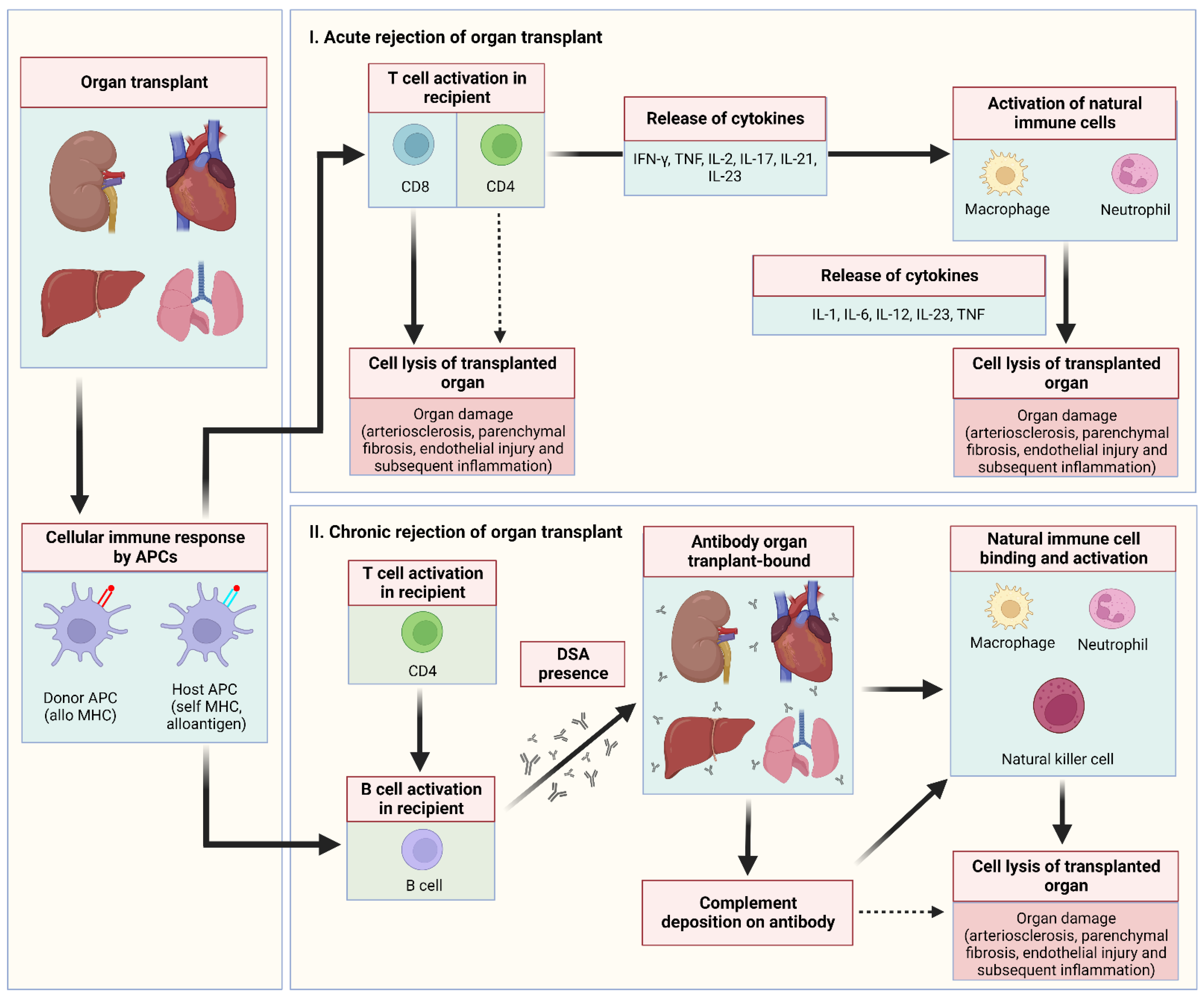
:1. Introduction
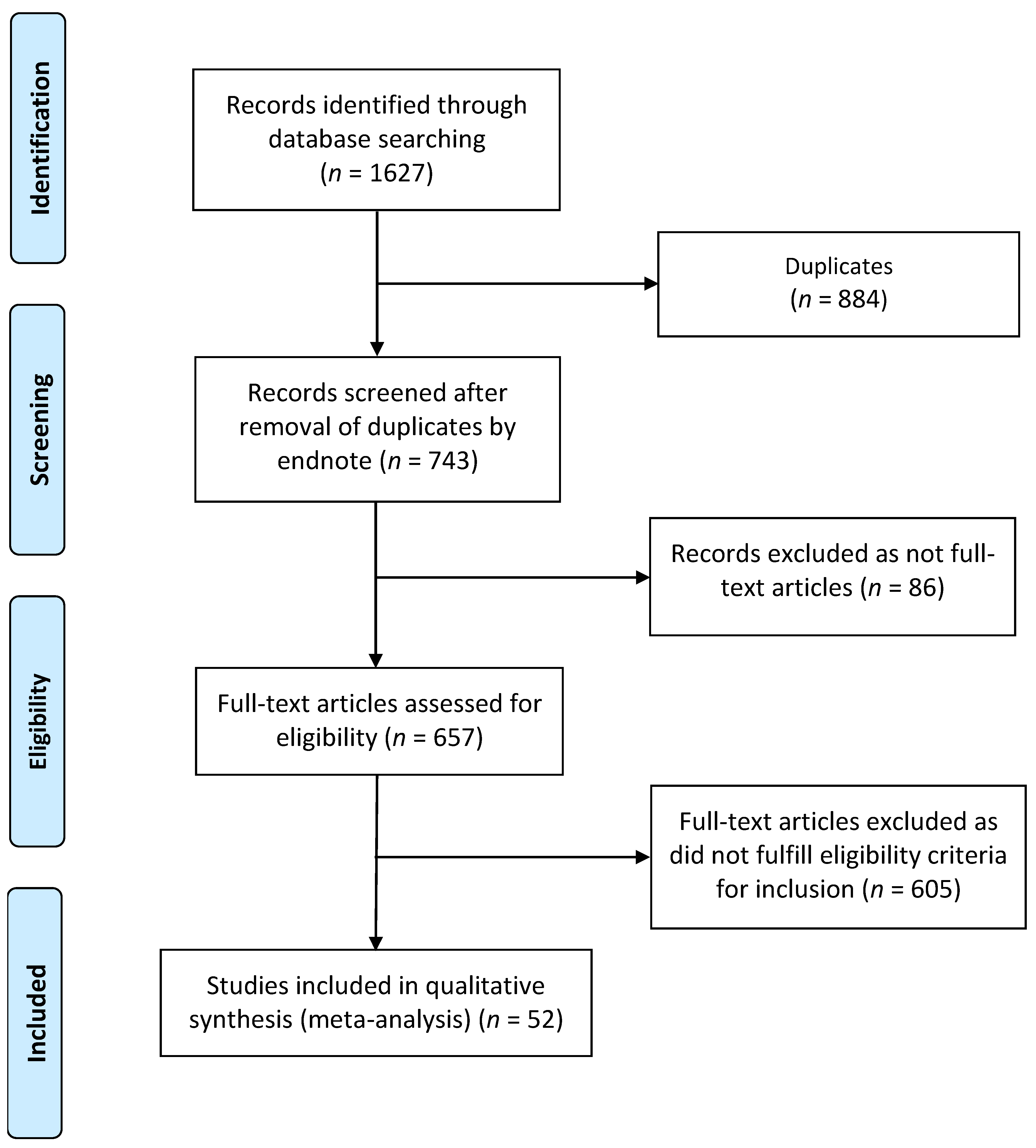
2. Methods
2.1. Design
2.2. Inclusion–Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Analysis
3. Results
3.1. Study Characteristics and Quality
3.2. Meta-Analysis of Organs Rejection Following COVID-19 Vaccination
3.3. Meta-Analysis of Organs Rejection after COVID-19 Infection
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACCR | Acute Cardiac Cellular Rejection |
| AHCR | Acute hepatic cellular rejection |
| APCR | acute pancreatic cellular rejection |
| ARCR | acute renal cellular rejection |
| COVID-19 | Coronavirus disease 2019 |
| NOS | Newcastle–Ottawa scale |
| PRISMA | Preferred Reporting Items for systematic reviews and meta-Analyses |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
References
- Castells, M.C.; Phillips, E.J. Maintaining safety with SARS-CoV-2 vaccines. N. Engl. J. Med. 2021, 384, 643–649. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dhawan, M.; Emran, T.B.; Rabaan, A.A.; Alhumaid, S. COVID-19 vaccine hesitancy–reasons and solutions to achieve a successful global vaccination campaign to tackle the ongoing pandemic. Hum. Vaccines Immunother. 2021, 17, 3495–3499. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Calina, D.; Poulas, K.; Docea, A.O.; Tsatsakis, A.M. Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol. Rep. 2021, 8, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.R.; Mohan, S.; Cohen, D.J.; Husain, S.A.; Dube, G.K.; Ratner, L.E.; Arcasoy, S.; Aversa, M.M.; Benvenuto, L.J.; Dadhania, D.M. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am. J. Transplant. 2020, 20, 1800–1808. [Google Scholar] [CrossRef]
- Tsapepas, D.; Paget, K.; Mohan, S.; Cohen, D.J.; Husain, S.A. Clinically significant COVID-19 following SARS-CoV-2 vaccination in kidney transplant recipients. Am. J. Kidney Dis. 2021, 78, 314–317. [Google Scholar] [CrossRef]
- Balidis, M.; Mikropoulos, D.; Gatzioufas, Z.; de Politis, P.B.; Sidiropoulos, G.; Vassiliadis, V. Acute corneal graft rejection after anti-severe acute respiratory syndrome-coronavirus-2 vaccination: A report of four cases. Eur. J. Ophthalmol. 2021, 11206721211064033. [Google Scholar] [CrossRef]
- Bau, J.T.; Churchill, L.; Pandher, M.; Benediktsson, H.; Tibbles, L.A.; Gill, S. Acute Kidney Allograft Rejection Following Coronavirus mRNA Vaccination: A Case Report. Transplant. Direct 2022, 8, e1274. [Google Scholar] [CrossRef]
- Del Bello, A.; Marion, O.; Delas, A.; Congy-Jolivet, N.; Colombat, M.; Kamar, N. Acute rejection after anti–SARS-CoV-2 mRNA vaccination in a patient who underwent a kidney transplant. Kidney Int. 2021, 100, 238–239. [Google Scholar] [CrossRef]
- Forshaw, T.R.J.; Jørgensen, C.; Kyhn, M.C.; Cabrerizo, J. Acute Bilateral Descemet Membrane Endothelial Keratoplasty Graft Rejection After the BNT162b2 mRNA COVID-19 Vaccine. Int. Med. Case Rep. J. 2022, 15, 201. [Google Scholar] [CrossRef]
- Hume, S.J.; Jackett, L.A.; Testro, A.G.; Gow, P.J.; Sinclair, M.J. A Case Series of Patients with Acute Liver Allograft Rejection After Anti–SARS-CoV-2 mRNA Vaccination. Transplantation 2022, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Akilesh, S.; Nast, C.C.; Yamashita, M.; Henriksen, K.; Charu, V.; Troxell, M.L.; Kambham, N.; Bracamonte, E.; Houghton, D.; Ahmed, N.I. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am. J. Kidney Dis. 2021, 77, 82–93.e1. [Google Scholar] [CrossRef] [PubMed]
- Barros, N.; Sharfuddin, A.A.; Powelson, J.; Yaqub, M.; Adebiyi, O.O.; Beeler, C.; Lutz, A.; Fridell, J.A. Rabbit Anti-Thymocyte Globulin Administration to Treat Rejection in Simultaneous Pancreas and Kidney Transplant Recipients with Recent COVID-19 Infection; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Bitton, K.; Dubois, M.; Courtin, R.; Panthier, C.; Gatinel, D. Descemet’s membrane endothelial keratoplasty (DMEK) rejection following COVID-19 infection: A case report. Am. J. Ophthalmol. Case Rep. 2021, 23, 101138. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.X.; Juthani, V. Acute corneal endothelial graft rejection with coinciding COVID-19 infection. Cornea 2021. [Google Scholar] [CrossRef] [PubMed]
- Kudose, S.; Batal, I.; Santoriello, D.; Xu, K.; Barasch, J.; Peleg, Y.; Canetta, P.; Ratner, L.E.; Marasa, M.; Gharavi, A.G. Kidney biopsy findings in patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1959–1968. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group*, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ott. Hosp. Res. Inst. 2011, 2, 1–12. [Google Scholar]
- Abousy, M.; Bohm, K.; Prescott, C.; Bonsack, J.M.; Rowhani-Farid, A.; Eghrari, A.O.; Bilateral, E.K. Rejection After COVID-19 Vaccine. Eye Contact Lens 2021, 47, 625–628. [Google Scholar] [CrossRef]
- Abu-Khader, A.; Wang, W.; Berka, M.; Galaszkiewicz, I.; Khan, F.; Berka, N. SARS-CoV-2 vaccination induces de novo donor-specific HLA antibodies in a renal transplant patient on waiting list: A case report. HLA 2022, 99, 25–30. [Google Scholar] [CrossRef]
- Al Jurdi, A.; Benedetti Gassen, R.; Borges, T.J.; Solhjou, Z.; Hullekes, F.; Tadeval Lape, I.; Efe, O.; Alghamdi, A.; Patel, P.; Choi, J.Y. Non-invasive monitoring for rejection in kidney transplant recipients after SARS-CoV-2 mRNA vaccination. Front. Immunol. 2022, 743. [Google Scholar] [CrossRef]
- Crnej, A.; Khoueir, Z.; Cherfan, G.; Saad, A. Acute corneal endothelial graft rejection following COVID-19 vaccination. J. Fr. d’Ophtalmol. 2021, 44, e445–e447. [Google Scholar] [CrossRef] [PubMed]
- De la Presa, M.; Govil, A.; Chamberlain, W.D.; Holland, E.J. Acute corneal epithelial rejection of LR-CLAL after SARS-CoV-2 vaccination. Cornea 2022, 41, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Eleiwa, T.K.; Haseeb, A.; Sharawy, A.I.; Higazy, M.T. Acute Corneal Graft Rejection Following COVID-19 Vaccination; Research Square: Durham, NC, USA, 2022. [Google Scholar]
- Fujimoto, H.; Kiryu, J. Incidence of corneal transplant rejection following BNT162b2 SARS-CoV-2 messenger RNA vaccination. J. Ophthalmol. Res. 2021, 4, 279–288. [Google Scholar] [CrossRef]
- Gouvea, L.; Slomovic, A.R.; Chan, C.C. “Smoldering” Rejection of Keratolimbal Allograft. Cornea 2022, 41, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L.; Brunn, J.A.; Jacobs, J.; Todd, P.K.; Askari, F.K.; Fontana, R.J. Guillain-Barré Syndrome After COVID-19 mRNA Vaccination in a Liver Transplantation Recipient with Favorable Treatment Response. Liver Transplant. 2022, 28, 134–137. [Google Scholar] [CrossRef]
- Jang, H.-W.; Bae, S.; Ko, Y.; Lim, S.J.; Kwon, H.E.; Jung, J.H.; yon Cho, H.; Go, H.; Kwon, H.; Kim, Y.H. Acute T cell-mediated rejection after administration of the BNT162b2 mRNA COVID-19 vaccine in a kidney transplant recipient: A case report. Korean J. Transplant. 2021, 35, 253–256. [Google Scholar] [CrossRef]
- Masset, C.; Lebot-Bouras, S.; Branchereau, J.; Renaudin, K.; Cantarovich, D. Pancreas allograft rejection occurring after ChAdOx1 nCoV-19 vaccine. Diabetes Metab. 2022, 48, 101303. [Google Scholar] [CrossRef]
- Molero-Senosiain, M.; Houben, I.; Savant, S.; Savant, V. Five Cases of Corneal Graft Rejection After Recent COVID-19 Vaccinations and a Review of the Literature. Cornea 2022, 41, 669–672. [Google Scholar] [CrossRef]
- Nahata, H.; Nagaraja, H.; Shetty, R. A case of acute endothelial corneal transplant rejection following immunization with ChAdOx1 nCoV-19 coronavirus vaccine. Indian J. Ophthalmol. 2022, 70, 1817–1818. [Google Scholar] [CrossRef]
- Nioi, M.; d’Aloja, E.; Fossarello, M.; Napoli, P.E. Dual corneal-graft rejection after mrna vaccine (Bnt162b2) for COVID-19 during the first six months of follow-up: Case report, state of the art and ethical concerns. Vaccines 2021, 9, 1274. [Google Scholar] [CrossRef]
- Parmar, D.P.; Garde, P.V.; Shah, S.M.; Bhole, P.K. Acute graft rejection in a high-risk corneal transplant following COVID-19 vaccination: A case report. Indian J. Ophthalmol. 2021, 69, 3757–3758. [Google Scholar] [CrossRef]
- Phylactou, M.; Li, J.-P.O.; Larkin, D.F. Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. Br. J. Ophthalmol. 2021, 105, 893–896. [Google Scholar] [CrossRef]
- Rajagopal, R.; Priyanka, T.M. Stromal rejection in penetrating keratoplasty following COVID-19 vector vaccine (Covishield)–A case report and review of literature. Indian J. Ophthalmol. 2022, 70, 319–321. [Google Scholar] [CrossRef]
- Rallis, K.I.; Ting, D.S.; Said, D.G.; Dua, H.S. Corneal graft rejection following COVID-19 vaccine. Eye 2021, 36, 1319–1320. [Google Scholar] [CrossRef]
- Ravichandran, S.; Natarajan, R. Corneal graft rejection after COVID-19 vaccination. Indian J. Ophthalmol. 2021, 69, 1953. [Google Scholar]
- Sarwar, R.; Adeyi, O.A.; Lake, J.; Lim, N. Acute cellular rejection in liver transplant recipients following vaccination against COVID-19: A case series. Liver Transplant. 2022, 28, 1388–1392. [Google Scholar] [CrossRef]
- Shah, A.P.; Dzhaber, D.; Kenyon, K.R.; Riaz, K.M.; Ouano, D.P.; Koo, E.H. Acute corneal transplant rejection after COVID-19 vaccination. Cornea 2022, 41, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Simão, M.F.; Kwitko, S. Corneal Graft Rejection After Inactivated SARS-CoV-2 Vaccine: Case Report. Cornea 2022, 41, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, M.; Lauterio, A.; Crocchiolo, R.; De Carlis, R.; Pugliano, M.; Centonze, L.; Ferla, F.; Zaniboni, M.; Veronese, S.; Podda, G.M. New-onset antibodies to platelet factor 4 following liver transplantation from a donor with vaccine-induced thrombotic thrombocytopenia. Liver Transplant. 2022, 28, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Vnučák, M.; Graňák, K.; Beliančinová, M.; Jeseňák, M.; Macháleková, K.K.; Benko, J.; Samoš, M.; Dedinská, I. Acute kidney rejection after anti-SARS-CoV-2 virus-vectored vaccine—Case report. NPJ Vaccines 2022, 7, 1–4. [Google Scholar] [CrossRef]
- Vyhmeister, R.; Enestvedt, C.K.; VanSandt, M.; Schlansky, B. Steroid-Resistant Acute Cellular Rejection of the Liver After Severe Acute Respiratory Syndrome Coronavirus 2 mRNA Vaccination. Liver Transplant. 2021, 27, 1339–1342. [Google Scholar] [CrossRef]
- Wasser, L.M.; Roditi, E.; Zadok, D.; Berkowitz, L.; Weill, Y. Keratoplasty rejection after the BNT162b2 messenger RNA vaccine. Cornea 2021, 40, 1070. [Google Scholar] [CrossRef]
- Yu, S.; Ritterband, D.C.; Mehta, I. Acute corneal transplant rejection after severe acute respiratory syndrome Coronavirus 2 mRNA-1273 vaccination. Cornea 2022, 41, 257–259. [Google Scholar] [CrossRef]
- Abuzeineh, M.; Tariq, A.; Rosenberg, A.; Brennan, D.C. Chronic active antibody-mediated rejection following COVID-19 infection in a kidney transplant recipient: A case report. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Anandh, U.; Gowrishankar, S.; Sharma, A.; Salama, A.; Dasgupta, I. Kidney transplant dysfunction in a patient with COVID-19 infection: Role of concurrent SARS-CoV-2 nephropathy, chronic rejection and vitamin C-mediated hyperoxalosis: Case report. BMC Nephrol. 2021, 22, 91. [Google Scholar] [CrossRef]
- Asti, A.L.; Lilleri, D.; Gregorini, M.; Zelini, P.; Pattonieri, E.F.; Sepe, V.; Libetta, C.; Islami, T.; Baldanti, F.; Rampino, T. Kidney transplant rejection rate in screened patients for anti-SARS-CoV-2 antibodies, during COVID-19 pandemic in Northern Italy. New Microbiol. 2021, 44, 184–186. [Google Scholar]
- Basic-Jukic, N.; Coric, M.; Bulimbasic, S.; Dika, Z.; Juric, I.; Furic-Cunko, V.; Katalinic, L.; Kos, J.; Fistrek, M.; Kastelan, Z. Histopathologic findings on indication renal allograft biopsies after recovery from acute COVID-19. Clin. Transplant. 2021, 35, e14486. [Google Scholar] [CrossRef]
- Behera, G.; Gokhale, T.; Babu, K.R. Acute Endothelial Graft Rejection Following COVID-19 Infection. Cureus 2021, 1, 3. [Google Scholar] [CrossRef]
- Hanson, P.J.; Liu-Fei, F.; Lai, C.; Toma, M.; McManus, B.M. COVID-19-positivity in a heart transplant recipient—antibody-mediated rejection or SARS-CoV-2-associated cardiac injury? Oxf. Med. Case Rep. 2022, 2, omab143. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, S.; Grins, E.; Larsson, H.; Nilsson, J.; Akbarshahi, H.; Silva, I.; Hyllen, S.; Wagner, D.; Sjögren, J.; Hansson, L. Lung transplant after 6 months on ECMO support for SARS-CoV-2-induced ARDS complicated by severe antibody-mediated rejection. BMJ Open Respir. Res. 2021, 8, e001036. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; An, Y.; Lu, X.; Wu, J. Poor Compliance Causes Acute Rejection in Kidney Transplant Recipients During COVID-19 Pandemic: 2 Cases Report. Patient Prefer. Adherence 2022, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Alteri, C.; Colagrossi, L.; Perricone, G.; Chiappetta, S.; Travi, G.; Campisi, D.; Pugliano, M.T.; Vecchi, M.; Orcese, C. Mild Course of SARS-CoV-2 Infection in a Liver Transplant Recipient Undergoing Plasma Exchange and Defibrotide for Acute Graft Rejection. Transplantation 2021, 105, e22–e24. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Smith, J.; Parajuli, S.; Garg, N.; Aziz, F.; Mandelbrot, D.; Djamali, A.; Zhong, W. Successful management of T-cell mediated rejection in a recent kidney transplant recipient with COVID-19 associated severe acute respiratory syndrome. Transpl. Infect. Dis. 2021, 23, e13598. [Google Scholar] [CrossRef]
- Moriyama, A.S.; de Queiroz Campos, M.S. Presumed DMEK Graft Rejection Associated with COVID-19 Infection. Cornea 2022, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Nourié, N.; Nassereddine, H.; Mouawad, S.; Chebbou, L.; Ghaleb, R.; Abbas, F.; Azar, H. Late antibody-mediated rejection in a kidney transplant recipient: COVID 19 induced? BMC Nephrol. 2022, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Palleschi, A.; Rosso, L.; Morlacchi, L.C.; Del Gobbo, A.; Ramondetta, M.; Gori, A.; Blasi, F.; Nosotti, M. Early acute rejection after lung transplantation mimicking viral pneumonia in the middle of COVID-19 pandemic: A case report. Int. J. Surg. Case Rep. 2020, 77, 80–85. [Google Scholar] [CrossRef]
- Singh, G.; Mathur, U. Acute graft rejection in a COVID-19 patient: Co-incidence or causal association? Indian J. Ophthalmol. 2021, 69, 985. [Google Scholar] [CrossRef]
- Vásquez-Jiménez, E.; Moguel-González, B.; Soto-Abraham, V.; Flores-Gama, C. Risk of acute rejection in kidney transplant recipients after COVID-19. J. Nephrol. 2022, 35, 367–369. [Google Scholar] [CrossRef] [PubMed]
- The International Society of Heart and Lung Transplantation. SARS-CoV-2 Vaccination in Heart and Lung Transplantation: Recommendations from the ISHLT COVID-19 Task Force 2021. Available online: https://ishlt.org/ishlt/media/Documents/COVID19_Vaccine-Recommendations_3-15-2021.pdf (accessed on 25 June 2022).
- Maghsoudlou, P.; Sood, G.; Akhondi, H. Cornea Transplantation; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Chinen, J.; Buckley, R.H. Transplantation immunology: Solid organ and bone marrow. J. Allergy Clin. Immunol. 2010, 125, S324–S335. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, A.; Massera, R.; Maloof, A. Stromal rejection in a deep anterior lamellar keratoplasty following influenza vaccination. Clin. Exp. Ophthalmol. 2015, 43, 838–839. [Google Scholar] [CrossRef]
- Steinemann, T.L.; Koffler, B.H.; Jennings, C.D. Corneal allograft rejection following immunization. Am. J. Ophthalmol. 1988, 106, 575–578. [Google Scholar] [CrossRef]
- Matoba, A. Corneal allograft rejection associated with herpes zoster recombinant adjuvanted vaccine. Cornea 2022, 41, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Vignapiano, R.; Vicchio, L.; Favuzza, E.; Cennamo, M.; Mencucci, R. Corneal graft rejection after yellow fever vaccine: A case report. Ocul. Immunol. Inflamm. 2021, 1–4. [Google Scholar] [CrossRef]
- Lee, E.H.; Li, J.Y. Immunization-Associated Corneal Transplantation Rejection: A Review. Cornea 2022, 41, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Burki, T. Global COVID-19 vaccine inequity. Lancet Infect. Dis. 2021, 21, 922–923. [Google Scholar] [CrossRef]
- Sakamoto, A.; Kawakami, R.; Kawai, K.; Gianatti, A.; Pellegrini, D.; Kutys, R.; Guo, L.; Mori, M.; Cornelissen, A.; Sato, Y. ACE2 (Angiotensin-Converting Enzyme 2) and TMPRSS2 (Transmembrane Serine Protease 2) expression and localization of SARS-CoV-2 infection in the human heart. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 542–544. [Google Scholar] [CrossRef]
- Thunders, M.; Delahunt, B. Gene of the month: TMPRSS2 (transmembrane serine protease 2). J. Clin. Pathol. 2020, 73, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Clement, C.; Arceneaux, L.; Lukiw, W.J. Angiotensin converting enzyme 2 (ACE2) expression in the aged brain and visual system. J. Aging Sci. 2021, 9. [Google Scholar]
- Rabaan, A.A.; Al-Ahmed, S.H.; Muhammad, J.; Khan, A.; Sule, A.A.; Tirupathi, R.; Mutair, A.A.; Alhumaid, S.; Al-Omari, A.; Dhawan, M. Role of inflammatory cytokines in COVID-19 patients: A review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines 2021, 9, 436. [Google Scholar] [CrossRef]
- Mahabadi, N.; Czyz, C.N.; Tariq, M.; Havens, S.J. Corneal Graft Rejection; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Sun, J.; Zhu, A.; Li, H.; Zheng, K.; Zhuang, Z.; Chen, Z.; Shi, Y.; Zhang, Z.; Chen, S.-B.; Liu, X. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microbes Infect. 2020, 9, 991–993. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Oesterreich, S.; Wilde, B.; Eisenberger, U.; Muelling, N.; Horn, P.A.; Heinemann, F.M.; Witzke, O. Sex-specific differences in HLA antibodies after pneumococcal vaccination in kidney transplant recipients. Vaccines 2019, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Cordero, E.; Bulnes-Ramos, A.; Aguilar-Guisado, M.; González Escribano, F.; Olivas, I.; Torre-Cisneros, J.; Gavaldá, J.; Aydillo, T.; Moreno, A.; Montejo, M. Effect of influenza vaccination inducing antibody mediated rejection in solid organ transplant recipients. Front. Immunol. 2020, 11, 1917. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Tsui, K.; Sunder, S.; Ganninger, A.; Tague, L.K.; Witt, C.A.; Byers, D.E.; Trulock, E.P.; Nava, R.; Puri, V. Pseudomonas aeruginosa and acute rejection independently increase the risk of donor-specific antibodies after lung transplantation. Am. J. Transplant. 2020, 20, 1028–1038. [Google Scholar] [CrossRef]
- Mulley, W.R.; Dendle, C.; Ling, J.E.; Knight, S.R. Does vaccination in solid-organ transplant recipients result in adverse immunologic sequelae? A systematic review and meta-analysis. J. Heart Lung Transplant. 2018, 37, 844–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roll, G.R.; Lunow-Luke, T.; Braun, H.J.; Buenaventura, O.; Mallari, M.; Stock, P.G.; Rajalingam, R. COVID-19 does not impact HLA antibody profile in a series of waitlisted renal transplant candidates. Hum. Immunol. 2021, 82, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, P.; Mothi, S.S.; Azzi, Y.; Haverly, M.; Farouk, S.S.; Pérez-Sáez, M.J.; Redondo-Pachón, M.D.; Murphy, B.; Florman, S.; Cyrino, L.G. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am. J. Transplant. 2020, 20, 3140–3148. [Google Scholar] [CrossRef]
- Mamode, N.; Ahmed, Z.; Jones, G.; Banga, N.; Motallebzadeh, R.; Tolley, H.; Marks, S.; Stojanovic, J.; Khurram, M.A.; Thuraisingham, R. Mortality rates in transplant recipients and transplantation candidates in a high-prevalence COVID-19 environment. Transplantation 2021, 105, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Yuan, Q.; Xia, Q.-X.; Zeng, X.-P.; Peng, J.-T.; Liu, J.; Xiao, X.-Y.; Jiang, G.-S.; Xiao, H.-Y. Identification of kidney transplant recipients with coronavirus disease 2019. Eur. Urol. 2020, 77, 742–747. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, X.; Ma, K.; Yang, J.; Guan, H.; Chen, S.; Chen, Z.; Chen, G. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am. J. Transplant. 2020, 20, 1859–1863. [Google Scholar] [CrossRef] [Green Version]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef]
- Alvarez-Aquino, F.G.; Shah, S. Lung transplantation: Challenges in the COVID-19 era, a review of the literature. Curr. Chall. Thorac. Surg. 2021, 3, 29. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Garout, M.A.; Al-Qaaneh, A.M.; Sule, A.A.; Tirupathi, R.; Mutair, A.A.; Alhumaid, S.; Hasan, A.; Dhawan, M. Diverse immunological factors influencing pathogenesis in patients with COVID-19: A review on viral dissemination, immunotherapeutic options to counter cytokine storm and inflammatory responses. Pathogens 2021, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Pszczolinski, R.; Moulin, B. Prediction of Vaccine Response and Development of a Personalized Anti-SARS-CoV-2 Vaccination Strategy in Kidney Transplant Recipients: Results from a Large Single-Center Study. J. Pers. Med. 2022, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Meunier, L.; Sanavio, M.; Dumortier, J.; Meszaros, M.; Faure, S.; Ursic Bedoya, J.; Echenne, M.; Boillot, O.; Debourdeau, A.; Pageaux, G.P. Mycophenolate mofetil decreases humoral responses to three doses of SARS-CoV-2 vaccine in liver transplant recipients. Liver Int. 2022, 42, 1872–1878. [Google Scholar] [CrossRef]
- Caillard, S.; Anglicheau, D.; Matignon, M.; Durrbach, A.; Greze, C.; Frimat, L.; Thaunat, O.; Legris, T.; Moal, V.; Westeel, P.F. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020, 98, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- American Society of Transplantation. Statement on COVID-19 Vaccination in Solid Organ Transplant Recipients (Updated 2 June 2021) 2021. Available online: https://www.myast.org/statement-covid-19-vaccination-solid-organ-transplant-recipients (accessed on 23 June 2022).
- Tsapepas, D.; Husain, S.A.; King, K.L.; Burgos, Y.; Cohen, D.J.; Mohan, S. Perspectives on COVID-19 vaccination among kidney and pancreas transplant recipients living in New York City. Am. J. Health-Syst. Pharm. 2021, 78, 2040–2045. [Google Scholar] [CrossRef]
- Oehler, D.; Bruno, R.R.; Boeken, U.; Westenfeld, R. Moderate acceptance of COVID-19 vaccination in patients pre-and post-heart transplantation: Experiences from a German Transplant Centre. Transpl. Infect. Dis. 2021, 23, e13681. [Google Scholar] [CrossRef]
- Giannini, E.G.; Marenco, S. High acceptance rate of COVID-19 vaccination in liver transplant recipients. J. Hepatol. 2021, 75, 483–484. [Google Scholar] [CrossRef]
- Costantino, A.; Invernizzi, F.; Centorrino, E.; Vecchi, M.; Lampertico, P.; Donato, M.F. COVID-19 vaccine acceptance among liver transplant recipients. Vaccines 2021, 9, 1314. [Google Scholar] [CrossRef]
- Dogan, N.; Hüsing-Kabar, A.; Schmidt, H.H.; Cicinnati, V.R.; Beckebaum, S.; Kabar, I. Acute allograft rejection in liver transplant recipients: Incidence, risk factors, treatment success, and impact on graft failure. J. Int. Med. Res. 2018, 46, 3979–3990. [Google Scholar] [CrossRef] [Green Version]
- Labarrere, C.A.; Jaeger, B.R. Biomarkers of heart transplant rejection: The good, the bad, and the ugly! Transl. Res. 2012, 159, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Redfield, R.; Kaufman, D.; Odorico, J. Diagnosis and treatment of pancreas rejection. Curr. Transplant. Rep. 2015, 2, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shostak, Y.; Shafran, N.; Heching, M.; Rosengarten, D.; Shtraichman, O.; Shitenberg, D.; Amor, S.M.; Yahav, D.; Zvi, H.B.; Pertzov, B. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir. Med. 2021, 9, e52–e53. [Google Scholar] [CrossRef]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021, 325, 1784–1786. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Lu, K.-C.; Kuo, K.-L. The efficacy of COVID-19 vaccines in chronic kidney disease and kidney transplantation patients: A narrative review. Vaccines 2021, 9, 885. [Google Scholar] [CrossRef]
- Tylicki, L.; Dębska-Ślizień, A.; Muchlado, M.; Ślizień, Z.; Gołębiewska, J.; Dąbrowska, M.; Biedunkiewicz, B. Boosting humoral immunity from mRNA COVID-19 vaccines in kidney transplant recipients. Vaccines 2021, 10, 56. [Google Scholar] [CrossRef]
- Caillard, S.; Thaunat, O.; Benotmane, I.; Masset, C.; Blancho, G. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: A case series. Ann. Intern. Med. 2022, 175, 455–456. [Google Scholar] [CrossRef]
- Alejo, J.L.; Mitchell, J.; Chiang, T.P.-Y.; Abedon, A.T.; Boyarsky, B.J.; Avery, R.K.; Tobian, A.A.; Levan, M.L.; Massie, A.B.; Garonzik-Wang, J.M. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: A case series. Transplantation 2021, 105, e280. [Google Scholar] [CrossRef]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of COVID-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef]
- O’Brien, M.P.; Forleo-Neto, E.; Musser, B.J.; Isa, F.; Chan, K.-C.; Sarkar, N.; Bar, K.J.; Barnabas, R.V.; Barouch, D.H.; Cohen, M.S. Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N. Engl. J. Med. 2021, 385, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Abella, B.S.; Jolkovsky, E.L.; Biney, B.T.; Uspal, J.E.; Hyman, M.C.; Frank, I.; Hensley, S.E.; Gill, S.; Vogl, D.T.; Maillard, I. Efficacy and safety of hydroxychloroquine vs. placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: A randomized clinical trial. JAMA Intern. Med. 2021, 181, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Virmani, T.; Pathak, V.; Sharma, A.; Pathak, K.; Kumar, G.; Pathak, D. Artificial Intelligence-Based Data-Driven Strategy to Accelerate Research, Development, and Clinical Trials of COVID Vaccine. BioMed Res. Int. 2022, 2022, 7205241. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Cao, C.; You, M.; Han, S.; Liu, Z.; Xiao, Y.; He, W.; Liu, C.; Peng, P.; Xue, Z. Artificial intelligence-assisted colorimetric lateral flow immunoassay for sensitive and quantitative detection of COVID-19 neutralizing antibody. Biosens. Bioelectron. 2022, 213, 114449. [Google Scholar] [CrossRef]

| Author, Year, Study Location | Study Design, Setting | Age (Years) a | Male, N (%) | Ethnicity b | Time from COVID-19 Vaccination to Organ Rejection (Days) | Comorbidities, N | Vaccine Brand and Dose | New Onset or Relapse | Clinical Presentation | Laboratory Findings | Biopsy Findings c | Imaging | Treatment Initiated after Rejection, N | NOS Score; Graft Failure; and Treatment Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ rejected: LIVER | ||||||||||||||
| Hughes et al. 2022 [27], United States | Retrospective case report, single centre | 65 | 1 [100] | 1 White (Caucasian) | 2 | 1 Cryptogenic cirrhosis 1 Liver transplant recipient 1 Coronary artery disease 1 Diabetes mellitus 1 Hyperlipidaemia | Pfizer-BioNTech, dose 1 [n = 1] | New-onset [n = 1] | 1 Extremity weakness 1 Paraesthesia ascending to bilateral hands 1 Hyporeflexia 1 Loss of pinprick sensation 1 Difficulty with walking 1 Bilateral cranial nerve 7 palsies 1 Acute inflammatory demyelinating polyneuropathy | 1 Raised liver enzymes 1 Raised bilirubin 1 Thrombocytopenia 1 Raised white blood cells 1 High C-reactive protein | Mild AHCR in patient’s graft [n = 1] | Innumerable new bilobar lesions [n = 1] | 1 IVIG 1 Steroid | (NOS, 7) No [n = 1] 1 survived |
| Hume et al. 2022 [11], Australia | Retrospective case-series, single centre | 30.7 ± 15.1 | 0 [0] | 3 Whites (Caucasians) | Mean [SD], 11.3 [3] | 1 Cryptogenic cirrhosis 1 Caroli’s disease 1 Autosomal recessive polycystic kidney disease 1 Biliary atresia | Pfizer-BioNTech, dose 1 [n = 3] | New-onset [n = 2] Relapsed [n = 1] | 1 Liver allograft failure 1 Positive PCR for SARS-CoV-2 | 3 Raised liver enzymes 3 Raised bilirubin | Moderate or severe AHCR in patient’s graft [n = 1] | Not reported [n = 3] | 3 Steroid 3 Tacrolimus 1 Mycophenolate mofetil 1 Ursodeosxycholic acid 1 Plasma exchange 1 Rituximab | (NOS, 8) No [n = 2] Yes [n = 1] 2 survived 1 died |
| Sarwar et al. 2022 [38], United States | Retrospective case-series, single centre | 54 (51–66) | 4 [80] | 5 Whites (Caucasians) | Mean [SD], 11.6 [4.6] | 5 Liver transplant recipients 3 Non-alcoholic steatohepatitis-related cirrhosis 2 Alcohol-related cirrhosis 2 History of acute cellular rejection | Moderna, dose 1 and dose 2 [n = 3] Pfizer-BioNTech, dose 1 and dose 2 [n = 2] | New-onset [n = 3] Relapsed [n = 2] | Not reported [n = 5] | 3 Raised liver enzymes 4 Raised bilirubin | Typical features of T cell-mediated AHCR including portal inflammation of predominantly mixed activated lymphocytes, portal vein phlebitis and bile duct injuries [n = 5] | Not performed [n = 5] | 9 Steroid 1 Everolimus 2 Tacrolimus 1 Cyclosporine 1 Mycophenolate mofetil | (NOS, 6) No [n = 5] 5 survived |
| Valsecchi et al. 2022 [41], Italy | Retrospective case report, single centre | 58 | 0 [0] | 1 White (Caucasian) | 44 | 1 Autoimmune cirrhosis 1 Grade II encephalopathy 1 Refractory ascites 1 End-stage liver disease 1 Liver transplant recipient | Pfizer-BioNTech, dose 1 [n = 1] | New-onset [n = 1] | 1 Worsened neurologic status 1 Vaccine-induced immune thrombotic thrombocytopenia 1 Graft-versus-host disorder 1 Transplantation-mediated alloimmune thrombocytopenia | 1 Low haemoglobin 1 Thrombocytopenia 1 High INR 1 High D-dimer 1 Raised liver enzymes 1 Positive for antibodies directed against (PF4) antibodies | Not performed [n = 1] | Small millimetric high density area on the occipital lobe [n = 1] | 1 Heparin 1 Fondaparinux 1 IVIG 1 Steroid | (NOS, 7) No [n = 1] 1 survived |
| Vyhmeister et al. 2021 [43], United States | Retrospective case report, single centre | 64 | 0 [0] | 1 White (Caucasian) | 11 | 1 Cirrhosis 1 Hepatitis C virus 1 Hepatocellular carcinoma 1 Liver transplant recipient | Moderna, dose 1 [n = 1] | New-onset [n = 1] | 1 Dark urine 1 Fatigue 1 Malaise | 1 Raised liver enzymes | Typical features of AHCR including mixed portal inflammation, bile duct injury and endotheliitis [n = 1] | Unremarkable [n = 1] | 1 Steroid 1 Azathioprine 1 Mycophenolate mofetil 1 Anti-thymocyte globulin | (NOS, 6) No [n = 1] 1 survived |
| Organ rejected: CORNEA | ||||||||||||||
| Abousy et al. 2021 [19], United States | Retrospective case report, single centre | 73 | 0 [0] | 1 White (Caucasian) | 14 | 1 Bilateral Descemet stripping endothelial keratoplasty | Pfizer-BioNTech, dose 2 [n = 1] | New-onset [n = 1] | 1 Bilateral decreased visual acuity 1 Ocular pain 1 Photophobia | Not performed [n = 1] | Not performed [n = 1] | Quiet conjunctiva and sclera [n = 1] Bilateral thickened corneas with Descemet folds [n = 1] | 1 Steroid 1 Sodium chloride hypertonicity | (NOS, 7) No [n = 1] 1 survived |
| Balidis et al. 2021 [7], Greece | Retrospective case reports, single centre | 66.5 (63.2–75) | 2 [50] | 4 Whites (Caucasians) | 7 (5.5–9.2) | 1 Pseudophakic bullous keratopathy 4 Penetrating keratoplasty 1 Fuch’s endothelial corneal dystrophy 1 Hyperdense nuclear cataract 1 Graft rejection on 3 different occasions 1 Herpes simplex keratitis 1 Diabetes mellitus 1 Diabetic macular oedema 1 Herpetic keratitis 1 Extensive post-herpetic corneal scarring | Moderna, dose 1 [n = 1] and dose 2 [n = 1] Oxford Uni-AstraZeneca, dose 1 [n = 2] | New-onset [n = 3] Relapsed [n = 1] | 2 Blurred vision 2 Gradual deterioration of vision | Not performed [n = 4] | Not performed [n = 4] | Subtle corneal oedema [n = 4] Small pigmented keratic precipitates [n = 4] Subepithelial bullae 1 Cells ( + ) in the anterior chamber [n = 1] Increased corneal thickness [n = 3] | 4 Steroid 2 Hypertonic eye drops | (NOS, 8) No [n = 4] 4 survived |
| Crnej et al. 2021 [22], Lebanon | Retrospective case report, single centre | 71 | 1 [100] | 1 Arab | 7 | 1 Hypertension 1 Smoking 1 Coronary artery disease 1 Descemet’s membrane endothelial keratoplasty | Pfizer-BioNTech, dose 1 [n = 1] | New-onset [n = 1] | 1 Painless decrease of vision | Not performed [n = 1] | Not performed [n = 1] | Diffuse corneal oedema [n = 1] | 1 Steroid 1 Valacyclovir | (NOS, 6) No [n = 1] 1 survived |
| de la Presa et al. 2022 [23], United States | Retrospective case report, single centre | 27 | 0 [0] | 1 White (Caucasian) | 15 | 1 No medical history | Moderna, dose 1 [n = 1] | New-onset [n = 1] | 1 Acute redness and irritation of the right eye | Not performed [n = 1] | Not performed [n = 1] | 1+ conjunctival hyperemia [n = 1] Irregular epithelial rejection line [n = 1] Epitheliopathy [n = 1] | 1 Steroid 1 Difluprednate 1 Mycophenolate mofetil | (NOS, 7) No [n = 1] 1 survived |
| Eleiwa et al. 2022 [24], Egypt | Retrospective case report, single centre | 81 | 1 [100] | 1 Arab | 3 | 1 Penetrating keratoplasty 1 Pseudophakic bullous keratopathy | Moderna, dose 2 [n = 1] | New-onset [n = 1] | 1 Painful pink eye 1 Rapid decline in vision 1 Mild flu-like illness | Not performed [n = 1] | Not performed [n = 1] | Diffuse corneal punctate staining [n = 1] Diffuse severe corneal graft oedema [n = 1] Descemet’s folds [n = 1] Scattered keratic precipitates [n = 1] | 1 Steroid 1 Tacrolimus 1 Acyclovir 1 Bandage contact lens was inserted | (NOS, 5) Yes [n = 1] 1 survived |
| Forshaw et al. 2022 [10], Denmark | Retrospective case report, single centre | 94 | 0 [0] | 1 White (Caucasian) | 14 | 1 Fuchs’ endothelial dystrophy 1 Bilateral Descemet membrane endothelial keratoplasty 1 Hypertension 1 Cataract operation | Pfizer-BioNTech, dose 1 [n = 1] | New-onset [n = 1] | 1 Rapid decline in vision 1 Ocular pain | Not performed [n = 1] | Not performed [n = 1] | Diffuse corneal oedema [n = 1] Increased corneal thickness [n = 1] | 1 Steroid 1 Antibiotics 1 Sodium chloride hypertonicity 1 Analgesics 1 re-Descemet membrane endothelial keratoplasty transplantation | (NOS, 8) Yes [n = 1] 1 survived |
| Fujimoto et al. 2021 [25], Japan | Retrospective cohort, multicentre | 80 (50–87) | 5 [71.4] | 7 Asians | Mean [SD], 69 [35.8] | 7 Penetrating keratoplasty 3 Descemet stripping automated endothelial keratoplasty 2 Anterior lamellar keratoplasty 2 Corneal limbal transplantation | Pfizer-BioNTech, dose 1 [n = 1] Pfizer-BioNTech, dose 2 [n = 6] | New-onset [n = 7] | 7 Painful pink eye 7 Rapid decline in vision | Not performed [n = 1] | Not performed [n = 1] | Bullous keratopathy [n = 1] Corneal stromal oedema [n = 7] Cells in the anterior chamber [n = 1] Keratic precipitates [n = 7] Increased corneal thickness [n = 7] | 6 Steroid 2 Tacrolimus 1 Acyclovir | (NOS, 7) No [n = 6] Yes [n = 1] 7 survived |
| Gouvea et al. 2022 [26], Canada | Retrospective case report, single centre | 72 | 1 [100] | 1 White (Caucasian) | 30 | 1 Total limbal stem cell deficiency 1 Penetrating keratoplasty | Pfizer-BioNTech, dose 2 [n = 1] | New-onset [n = 1] | 1 Rapid decline in vision | Not performed [n = 1] | Not performed [n = 1] | Circumferential perilimbal engorgement [n = 1] Stagnation [n = 1] Tortuosity of vessels with mild chemosis [n = 1] | 1 Difluprednate 1 Tacrolimus | (NOS, 6) No [n = 1] 1 survived |
| Molero-Senosiain et al. 2022 [30], United Kingdom | Retrospective case-series, single centre | 61 (51.5–77) | 2 [40] | 4 Whites (Caucasians) 1 Asian | Mean [SD], 16.86 [6.96] for Pfizer-BioNTech Mean [SD], 17 [11.89] for Oxford Uni-AstraZeneca | 2 Descemet stripping automated endothelial keratoplasty 2 Fuchs endothelial dystrophy 3 Penetrating keratoplasty 3 Keratoconus | Pfizer-BioNTech, dose 1 [n = 3] Oxford Uni-AstraZeneca, dose 2 [n = 2] | New-onset [n = 5] | 5 Blurred vision | Not performed [n = 1] | Not performed [n = 1] | Diffuse corneal graft oedema [n = 5] Descemet folds [n = 2] Localized keratic precipitates [n = 1] Mild anterior chamber reaction [n = 1] | 5 Steroid | (NOS, 8) No [n = 5] 5 survived |
| Nahata et al. 2022 [31], India | Retrospective case report, single centre | 28 | 0 [0] | 1 Indian | 14 | 1 Pellucid marginal degeneration 1 Femtosecond laser enabled keratoplasty | Oxford Uni-AstraZeneca, dose 1 [n = 1] | New-onset [n = 1] | 1 Ocular pain 1 Eye redness 1 Blurring of vision | Not performed [n = 1] | Not performed [n = 1] | Stromal oedema with Descemet’s membrane folds [n = 1] Khodadoust line with anterior chamber cells [n = 1] Flare [n = 1] | 1 Steroid 1 Cycloplegics | (NOS, 6) No [n = 1] 1 survived |
| Nioi et al. 2021 [32], Italy | Retrospective case report, single centre | 44 | 0 [0] | 1 White (Caucasian) | 13 | 1 Penetrating keratoplasty 1 Keratoconus | Pfizer-BioNTech, dose 1 [n = 1] | New-onset [n = 1] | 1 Blurred vision 1 Eye redness 1 Eye discomfort | 1 Vitamin D deficiency | Not performed [n = 1] | Ciliary injection [n = 1] Diffuse corneal oedema within the graft [n = 1] Keratic precipitates [n = 1] Descemet folds [n = 1] Anterior chamber cells [n = 1] | 1 Steroid 1 Vitamin D supplement | (NOS, 8) No [n = 1] 1 survived |
| Parmar et al. 2021 [33], India | Retrospective case report, single centre | 35 | 1 [100] | 1 Indian | 2 | 1 Penetrating keratoplasty | Oxford Uni-AstraZeneca, dose 1 [n = 1] | New-onset [n = 1] | 1 Diminished vision | Not performed [n = 1] | Not performed [n = 1] | Microcystic epithelial and stromal corneal graft oedema [n = 1] Few fresh endothelial keratic precipitates [n = 1] | 1 Steroid 1 Cycloplegics | (NOS, 6) No [n = 1] 1 survived |
| Phylactou et al. 2021 [34], United Kingdom | Retrospective case reports, single centre | 66 and 83 | 0 [0] | 2 Whites (Caucasians) | 7 and 21 | 1 Human immunodeficiency virus infection 2 Fuchs endothelial corneal dystrophy 2 Descemet’s membrane endothelial keratoplasty 1 Cataract operation | Pfizer-BioNTech, dose 1 [n = 1] Pfizer-BioNTech, dose 2 [n = 1] | New-onset [n = 2] | 2 Blurred vision 2 Eye redness 2 Photophobia 1 Ocular pain | Not performed [n = 1] | Not performed [n = 1] | Moderate conjunctival injection [n = 2] Diffuse corneal oedema [n = 1] Fine keratic precipitates [n = 2] Anterior chamber inflammation [n = 2] | 2 Steroid | (NOS, 8) No [n = 2] 2 survived |
| Rajagopal et al. 2022 [35], India | Retrospective case report, single centre | 79 | 1 [100] | 1 Indian | 42 | 1 Penetrating keratoplasty 1 Removed right eye 1 Endophthalmitis 1 Descemet’s stripping endothelial keratoplasty 1 Pseudophakic bullous keratopathy 1 Hodgkin’s lymphoma | Oxford Uni-AstraZeneca, dose 2 [n = 1] | New-onset [n = 1] | 1 Diminished vision | Not performed [n = 1] | Not performed [n = 1] | Central stromal oedema [n = 1] | 1 Steroid | (NOS, 6) No [n = 1] 1 survived |
| Rallis et al. 2021 [36], United Kingdom | Retrospective case report, single centre | 68 | 0 [0] | 1 White (Caucasian) | 4 | 1 Bilateral lamellar Descemet Stripping Automated Endothelial Keratoplasty 1 Fuchs’ corneal endothelial dystrophy 1 Left re-do penetrating keratoplasty | Pfizer-BioNTech, dose 1 [n = 1] | New-onset [n = 1] | 1 Painful red eye 1 Rapid deterioration of vision 1 Moderate systemic reactions 1 Chills 1 Myalgia 1 Tiredness | Not performed [n = 1] | Not performed [n = 1] | Conjunctival injection [n = 1] Corneal graft haze [n = 1] Diffuse corneal oedema [n = 1] Descemet’s folds [n = 1] Scattered keratic precipitates [n = 1] Anterior chamber inflammation [n = 1] 1+ cells in anterior chamber [n = 1] | 1 Steroid 1 Acyclovir | (NOS, 8) No [n = 1] 1 survived |
| Ravichandran et al. 2021 [37], India | Retrospective case report, single centre | 62 | 1 [[100]] | 1 Indian | 21 | 1 Penetrating keratoplasty | Oxford Uni-AstraZeneca, dose 1 [n = 1] | New-onset [n = 1] | 1 Congestion and diminished vision | Not performed [n = 1] | Not performed [n = 1] | Khodadoust’s rejection line [n = 1] Corneal oedema [n = 1] Anterior chamber inflammation [n = 1] | 1 Not reported [n = 1] | (NOS, 6) No [n = 1] 1 survived |
| Shah et al. 2022 [39], United States | Retrospective case reports, single centre | 71.5 (63–76.2) | 2 [50] | 3 Whites (Caucasians) 1 Black | 14 (10.2–19.2) | 2 Descemet’s membrane endothelial keratoplasty 1 Pseudophakic bullous keratopathy 1 Contact lens–related Aspergillus keratitis 1 Tectonic sclerokeratoplasty 2 Penetrating keratoplasty 2 Cataract operation 1 Chamber intraocular lens placement 1 Accidental blunt trauma (limited keratoplasty wound dehiscence) 1 Type 2 diabetes mellitus 1 Nonprogressive Salzmann nodular degeneration (left eye) 1 Fuchs endothelial corneal dystrophy 1 Multiple sclerosis | Moderna, dose 1 [n = 1] Moderna, dose 2 [n = 3] | New-onset [n = 4] | 4 Decreased vision in the operated eye 1 Photophobia 1 Brow ache | Not performed [n = 1] | Not performed [n = 1] | Khodadoust’s rejection line [n = 2] Microcystic epithelial and stromal oedema [n = 4] Descemet membrane folds [n = 1] Keratic precipitates [n = 3] Conjunctival injection [n = 2] Anterior chamber cells [n = 1] | 3 Steroid 1 Difluprednate | (NOS, 8) No [n = 4] 4 survived |
| Simão et al. 2022 [40], Brazil | Retrospective case report, single centre | 63 | 0 [0] | 1 Hispanic | 1 | 1 Penetrating keratoplasty 1 Laser in situ keratomileusis 1 Acanthamoeba keratitis 1 Radial keratotomy 1 Fungal keratitis 1 Cataract operation 1 Intraocular lens implantation 1 Trabeculectomy with mitomycin-C 1 Pupilloplasty 1 Glaucoma 1 History of vaccination included influenza vaccine | Sinovac-CoronaVac, dose 1 [n = 1] | Relapsed [n = 1] | 1 Blurred vision 1 Ocular pain 1 Photophobia 1 Eye redness 1 Myalgia | Not performed [n = 1] | Not performed [n = 1] | Corneal oedema [n = 1] Interface fluid accumulation [n = 1] Ciliary injection [n = 1] Increased corneal thickness [n = 1] Anterior chamber reaction [n = 1] | 1 Steroid 1 Polydimethylsiloxane 1 Tacrolimus 1 Timolol 1 Bimatoprost | (NOS, 6) Yes [n = 1] 1 survived |
| Wasser et al. 2021 [44], Israel | Retrospective case reports, single centre | 73 and 56 | 2 [100] | 2 Jewish | 13 and 14 | 2 Penetrating keratoplasty 1 Keratoconus 1 Regraft due to late endothelial failure 1 Keratoconus | Pfizer-BioNTech, dose 1 [n = 2] | New-onset [n = 2] | 1 Eye discomfort 1 Blurred vision 1 Eye redness | Not performed [n = 1] | Not performed [n = 1] | Ciliary injection [n = 1] Corneal oedema [n = 2] Descemet folds [n = 1] Keratic precipitates [n = 2] Anterior chamber cells [n = 1] | 2 Steroid | (NOS, 6) No [n = 2] 2 survived |
| Yu et al. 2022 [45], United States | Retrospective case report, single centre | 51 | 1 [100] | 1 White (Caucasian) | 3 | 1 Keratoconus 1 Penetrating keratoplasty 1 Radial keratotomy 1 Glaucoma | Moderna, dose 1 [n = 1] | New-onset [n = 1] | 1 Eye pain 1 Photophobia 1 Blurred vision | Not performed [n = 1] | Not performed [n = 1] | Corneal oedema [n = 1] Endothelial keratic precipitates [n = 1] | 1 Steroid | (NOS, 7) Yes [n = 1] 1 survived |
| Organ rejected: KIDNEY | ||||||||||||||
| Abu-Khader et al. 2022 [20], Canada | Retrospective case report, single centre | 42 | 1 [100] | 1 White (Caucasian) | 18 | 1 Renal transplant waitlist 1 History of vaccination included influenza, pneumococcal conjugate; and pneumococcal polysaccharide 23 vaccines | Pfizer-BioNTech, dose 1 [n = 1] | New-onset [n = 1] | 1 No clinical presentation | 1 Presence of de novo donor-specific antibodies and strongly positive T and B cells | Not performed [n = 1] | Not performed [n = 1] | 1 Transplant team cancelled the surgery | (NOS, 6) No [n = 1] 1 survived |
| Al Jurdi et al. 2022 [21], United States | Prospective cohort, multicentre | Not reported [n = 1] | Not reported [n = 1] | Not reported [n = 1] | 40 | Not reported [n = 1] | Pfizer-BioNTech, dose 1 [n = 1] | New-onset [n = 1] | Not reported [n = 1] | 1 High creatinine 1 High urinary CXCL9 mRNA | Not reported [n = 1] | Not reported [n = 1] | 1 Tacrolimus 1 Belatacept | (NOS, 6) 1 outcome was not reported |
| Bau et al. 2022 [8], Canada | Retrospective case report, single centre | 53 | 1 [100] | 1 White (Caucasian) | 1 | 1 Hypertension 1 Obstructive sleep apnea 1 Obesity 1 End-stage kidney disease 1 Preemptive living-related kidney transplant | Moderna, dose 2 [n = 1] | New-onset [n = 1] | 1 Fatigue 1 Muscle aches 1 Low blood pressure 1 Acute tubular injury 1 Minimal tubular atrophy | 1 High creatinine 1 New mild proteinuria | Histopathological features were consistent with severe T-cell mediated ARCR [n = 1] | Unremarkable [n = 1] | 1 IV fluids 1 Steroid 1 Antithymocyte globulin 1 IVIG 1 Plasmapheresis | (NOS, 8) No [n = 1] 1 survived |
| Del Bello et al. 2021 [9], France | Retrospective case report, single centre | 23 | 0 [0] | 1 White (Caucasian) | 8 | 1 Nephronophthisis | Pfizer-BioNTech, dose 2 [n = 1] | New-onset [n = 1] | 1 Impaired kidney function | 1 High creatinine 1 Presence of de novo donor-specific antibodies | Histopathological features were consistent with ARCR [n = 1] | Not performed [n = 1] | 1 Steroid 1 Polyclonal antibodies | (NOS, 8) No [n = 1] 1 survived |
| Jang et al. 2021 [28], South Korea | Retrospective case report, single centre | 78 | 0 [0] | 1 Asian | 15 | 1 Hypertension 1 Herpes zoster infection | Pfizer-BioNTech, dose 2 [n = 1] | New-onset [n = 1] | 1 Headache 1 Fever | 1 High creatinine | Histopathological features were consistent with ARCR [n = 1] | Swelling of the transplanted kidney [n = 1] | 1 Steroid | (NOS, 7) No [n = 1] 1 survived |
| Vnučák et al. 2022 [42], Slovakia | Retrospective case report, single centre | 25 | 0 [0] | 1 White (Caucasian) | 14 | 1 Diabetic kidney disease 1 End-stage kidney disease 1 Type 1 diabetes mellitus 1 Hypertension 1 Autoimmune thyroiditis | Oxford Uni-AstraZeneca, dose 1 [n = 1] | New-onset [n = 1] | 1 Fatigue 1 General weakness 1 Vomiting 1 Inability to eat or drink 1 High risk of septic complications | 1 High creatinine 1 High urea 1 Low haemoglobin 1 High C-reactive protein 1 Low pH 1 Presence of de novo donor-specific antibodies 1 Leukocytosis 1 Escherichia coli (urine culture) | Histopathological features were consistent with ARCR [n = 1] | Unremarkable [n = 1] | 1 Steroid 1 IV fluids 1 Immunosuppressants 1 IVIG 1 Plasmapheresis 1 Diuretics 1 Rituximab | (NOS, 7) Yes [n = 1] 1 survived |
| Organ rejected: PANCREAS | ||||||||||||||
| Masset et al. 2021 [29], France | Retrospective case report, single centre | 51 | 0 [0] | 1 White (Caucasian) | 1 | 1 Type 1 diabetes mellitus | Oxford Uni-AstraZeneca, dose 1 [n = 1] | New-onset [n = 1] | 1 Weakness 1 Fever 1 Polyuria 1 Polydipsia 1 Hyperglycemia 1 Ketoacidosis | 1 Elevation of lipasemia 1 Decline of the C-peptide level 1 Eosinophilia 1 Positive auto-antibodies for anti-ZnT8, anti-GAD65 and anti-islet cell | Histopathological features were consistent with APCR [n = 1] | Unremarkable [n = 1] | 1 Steroid 1 Antithymocyte globulin | (NOS, 8) No [n = 1] 1 survived |
| Author, Year, Study Location | Study Design, Setting | Age (Years) a | Male, N (%) | Ethnicity b | Method Used to Detect COVID-19 | Symptoms of COVID-19 Infection | Time from COVID-19 Infection to Organ Rejection (Days) | Comorbidities, N | Clinical Presentation | Laboratory Findings | Biopsy Findings c | Imaging | Treatment initiated after rejection, n | NOS score; Graft Failure; and Treatment Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ rejected: KIDNEY | ||||||||||||||
| Abuzeineh et al. 2021 [46], United States | Retrospective case report, single centre | 45 | 1 [100] | 1 Black | rt-PCR [n = 1] | 1 Fever 1 Watery diarrhoea 1 Nausea 1 Vomiting 1 Loss of taste and smell 1 Increased lethargy 1 Reduced oral intake 1 Dry mucous membranes 1 Hypoxemia | 73 | 1 Diabetes mellitus 1 End-stage kidney disease 1 Hypertensive nephrosclerosis | 1 Weight gain 1 Bilateral lower limb and scrotal oedema 1 Hypertension | 1 Presence of de novo donor-specific antibodies 1 Elevated plasma donor-derived cell-free deoxyribonucleic acid 1 High creatinine 1 High body urea nitrogen 1 High ferritin 1 High erythrocyte sedimentation rate 1 High C-reactive protein 1 High interleukin-6 1 Proteinuria | Histopathological features were consistent with ARCR [n = 1] | Bilateral coarse crepitations over lower lung zones [n = 1] Bilateral peripheral patchy opacities [n = 1] Mild hydronephrosis (renal allograft) [n = 1] | 1 IVIG 1 Mycophenolate mofetil 1 IV fluids 1 Oxygen supplementation 1 Antibiotics 1 Antifungals 1 Acyclovir 1 Prone position 1 Tocilizumab 1 Inserted Foley’s catheter 1 Diuretics | (NOS, 7) No [n = 1] 1 survived |
| Akilesh et al. 2021 [12], United States | Retrospective case-series, multicentre | 47 and 54 | 1 [50] | 1 Black and 1 Asian | rt-PCR [n = 2] | 1 Sore throat 1 Nasal congestion 1 Anosmia 1 Cough 1 Malaise 1 Pleuritic chest 1 Pain 2 Fever 1 Nausea 1 Vomiting 1 Acute respiratory failure | 4 and 42 | 1 Human immunodeficiency virus infection 2 Hypertension 1 Diabetes mellitus 1 Focal segmental glomerulosclerosis | 2 Acute kidney injury 1 Oedema | 2 High creatinine 1 Low haemoglobin 1 Thrombocytopenia 2 Proteinuria 1 Presence of de novo donor-specific antibodies | Histopathological features were consistent with ARCR [n = 2] | Immunoglobulin A nephropathy [n = 1] Focal segmental glomerulosclerosis [n = 1] Thrombotic microangiopathy [n = 1] | 2 Steroid 2 Antihypertensives 2 Diuretics 2 Haemodialysis 1 IVIG 1 Rituximab 1 Plasma exchange | (NOS, 8) No [n = 2] 2 survived |
| Anandh et al. 2021 [47], India | Retrospective case report, single centre | 56 | 1 [100] | 1 Indian | rt-PCR [n = 1] | 1 Fever 1 Diarrhoea 1 Tachypnea 1 Low oxygen saturations | 14 | 1 High dose of supplemental Vitamin C 1 Ischemic heart disease 1 Percutaneous transluminal coronary angioplasty | 1 Reduced urine output 1 Swelling of legs 1 Progressive breathlessness 1 Acute tubular injury 1 Extensive oxalate crystal deposition 1 Deterioration of cardiac function (Ejection fraction of 20%) | 1 High creatinine 1 Raised serum pro-BNP level 1 High C-reactive protein 1 High D-dimer | Histopathological features were consistent with ARCR [n = 1] Presence of extensive oxalate deposition in the tubules [n = 1] | Spherical spiked particles in the glomerular capillary endothelium [n = 1] Tubulo-reticular inclusions [n = 1] Moderate left ventricular dysfunction [n = 1] Mosaic attenuation of both lungs [n = 1] Ground glass opacities [n = 1] | 1 IV fluids 1 Antibiotics 1 Steroid 1 HCQ 1 Zinc 1 Vitamin C 1 Tacrolimus 1 Haemodialysis 1 Anticoagulation 1 Remdesivir | (NOS, 7) Yes [n = 1] 1 died |
| Asti et al. 2021 [48], Italy | Retrospective case-series, multicentre | 59 and 51 | 2 [100] | 2 Whites (Caucasians) | IgG anti SARS-CoV-2 and SARS-CoV-2 nucleic capsid protein [n = 2] | 2 Fever 1 Cough 2 Diarrhoea 1 Nausea 1 Phlegm 1 Asthenia 2 Dyspnoea 1 Conjunctivitis | Not reported [n = 2] | Not reported [n = 2] | Not reported [n = 2] | Not reported [n = 2] | Not reported [n = 2] | Not reported [n = 2] | 1 Cyclosporine 2 Steroids 1 Tacrolimus | (NOS, 7) No [n = 2] 2 survived |
| Barros et al. 2020 [13], United States | Retrospective case reports, single centre | 53 and 46 | 1 [50] | 2 Whites (Caucasians) | rt-PCR and IgG anti SARS-CoV-2 [n = 2] | 1 Mild COVID-19 1 Asymptomatic COVID-19 | 20 and not reported [n = 1] | 2 Simultaneous pancreas and kidney transplant | Not reported [n = 2] | 1 Elevated lipase levels 1 High creatinine 2 High HbA1c 1 Presence of de novo donor-specific antibodies | Not reported [n = 2] | Fat stranding surrounding both kidney and pancreas allografts [n = 1] | 1 Steroid 1 Plasma exchange 1 Rituximab 1 IVIG 2 Anti-thymocyte globulin 1 Haemodialysis 1 Stent placement | (NOS, 8) No [n = 2] 2 survived |
| Basic-Jukic et al. 2021 [49], Croatia | Retrospective case-series, multicentre | 40, 53 and 31 | 1 [33.3] | 3 Whites (Caucasians) | rt-PCR [n = 3] | 2 Fever 1 Cough 1 Dyspnoea 1 Diarrhoea 1 Asymptomatic COVID-19 | Not reported [n = 3] | 1 Lupus nephropathy 1 Autosomal dominant polycystic kidney disease 1 Unknown primary kidney disease 3 Arterial hypertension 1 Diabetes mellitus 1 Peripheral upper arm embolization 1 Disseminated cryptococcal infection | 1 Acute tubular injury 1 Proteinuria 3 Peripheral oedema | 1 High C-reactive protein 3 High leucocytes 1 High D-dimer | Inflammatory infiltration within the tubulointerstitial department [n = 1] Mononuclear infiltration [n = 1] Mild tubulitis [n = 1] Capillaritis [n = 1] | Bilateral imaging confirmed pneumonia [n = 3] | 3 Anticoagulation 1 Antibiotics 1 Haemodialysis 2 IVIG | (NOS, 7) No [n = 3] 3 survived |
| Kudose et al. 2020 [16], United States | Retrospective case-series, multicentre | 54 | 1 [100] | 1 Balck | rt-PCR [n = 1] | 1 Asymptomatic COVID-19 | Not reported [n = 1] | 1 End-stage kidney disease 1 IgA nephropathy 1 Hypertension 1 Obesity | 1 Acute kidney injury | 1 High creatinine 1 Low haemoglobin | Severe lymphocytic tubulitis [n = 1] Focal interstitial fibrosis [n = 1] Mild vascular sclerosis [n = 1] | Unremarkable [n = 1] | 1 Tocilizumab 1 IVIG 1 Steroids | (NOS, 8) No [n = 1] 1 survived |
| Ma et al. 2022 [53], China | Retrospective case report, single centre | 32 and 33 | 2 [100] | 2 Asian | rt-PCR [n = 2] | 1 Nausea 1 Vomiting 1 Diarrhoea | Not reported [n = 2] | 1 IgA nephropathy | 1 Glomerulonephritis 1 Polyuria 1 Foamy urine 1 Nocturia 1 Stomachache 1 Reduced urine output | 2 High creatinine 2 Proteinuria 1 High C-reactive protein | Histopathological features were consistent with ARCR [n = 2] | Not reported [n = 2] | 2 Steroids 2 Mycophenolate mofetil 2 Tacrolimus 1 IVIG 1 Antithymocyte globulin | (NOS, 6) No [n = 2] 2 survived |
| Mohamed et al. 2021 [55], United States | Retrospective case report, single centre | 33 | 0 [0] | 1 White (Caucasian) | rt-PCR and IgG anti SARS-CoV-2 [n = 1] | 1 Shortness of breath 1 Pulse-oximetry (SpO2) ranging from 55–78% 1 Hypoxia 1 Tachypnea 1 Labored breathing 1 2 plus pitting oedema | 5 | 1 Congenital single kidney 1 Minimal change disease 1 Non-ischemic cardiomyopathy 1 Mitral valve repair 1 Obstructive sleep apnea 1 Failed living-related kidney transplant 1 Ureteric stent | 1 Acute kidney injury 1 Isolated vasculitis | 1 High creatinine 1 High D-dimer 1 Hematuria 1 1 Isolation of E. Faecium (bacteriuria) | Histopathological features were consistent with ARCR [n = 1] | New diffuse airspace opacities [n = 1] Severe intimal arteritis and hyperplasia [n = 1] | 1 Endotracheal intubation 1 Mechanical ventilation 1 Bilevel positive airway pressure 1 Convalescent plasma 1 Remdesivir 1 Antibiotics 1 Oxygen supplementation 1 Steroid | (NOS, 8) No [n = 1] 1 survived |
| Nourié et al. 2022 [57], Lebanon | Retrospective case report, single centre | 54 | 1 [100] | 1 Arab | rt-PCR [n = 1] | 1 Fatigue 1 Fever | Not reported [n = 1] | 1 Focal and segmental glomerulosclerosis 1 Haemodialysis | 1 Global glomerulitis 1 Moderate capillaritis 1 Thrombotic microangiopathy affecting arterioles and glomeruli | 1 High C-reactive protein 1 Raised white blood cells 1 High creatinine 1 Presence of de novo donor-specific antibodies | Histopathological features were consistent with ARCR [n = 1] | Multiple well-defined ground glass opacities [n = 1] | 1 Acetaminophen 1 Oral hydration 1 Mycophenolate mofetil 1 Tacrolimus 1 Steroids 1 IVIG 1 Plasma exchange | (NOS, 6) No [n = 1] 1 survived |
| Vásquez-Jiménez et al. 2022 [60], Mexico | Retrospective case-series, single centre | 34 (30–37) | 10 (71.4) | 14 Hispanics | rt-PCR [n = 14] | Not reported [n = 14] | Not reported [n = 14] | 1 Hypertension 2 Retransplants 4 Previous rejections | 8 Acute kidney injuries | Not reported [n = 14] | Histopathological features were consistent with ARCR [n = 14] | Tubulitis [n = 14] Glomerulitis [n = 14] Inflammation in non-scarred cortex [n = 13] Peritubular capillaritis [n = 13] Tubular atrophy [n = 13] Chronic glomerulopathy [n = 4] Endarteritis [n = 3] | 10 Steroids 10 Mycophenolate mofetil 10 Tacrolimus 2 Azathioprine 2 Anti-thymocyte globulin 5 Rituximab | (NOS, 6) No [n = 14] 14 survived |
| Organ rejected: LIVER | ||||||||||||||
| Merli et al. 2021 [54], Italy | Retrospective case report, single centre | 50 | 0 [0] | 1 White (Caucasian) | rt-PCR and IgG anti SARS-CoV-2 [n = 1] | 1 Fever | Not reported [n = 1] | 1 Sclerosing cholangitis 1 Refractory ascites 1 Tacrolimus-induced sinusoidal obstruction syndrome | Not reported [n = 1] | 14 Presence of de novo donor-specific antibodies | Histopathological features were consistent with AHCR [n = 1] | Not reported [n = 1] | 1 Anticoagulation 1 Defibrotide 1 Plasma exchange 1 Human albumin 1 IVIG 1 Velpatasvir and sofosbuvir | (NOS, 7) No [n = 1] 1 survived |
| Organ rejected: CORNEA | ||||||||||||||
| Behera et al. 2021 [50], India | Retrospective case report, single centre | 57 | 0 [0] | 1 Indian | rt-PCR [n = 1] | 1 Nausea 1 Vomiting 1 Cough 1 Mild breathlessness | 2 | 1 Penetrating keratoplasty | 1 Acute-onset painful diminution of vision 1 Injury with vegetative matter | 1 Isolation of Candida species (cornea) | Not performed [n = 1] | Central corneal ulcer [n = 1] Stromal thinning [n = 1] Ground glass opacities [n = 1] Keratic precipitates [n = 1] Posterior synechiae [n = 1] Inflammatory iris nodules 3+ [n = 1] Anterior chamber cells [n = 1] | 1 Antibiotics 1 Antifungals 1 Steroid 1 Cycloplegics 1 Lubricants 1 Anticoagulation 1 Oxygen supplementation | (NOS, 6) Yes [n = 1] 1 survived |
| Bitton et al. 2021 [14], France | Retrospective case report, single centre | 60 | 0 [0] | 1 White (Caucasian) | rt-PCR and IgG anti SARS-CoV-2 [n = 1] | 1 Anosmia 1 Fever 1 Arthralgia | 21 | 1 Fuch’s dystrophy 1 Descemet’s Membrane Endothelial Keratoplasty | 1 Eye redness 1 Vision loss | Not reported [n = 1] | Not performed [n = 1] | Mild conjunctival hyperemia [n = 1] Multiple granulomatous keratic precipitates [n = 1] Deep anterior chamber with 1+ cells [n = 1] Increased corneal thickness [n = 1] | 1 Steroid 1 Cyclosporine | (NOS, 6) No [n = 1] 1 survived |
| Jin et al. 2021 [15], United States | Retrospective case report, single centre | 31 | 0 [0] | 1 Black | rt-PCR and IgG anti SARS-CoV-2 [n = 1] | 1 Dysgeusia 1 Fever | 5 | 1 Asthma 1 Obstructive sleep apnea 1 Obesity 1 Bilateral keratoconus 1 Penetrating keratoplasty | 1 Ocular pain 1 Eye redness 1 Worsened vision | Not reported [n = 1] | Not performed [n = 1] | Conjunctival injection [n = 1] Increased corneal thickness [n = 1] Microcystic and stromal oedema [n = 1] Diffuse keratic precipitates [n = 1] | 1 Steroid | (NOS, 7) No [n = 1] 1 survived |
| Moriyama et al. 2022 [56], Brazil | Retrospective case report, single centre | 77 and 69 | 0 [0] | 2 Whites (Caucasians) | rt-PCR [n = 2] | Not reported [n = 1] | Not reported [n = 1] | 2 Descemet’s membrane endothelial keratoplasty 2 Fuchs dystrophy 2 Age-related macular degeneration 1 Glaucoma | 2 Conjunctivitis 1 Mild ocular discomfort 1 Tearing 1 Eye redness 2 Worsened vision 1 Mild transient inflammatory ocular symptoms | Not reported [n = 1] | Not performed [n = 1] | Mild corneal oedema [n = 2] | 2 Steroid 1 A new Descemet membrane endothelial keratoplasty procedure | (NOS, 6) No [n = 1] Yes [n = 1] 2 survived |
| Singh et al. 2021 [59], India | Retrospective case report, single centre | 32 | 1 [100] | 1 Indian | rt-PCR [n = 1] | 1 Sore throat 1 Fever 1 Malaise 1 Acute respiratory distress syndrome | 21 | 1 Penetrating keratoplasty 1 Cataract operation 1 Posterior chamber intraocular lens implantation 1 Glaucoma | 1 Diminished vision 1 Eye redness 1 Eye discomfort | 1 High interleukin-6 1 High C-reactive protein 1 High lactate dehydrogenase | Not performed [n = 1] | Multiple epithelial bullae [n = 1] Diffuse stromal oedema [n = 1] Few descemet folds [n = 1] Keratic precipitates [n = 1] | 1 Steroid | (NOS, 6) No [n = 1] 1 survived |
| Organ rejected: HEART | ||||||||||||||
| Hanson et al. 2022 [51], Canada | Retrospective case report, single centre | 57 | 0 [0] | 1 White (Caucasian) | rt-PCR [n = 1] | 1 Hypoxemia 1 Shortness of breath | 7 | 1 Ischemic cardiomyopathy 1 Heart failure 1 Cardiogenic shock 1 Deterioration of cardiac function (Ejection fraction of 11%) 1 Ex-smoker 1 Atrial fibrillation 1 Diabetes mellitus 1 Chronic kidney disease 1 Transient ischemic attack 1 Chronic obstructive pulmonary disease | 1 Increased oxygen requirements | 1 Presence of de novo donor-specific antibodies | Histopathological features were consistent with ACCR [n = 1] | Pleural effusion [n = 1] Ground-glass lung phenotype [n = 1] | 1 Steroid 1 Tacrolimus 1 Mycophenolate mofetil 1 Acetylsalicylic acid 1 Pravastatin | (NOS, 7) No [n = 1] 1 survived |
| Organ rejected: LUNG | ||||||||||||||
| Lindstedt et al. 2021 [52], Sweden | Retrospective case report, single centre | 62 | 1 [100] | 1 White (Caucasian) | rt-PCR [n = 1] | 1 Hypoxia 1 Dyspnoea 1 Cough 1 Fever 1 SARS-CoV-2-induced acute respiratory distress syndrome | Not reported [n = 1] | 1 Diabetes mellitus 1 Myocardial infarction | 1 Cerebral haemorrhage 1 Bloodstream infections 1 Respiratory failure 1 End-stage lung disease 1 Development of cor pulmonale | 1 Presence of de novo donor-specific antibodies | Non-specific inflammation [n = 1] Scattered fibrosis deposits [n = 1] | Progressive lung disease [n = 1] Bilateral airspace opacities [n = 1] Diffuse consolidation [n = 1] Air bronchograms [n = 1] Ground-glass opacities [n = 1] Consolidation [n = 1] Interstitial thickening [n = 1] | 1 Steroid 1 Plasmapheresis 1 Endotracheal intubation 1 Rituximab 1 IVIG 1 Tacrolimus 1 Remdesivir 1 Prone position 1 Extracorporeal membrane oxygenation (for 6 months) 1 Percutaneous tracheostomy 1 Dornase alfa 1 Mechanical ventilation | (NOS, 7) Yes [n = 1] 1 died |
| Palleschi et al. 2020 [58], Italy | Retrospective case report, single centre | 31 | 1 [100] | 1 White (Caucasian) | Not reported [n = 1] | 1 Fever | Not reported [n = 1] | 1 Cystic fibrosis | 1 Bilateral bronchorrhea 1 Persistent hyperpyrexia 1 Mild respiratory failure 1 Dyspnoea | 1 Presence of de novo donor-specific antibodies 1 Chronic colonization of Pseudomonas aeruginosa and Mycobacterium kansasii | Not performed [n = 1] | Bilateral confluent diffuse airspace opacities [n = 1] | 1 Mechanical ventilation 1 Oxygen supplementation 1 Tacrolimus 1 Steroids 1 Azathioprine 1 Antibiotics 1 Antifungals 1 Ethambutol 1 Plasmapheresis 1 Endotracheal intubation | (NOS, 7) Yes [n = 1] 1 died |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhumaid, S.; Rabaan, A.A.; Dhama, K.; Yong, S.J.; Nainu, F.; Hajissa, K.; Dossary, N.A.; Alajmi, K.K.; Saggar, A.E.A.; AlHarbi, F.A.; et al. Solid Organ Rejection following SARS-CoV-2 Vaccination or COVID-19 Infection: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1289. https://doi.org/10.3390/vaccines10081289
Alhumaid S, Rabaan AA, Dhama K, Yong SJ, Nainu F, Hajissa K, Dossary NA, Alajmi KK, Saggar AEA, AlHarbi FA, et al. Solid Organ Rejection following SARS-CoV-2 Vaccination or COVID-19 Infection: A Systematic Review and Meta-Analysis. Vaccines. 2022; 10(8):1289. https://doi.org/10.3390/vaccines10081289
Chicago/Turabian StyleAlhumaid, Saad, Ali A. Rabaan, Kuldeep Dhama, Shin Jie Yong, Firzan Nainu, Khalid Hajissa, Nourah Al Dossary, Khulood Khaled Alajmi, Afaf E. Al Saggar, Fahad Abdullah AlHarbi, and et al. 2022. "Solid Organ Rejection following SARS-CoV-2 Vaccination or COVID-19 Infection: A Systematic Review and Meta-Analysis" Vaccines 10, no. 8: 1289. https://doi.org/10.3390/vaccines10081289
APA StyleAlhumaid, S., Rabaan, A. A., Dhama, K., Yong, S. J., Nainu, F., Hajissa, K., Dossary, N. A., Alajmi, K. K., Saggar, A. E. A., AlHarbi, F. A., Aswany, M. B., Alshayee, A. A., Alrabiah, S. A., Saleh, A. M., Alqarni, M. A., Gharib, F. M. A., Qattan, S. N., Almusabeh, H. M., AlGhatm, H. Y., ... Al Mutair, A. (2022). Solid Organ Rejection following SARS-CoV-2 Vaccination or COVID-19 Infection: A Systematic Review and Meta-Analysis. Vaccines, 10(8), 1289. https://doi.org/10.3390/vaccines10081289









