Association between Stroke Risk and Influenza Vaccination in Patients with Gout: A Nationwide Population-Based Study
Abstract
1. Introduction
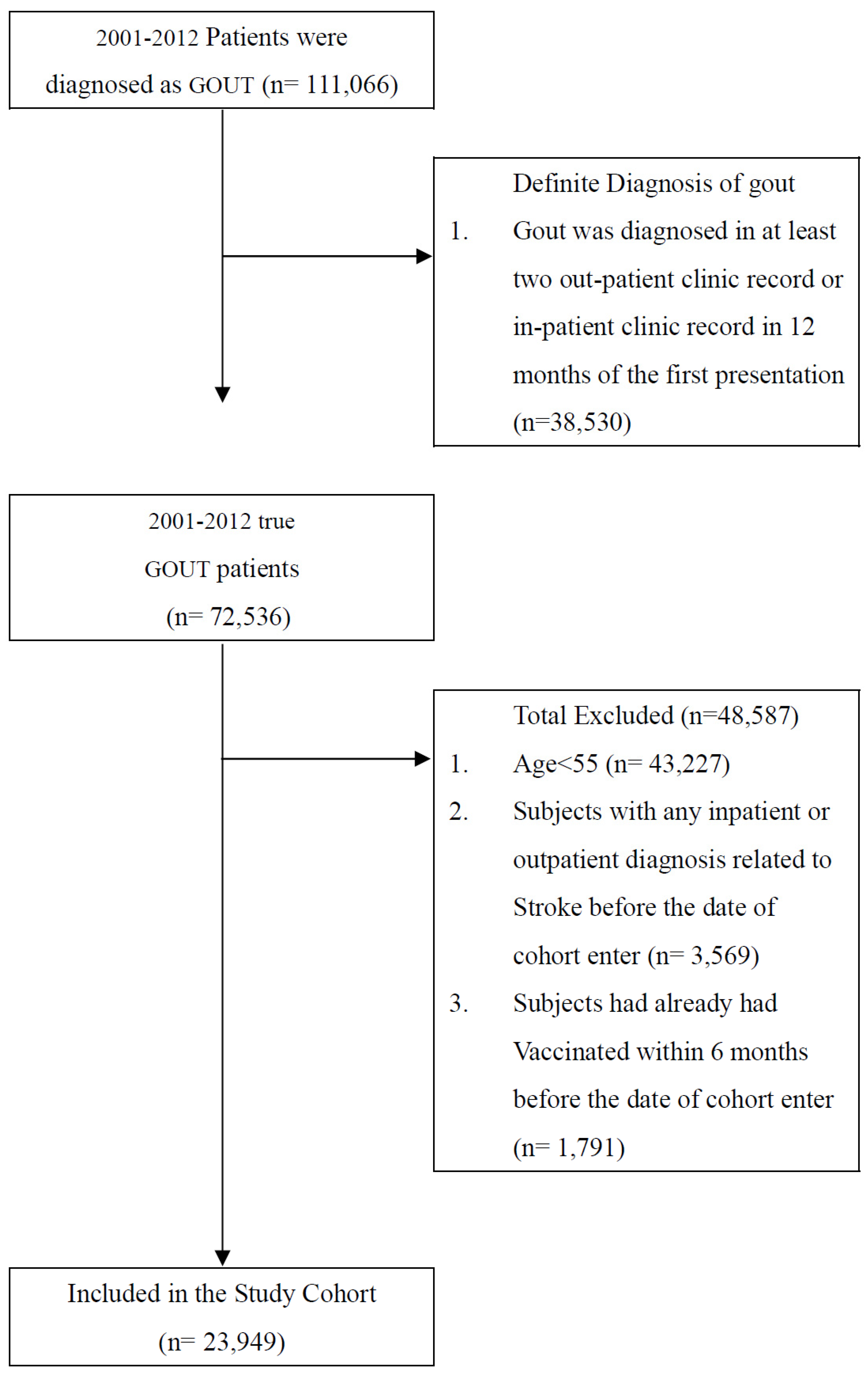
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. Risk of Stroke in Vaccinated and Unvaccinated Patients in Accordance with Age and Sex
3.3. Association between Number of Influenza Vaccinations and Risk Reduction of Hemorrhagic Stroke
3.4. Association between Number of Influenza Vaccinations and Reduction of Ischemic Stroke Risk
4. Discussion
4.1. Main Findings
4.2. Association between Stroke Development and Gout
4.3. Association between Stroke Risk and Influenza Vaccination
4.4. Different Postvaccination Stroke Risk Depending on Age and Sex
4.5. Effect of Medications on Stroke Risk
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, E.; Hoy, D.; Cross, M.; Merriman, T.R.; Vos, T.; Buchbinder, R.; Woolf, A.; March, L. The global burden of gout: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1470–1476. [Google Scholar] [CrossRef]
- Kuo, C.F.; Grainge, M.J.; Zhang, W.; Doherty, M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 2015, 11, 649–662. [Google Scholar] [CrossRef]
- Chu, N.F.; Wang, D.J.; Liou, S.H.; Shieh, S.M. Relationship between hyperuricemia and other cardiovascular disease risk factors among adult males in Taiwan. Eur. J. Epidemiol. 2000, 16, 13–17. [Google Scholar] [CrossRef]
- Zhong, C.; Zhong, X.; Xu, T.; Xu, T.; Zhang, Y. Sex-Specific Relationship Between Serum Uric Acid and Risk of Stroke: A Dose-Response Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e005042. [Google Scholar] [CrossRef]
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef]
- Toschke, A.M.; Heuschmann, P.U.; Wood, O.; Wolfe, C.D. Temporal relationship between influenza infections and subsequent first-ever stroke incidence. Age Ageing 2009, 38, 100–103. [Google Scholar] [CrossRef]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef]
- Lee, K.R.; Bae, J.H.; Hwang, I.C.; Kim, K.K.; Suh, H.S.; Ko, K.D. Effect of Influenza Vaccination on Risk of Stroke: A Systematic Review and Meta-Analysis. Neuroepidemiology 2017, 48, 103–110. [Google Scholar] [CrossRef]
- Naghavi, M.; Wyde, P.; Litovsky, S.; Madjid, M.; Akhtar, A.; Naguib, S.; Siadaty, M.S.; Sanati, S.; Casscells, W. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation 2003, 107, 762–768. [Google Scholar] [CrossRef]
- Kao, P.F.; Liu, J.C.; Hsu, Y.P.; Sung, L.C.; Yang, T.Y.; Hao, W.R.; Lin, Y.C.; Wu, S.Y. Influenza vaccination might reduce the risk of ischemic stroke in patients with atrial fibrillation: A population-based cohort study. Oncotarget 2017, 8, 112697–112711. [Google Scholar] [CrossRef]
- Liu, J.C.; Wang, T.J.; Sung, L.C.; Kao, P.F.; Yang, T.Y.; Hao, W.R.; Chen, C.C.; Hsu, Y.P.; Wu, S.Y. Influenza vaccination reduces hemorrhagic stroke risk in patients with atrial fibrillation: A population-based cohort study. Int. J. Cardiol. 2017, 232, 315–323. [Google Scholar] [CrossRef]
- Seminog, O.O.; Goldacre, M.J. Gout as a risk factor for myocardial infarction and stroke in England: Evidence from record linkage studies. Rheumatology 2013, 52, 2251–2259. [Google Scholar] [CrossRef]
- Singh, J.A.; Ramachandaran, R.; Yu, S.; Yang, S.; Xie, F.; Yun, H.; Zhang, J.; Curtis, J.R. Is gout a risk equivalent to diabetes for stroke and myocardial infarction? A retrospective claims database study. Arthritis Res. Ther. 2017, 19, 228. [Google Scholar] [CrossRef]
- Kuo, C.F.; Grainge, M.J.; Mallen, C.; Zhang, W.; Doherty, M. Impact of gout on the risk of atrial fibrillation. Rheumatology 2016, 55, 721–728. [Google Scholar] [CrossRef][Green Version]
- Feig, D.I.; Nakagawa, T.; Karumanchi, S.A.; Oliver, W.J.; Kang, D.H.; Finch, J.; Johnson, R.J. Hypothesis: Uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int. 2004, 66, 281–287. [Google Scholar] [CrossRef]
- Johnson, R.J.; Kang, D.H.; Feig, D.; Kivlighn, S.; Kanellis, J.; Watanabe, S.; Tuttle, K.R.; Rodriguez-Iturbe, B.; Herrera-Acosta, J.; Mazzali, M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003, 41, 1183–1190. [Google Scholar] [CrossRef]
- Kanellis, J.; Watanabe, S.; Li, J.H.; Kang, D.H.; Li, P.; Nakagawa, T.; Wamsley, A.; Sheikh-Hamad, D.; Lan, H.Y.; Feng, L.; et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 2003, 41, 1287–1293. [Google Scholar] [CrossRef]
- Kanellis, J.; Kang, D.H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin. Nephrol. 2005, 25, 39–42. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, S.K.; Lee, I.K.; Johnson, R.J. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005, 16, 3553–3562. [Google Scholar] [CrossRef]
- Gersch, C.; Palii, S.P.; Kim, K.M.; Angerhofer, A.; Johnson, R.J.; Henderson, G.N. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids 2008, 27, 967–978. [Google Scholar] [CrossRef]
- Neogi, T.; Ellison, R.C.; Hunt, S.; Terkeltaub, R.; Felson, D.T.; Zhang, Y. Serum uric acid is associated with carotid plaques: The National Heart, Lung, and Blood Institute Family Heart Study. J. Rheumatol. 2009, 36, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Gür, M.; Sahin, D.Y.; Elbasan, Z.; Kalkan, G.Y.; Yıldız, A.; Kaya, Z.; Özaltun, B.; Çaylı, M. Uric acid and high sensitive C-reactive protein are associated with subclinical thoracic aortic atherosclerosis. J. Cardiol. 2013, 61, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, D.; Gullu, H.; Caliskan, M.; Yildirim, E.; Bilgi, M.; Ulus, T.; Sezgin, N.; Muderrisoglu, H. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int. J. Clin. Pract. 2005, 59, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Grau, A.J.; Fischer, B.; Barth, C.; Ling, P.; Lichy, C.; Buggle, F. Influenza vaccination is associated with a reduced risk of stroke. Stroke 2005, 36, 1501–1506. [Google Scholar] [CrossRef]
- Van Lenten, B.J.; Wagner, A.C.; Nayak, D.P.; Hama, S.; Navab, M.; Fogelman, A.M. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation 2001, 103, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Chao, T.F.; Liu, C.J.; Chen, S.J.; Chung, F.P.; Liao, J.N.; Tuan, T.C.; Chen, T.J.; Chen, S.A. The association between influenza infection, vaccination, and atrial fibrillation: A nationwide case-control study. Heart Rhythm 2016, 13, 1189–1194. [Google Scholar] [CrossRef]
- Ariesen, M.J.; Claus, S.P.; Rinkel, G.J.; Algra, A. Risk factors for intracerebral hemorrhage in the general population: A systematic review. Stroke 2003, 34, 2060–2065. [Google Scholar] [CrossRef]
- Grysiewicz, R.A.; Thomas, K.; Pandey, D.K. Epidemiology of ischemic and hemorrhagic stroke: Incidence, prevalence, mortality, and risk factors. Neurol. Clin. 2008, 26, 871–895. [Google Scholar] [CrossRef]
- Sturgeon, J.D.; Folsom, A.R.; Longstreth, W.T., Jr.; Shahar, E.; Rosamond, W.D.; Cushman, M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007, 38, 2718–2725. [Google Scholar] [CrossRef]
- Yen, F.S.; Hsu, C.C.; Li, H.L.; Wei, J.C.; Hwu, C.M. Urate-lowering therapy may mitigate the risks of hospitalized stroke and mortality in patients with gout. PLoS ONE 2020, 15, e0234909. [Google Scholar] [CrossRef]
- Lei, H.; Gao, Q.; Liu, S.R.; Xu, J. The Benefit and Safety of Aspirin for Primary Prevention of Ischemic Stroke: A Meta-Analysis of Randomized Trials. Front. Pharmacol. 2016, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Shrank, W.H.; Patrick, A.R.; Brookhart, M.A. Healthy user and related biases in observational studies of preventive interventions: A primer for physicians. J. Gen. Intern. Med. 2011, 26, 546–550. [Google Scholar] [CrossRef] [PubMed]
| Whole Cohort (n = 23,949) | Unvaccinated (n = 12,300) | Vaccinated (n = 11,649) | pa | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age, years (Mean ± SD) | 66.75 (8.36) | 64.22 (8.39) | 69.42 (7.44) | <0.001 | |||
| 55–64 | 11,658 | 48.68 | 7973 | 64.82 | 3685 | 31.63 | <0.001 |
| 65–74 | 7982 | 33.33 | 2711 | 22.04 | 5271 | 45.25 | |
| ≥75 | 4309 | 17.99 | 1616 | 13.14 | 2693 | 23.12 | |
| Gender | |||||||
| Female | 10,225 | 42.69 | 5194 | 42.23 | 5031 | 43.19 | 0.133 |
| Male | 13,724 | 57.31 | 7106 | 57.77 | 6618 | 56.81 | |
| CCI | |||||||
| 0 | 7243 | 30.24 | 3784 | 30.76 | 3459 | 29.69 | 0.301 |
| 1 | 6328 | 26.42 | 3215 | 26.14 | 3113 | 26.72 | |
| 2 | 4498 | 18.78 | 2311 | 18.79 | 2187 | 18.77 | |
| ≥3 | 5880 | 24.55 | 2990 | 24.31 | 2890 | 24.81 | |
| Comorbidities | |||||||
| Diabetes | 6575 | 27.45 | 3414 | 27.76 | 3161 | 27.14 | 0.282 |
| Hypertension | 14,888 | 62.17 | 7259 | 59.02 | 7629 | 65.49 | <0.001 |
| Dyslipidemia | 8985 | 37.52 | 4855 | 39.47 | 4130 | 35.45 | <0.001 |
| Atrial fibrillation | 1891 | 7.90 | 748 | 6.08 | 1143 | 9.81 | <0.001 |
| Allopurinol | |||||||
| <28 days | 17,859 | 74.57 | 9766 | 79.40 | 8093 | 69.47 | <0.001 |
| ≥28 days | 6090 | 25.43 | 2534 | 20.60 | 3556 | 30.53 | |
| Benzbromarone | |||||||
| <28 days | 12,633 | 52.75 | 7175 | 58.33 | 5458 | 46.85 | <0.001 |
| ≥28 days | 11,316 | 47.25 | 5125 | 41.67 | 6191 | 53.15 | |
| Colchicine | |||||||
| <28 days | 16,113 | 67.28 | 8621 | 70.09 | 7492 | 64.31 | <0.001 |
| ≥28 days | 7836 | 32.72 | 3679 | 29.91 | 4157 | 35.69 | |
| Aspirin | |||||||
| <28 days | 13,284 | 55.47 | 7937 | 64.53 | 5347 | 45.90 | <0.001 |
| ≥28 days | 10,665 | 44.53 | 4363 | 35.47 | 6302 | 54.10 | |
| Statin | |||||||
| <28 days | 14,272 | 59.59 | 7758 | 63.07 | 6514 | 55.92 | <0.001 |
| ≥28 days | 9677 | 40.41 | 4542 | 36.93 | 5135 | 44.08 | |
| RAA | |||||||
| <28 days | 9517 | 39.74 | 5972 | 48.55 | 3545 | 30.43 | <0.001 |
| ≥28 days | 14,432 | 60.26 | 6328 | 51.45 | 8104 | 69.57 | |
| Metformin | |||||||
| <28 days | 18,063 | 75.42 | 9543 | 77.59 | 8520 | 73.14 | <0.001 |
| ≥28 days | 5886 | 24.58 | 2757 | 22.41 | 3129 | 26.86 | |
| Level of Urbanization | |||||||
| Urban | 16,573 | 69.20 | 9159 | 74.46 | 7414 | 63.64 | <0.001 |
| Suburban | 4713 | 19.68 | 2155 | 17.52 | 2558 | 21.96 | |
| Rural | 2663 | 11.12 | 986 | 8.02 | 1677 | 14.40 | |
| Monthly income (NT$) | |||||||
| 0 | 2138 | 8.93 | 912 | 7.41 | 1226 | 10.52 | <0.001 |
| 1–21,000 | 6642 | 27.73 | 2925 | 23.78 | 3717 | 31.91 | |
| 21,000–33,300 | 7593 | 31.70 | 3359 | 27.31 | 4234 | 36.35 | |
| ≥33,301 | 7576 | 31.63 | 5104 | 41.50 | 2472 | 21.22 | |
| All Group (n = 23,949) | Unvaccinated (Total Follow-Up 65,692.6 Person-Years) | Vaccinated (Total Follow-Up 92,060.5 Person-Years) | Adjusted HR † (95% C.I.) | ||||
|---|---|---|---|---|---|---|---|
| No. of Patients with Stroke | Incidence Rate (per 105 Person-Years) (95% C.I.) | No. of Patients with Stroke | Incidence Rate (per 105 Person-Years) (95% C.I.) | ||||
| Whole cohort | |||||||
| All stroke | 1500 | 2283.4 | (2167.8, 2398.9) | 2084 | 2263.7 | (2166.5, 2360.9) | 0.59 (0.55, 0.63) *** |
| Hemorrhagic stroke | 194 | 295.3 | (253.8, 336.9) | 241 | 261.8 | (228.7, 294.8) | 0.60 (0.49, 0.73) *** |
| Ischemic stroke | 1014 | 1543.6 | (1448.5, 1638.6) | 1473 | 1600.0 | (1518.3, 1681.7) | 0.60 (0.55, 0.65) *** |
| Age, 55–64 a | |||||||
| All stroke | 757 | 1652.7 | (1534.9, 1770.4) | 430 | 1273.9 | (1153.5, 1394.3) | 0.54 (0.48, 0.61) *** |
| Hemorrhagic stroke | 119 | 259.8 | (213.1, 306.5) | 66 | 195.5 | (148.4, 242.7) | 0.53 (0.39, 0.72) *** |
| Ischemic stroke | 495 | 1080.7 | (985.5, 1175.9) | 282 | 835.4 | (737.9, 932.9) | 0.53 (0.46, 0.62) *** |
| Age, 65–74 b | |||||||
| All stroke | 418 | 3133.0 | (2832.6, 3433.3) | 1061 | 2568.4 | (2413.8, 2722.9) | 0.63 (0.56, 0.71) *** |
| Hemorrhagic stroke | 42 | 314.8 | (219.6, 410.0) | 108 | 261.4 | (212.1, 310.7) | 0.64 (0.45, 0.92) * |
| Ischemic stroke | 300 | 2248.5 | (1994.1, 2503.0) | 764 | 1849.4 | (1718.3, 1980.9) | 0.63 (0.55, 0.72) *** |
| Age, ≥75 c | |||||||
| All stroke | 325 | 4965.0 | (4425.2, 5504.8) | 593 | 3489.1 | (3208.3, 3769.9) | 0.57 (0.50, 0.65) *** |
| Hemorrhagic stroke | 33 | 504.1 | (332.1, 676.1) | 67 | 394.2 | (299.8, 488.6) | 0.67 (0.44, 1.02) |
| Ischemic stroke | 219 | 3345.7 | (2902.5, 3788.8) | 427 | 2512.4 | (2274.4, 2750.7) | 0.60 (0.51, 0.71) *** |
| Female d | |||||||
| All stroke | 571 | 2092.0 | (1920.4, 2263.6) | 838 | 2104.2 | (1961.7, 2246.6) | 0.62 (0.55, 0.69) *** |
| Hemorrhagic stroke | 57 | 208.8 | (154.6, 263.0) | 94 | 236.0 | (188.3, 283.7) | 0.76 (0.54, 1.08) |
| Ischemic stroke | 404 | 1480.1 | (1335.8, 1624.5) | 587 | 1473.9 | (1354.7, 1593.2) | 0.60 (0.52, 0.68) *** |
| Male e | |||||||
| All stroke | 929 | 2419.4 | (2263.8, 2575.0) | 1246 | 2385.4 | (2252.9, 2517.8) | 0.57 (0.52, 0.63) *** |
| Hemorrhagic stroke | 137 | 356.8 | (297.0, 416.5) | 147 | 281.7 | (235.9, 326.) | 0.54 (0.42, 0.69) *** |
| Ischemic stroke | 610 | 1588.6 | (1462.6, 1714.7) | 886 | 1696.2 | (1584.5, 1807.9) | 0.60 (0.53, 0.67) *** |
| Unvaccinated | Vaccinated | p for Trend | |||
|---|---|---|---|---|---|
| 1 | 2–3 | ≥4 | |||
| Adjusted HR (95%C.I.) | Adjusted HR (95%C.I.) | Adjusted HR (95%C.I.) | Adjusted HR (95%C.I.) | ||
| Main model † | 1.00 | 0.81 (0.61, 1.08) | 0.80 (0.62, 1.02) | 0.37 (0.28, 0.48) *** | <0.001 |
| Additional covariates ‡ | |||||
| Main model + Allopurinol | 1.00 | 0.82 (0.62, 1.09) | 0.80 (0.63, 1.03) | 0.37 (0.28, 0.49) *** | <0.001 |
| Main model + Benzbromarone | 1.00 | 0.83 (0.62, 1.10) | 0.81 (0.63, 1.04) | 0.38 (0.29, 0.50) *** | <0.001 |
| Main model + Colchicine | 1.00 | 0.81 (0.61, 1.08) | 0.80 (0.62, 1.02) | 0.37 (0.28, 0.49) *** | <0.001 |
| Main model + Aspirin | 1.00 | 0.82 (0.62, 1.10) | 0.82 (0.63, 1.05) | 0.38 (0.29, 0.50) *** | <0.001 |
| Subgroup effects | |||||
| Age, years | |||||
| 55–64 | 1.00 | 0.51 (0.31, 0.84) ** | 0.70 (0.47, 1.05) | 0.38 (0.23, 0.64) *** | <0.001 |
| ≥65 | 1.00 | 1.07 (0.74, 1.54) | 0.89 (0.64, 1.24) | 0.39 (0.28, 0.55) *** | <0.001 |
| Sex | |||||
| Female | 1.00 | 1.10 (0.69, 1.74) | 0.96 (0.63, 1.46) | 0.43 (0.27, 0.69) *** | <0.001 |
| Male | 1.00 | 0.70 (0.49, 1.00) | 0.74 (0.54, 1.01) | 0.34 (0.24, 0.49) *** | <0.001 |
| Diabetes | |||||
| No | 1.00 | 0.75 (0.53, 1.05) | 0.76 (0.57, 1.02) | 0.37 (0.27, 0.51) *** | <0.001 |
| Yes | 1.00 | 0.99 (0.59, 1.68) | 0.87 (0.54, 1.40) | 0.33 (0.19, 0.58) *** | <0.001 |
| Dyslipidemia | |||||
| No | 1.00 | 0.89 (0.64, 1.23) | 0.74 (0.55, 1.00) | 0.41 (0.30, 0.56) *** | <0.001 |
| Yes | 1.00 | 0.66 (0.37, 1.18) | 0.97 (0.62, 1.53) | 0.28 (0.16, 0.51) *** | <0.001 |
| Hypertension | |||||
| No | 1.00 | 0.65 (0.40, 1.04) | 0.65 (0.42, 0.99) * | 0.38 (0.25, 0.60) *** | <0.001 |
| Yes | 1.00 | 0.94 (0.66, 1.34) | 0.89 (0.65, 1.22) | 0.36 (0.25, 0.51) *** | <0.001 |
| Atrial fibrillation | |||||
| No | 1.00 | 0.81 (0.60, 1.09) | 0.76 (0.58, 0.99) * | 0.35 (0.26, 0.47) *** | <0.001 |
| Yes | 1.00 | 0.93 (0.35, 2.51) | 1.27 (0.57, 2.84) | 0.59 (0.25, 1.38) | 0.299 |
| Allopurinol | |||||
| <28 days | 1.00 | 0.74 (0.52, 1.04) | 0.84 (0.63, 1.12) | 0.33 (0.24, 0.46) *** | <0.001 |
| ≥28 days | 1.00 | 1.09 (0.65, 1.84) | 0.76 (0.46, 1.26) | 0.50 (0.31, 0.82) ** | 0.003 |
| Benzbromarone | |||||
| <28 days | 1.00 | 0.89 (0.61, 1.29) | 0.77 (0.54, 1.09) | 0.46 (0.32, 0.66) *** | <0.001 |
| ≥28 days | 1.00 | 0.75 (0.49, 1.15) | 0.86 (0.60, 1.24) | 0.31 (0.20, 0.47) *** | <0.001 |
| Colchicine | |||||
| <28 days | 1.00 | 0.69 (0.47, 1.01) | 0.73 (0.52, 1.00) | 0.33 (0.23, 0.47) *** | <0.001 |
| ≥28 days | 1.00 | 1.04 (0.68, 1.61) | 0.93 (0.62, 1.38) | 0.44 (0.28, 0.68) *** | <0.001 |
| Aspirin | |||||
| <28 days | 1.00 | 0.75 (0.51, 1.10) | 0.74 (0.52, 1.05) | 0.32 (0.21, 0.49) *** | <0.001 |
| ≥28 days | 1.00 | 0.95 (0.62, 1.45) | 0.93 (0.64, 1.34) | 0.45 (0.31, 0.67) *** | <0.001 |
| Statin | |||||
| <28 days | 1.00 | 0.71 (0.49, 1.01) | 0.76 (0.56, 1.03) | 0.38 (0.27, 0.54) *** | <0.001 |
| ≥28 days | 1.00 | 1.10 (0.69, 1.75) | 0.93 (0.60, 1.44) | 0.39 (0.24, 0.63) *** | <0.001 |
| RAA | |||||
| <28 days | 1.00 | 0.65 (0.36, 1.15) | 0.70 (0.42, 1.16) | 0.40 (0.23, 0.71) ** | 0.002 |
| ≥28 days | 1.00 | 0.86 (0.62, 1.19) | 0.81 (0.61, 1.09) | 0.35 (0.26, 0.48) *** | <0.001 |
| Metformin | |||||
| <28 days | 1.00 | 0.70 (0.50, 0.98) * | 0.74 (0.55, 0.99) * | 0.36 (0.26, 0.50) *** | <0.001 |
| ≥28 days | 1.00 | 1.29 (0.75, 2.23) | 1.04 (0.63, 1.74) | 0.42 (0.24, 0.73) ** | 0.003 |
| Unvaccinated | Vaccinated | p for Trend | |||
|---|---|---|---|---|---|
| 1 | 2–3 | ≥4 | |||
| Adjusted HR (95%C.I.) | Adjusted HR (95%C.I.) | Adjusted HR (95%C.I.) | Adjusted HR (95%C.I.) | ||
| Main model † | 1.00 | 0.83 (0.74, 0.94) ** | 0.73 (0.65, 0.81) *** | 0.42 (0.38, 0.47) *** | <0.001 |
| Additional covariates ‡ | |||||
| Main model + Allopurinol | 1.00 | 0.83 (0.74, 0.94) ** | 0.73 (0.65, 0.81) *** | 0.42 (0.38, 0.47) *** | <0.001 |
| Main model + Benzbromarone | 1.00 | 0.84 (0.74, 0.94) ** | 0.73 (0.66, 0.82) *** | 0.43 (0.38, 0.47) *** | <0.001 |
| Main model + Colchicine | 1.00 | 0.83 (0.74, 0.94) ** | 0.73 (0.65, 0.81) *** | 0.42 (0.38, 0.47) *** | <0.001 |
| Main model + Aspirin | 1.00 | 0.78 (0.70, 0.88) *** | 0.66 (0.59, 0.73) *** | 0.37 (0.34, 0.42) *** | <0.001 |
| Subgroup effects | |||||
| Age, years | |||||
| 55–64 | 1.00 | 0.60 (0.48, 0.75) *** | 0.65 (0.53, 0.80) *** | 0.37 (0.29, 0.48) *** | <0.001 |
| ≥65 | 1.00 | 0.94 (0.82, 1.09) | 0.76 (0.67, 0.87) *** | 0.43 (0.38, 0.49) *** | <0.001 |
| Sex | |||||
| Female | 1.00 | 0.90 (0.75, 1.08) | 0.67 (0.56, 0.79) *** | 0.43 (0.37, 0.51) *** | <0.001 |
| Male | 1.00 | 0.78 (0.67, 0.92) ** | 0.77 (0.67, 0.89) *** | 0.41 (0.36, 0.48) *** | <0.001 |
| Diabetes | |||||
| No | 1.00 | 0.79 (0.68, 0.91) ** | 0.69 (0.60, 0.79) *** | 0.43 (0.38, 0.49) *** | <0.001 |
| Yes | 1.00 | 0.92 (0.75, 1.13) | 0.80 (0.66, 0.96) * | 0.38 (0.31, 0.46) *** | <0.001 |
| Dyslipidemia | |||||
| No | 1.00 | 0.85 (0.73, 0.98) * | 0.69 (0.60, 0.79) *** | 0.45 (0.40, 0.52) *** | <0.001 |
| Yes | 1.00 | 0.78 (0.64, 0.96) * | 0.80 (0.66, 0.96) * | 0.34 (0.28, 0.42) *** | <0.001 |
| Hypertension | |||||
| No | 1.00 | 0.78 (0.62, 0.97) * | 0.68 (0.56, 0.84) *** | 0.50 (0.41, 0.61) *** | <0.001 |
| Yes | 1.00 | 0.85 (0.74, 0.98) * | 0.72 (0.64, 0.82) *** | 0.37 (0.33, 0.42) *** | <0.001 |
| Atrial fibrillation | |||||
| No | 1.00 | 0.85 (0.75, 0.97) * | 0.77 (0.69, 0.87) *** | 0.42 (0.38, 0.48) *** | <0.001 |
| Yes | 1.00 | 0.69 (0.51, 0.93) * | 0.52 (0.39, 0.69) *** | 0.41 (0.32, 0.54) *** | <0.001 |
| Allopurinol | |||||
| <28 days | 1.00 | 0.89 (0.77, 1.03) | 0.75 (0.65, 0.85) *** | 0.43 (0.38, 0.49) *** | <0.001 |
| ≥28 days | 1.00 | 0.72 (0.58, 0.90) ** | 0.70 (0.58, 0.85) *** | 0.42 (0.35, 0.50) *** | <0.001 |
| Benzbromarone | |||||
| <28 days | 1.00 | 0.86 (0.73, 1.02) | 0.78 (0.67, 0.90) ** | 0.41 (0.35, 0.48) *** | <0.001 |
| ≥28 days | 1.00 | 0.80 (0.67, 0.95) ** | 0.70 (0.60, 0.82) *** | 0.45 (0.39, 0.52) *** | <0.001 |
| Colchicine | |||||
| <28 days | 1.00 | 0.89 (0.77, 1.04) | 0.76 (0.67, 0.88) *** | 0.40 (0.35, 0.46) *** | <0.001 |
| ≥28 days | 1.00 | 0.74 (0.60, 0.90) ** | 0.67 (0.56, 0.81) *** | 0.46 (0.39, 0.55) *** | <0.001 |
| Aspirin | |||||
| <28 days | 1.00 | 0.90 (0.71, 1.13) | 0.68 (0.54, 0.86) *** | 0.35 (0.27, 0.45) *** | <0.001 |
| ≥28 days | 1.00 | 0.76 (0.66, 0.87) *** | 0.66 (0.59, 0.75) *** | 0.40 (0.35, 0.45) *** | <0.001 |
| Statin | |||||
| <28 days | 1.00 | 0.86 (0.73, 1.01) | 0.77 (0.67, 0.90) ** | 0.45 (0.38, 0.52) *** | <0.001 |
| ≥28 days | 1.00 | 0.78 (0.66, 0.93) ** | 0.68 (0.58, 0.80) *** | 0.41 (0.35, 0.48) *** | <0.001 |
| RAA | |||||
| <28 days | 1.00 | 0.94 (0.75, 1.19) | 0.79 (0.64, 0.99) * | 0.46 (0.36, 0.58) *** | <0.001 |
| ≥28 days | 1.00 | 0.78 (0.68, 0.90) ** | 0.70 (0.62, 0.79) *** | 0.41 (0.36, 0.46) *** | <0.001 |
| Metformin | |||||
| <28 days | 1.00 | 0.78 (0.68, 0.90) *** | 0.70 (0.62, 0.80) *** | 0.43 (0.38, 0.49) *** | <0.001 |
| ≥28 days | 1.00 | 0.95 (0.77, 1.17) | 0.78 (0.65, 0.95) * | 0.40 (0.33, 0.49) *** | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Chou, K.-T.; Liu, J.-C.; Chiu, C.-C.; Yang, T.-Y.; Lin, C.-H.; Fang, Y.-A.; Jian, W.; Lei, M.-H.; Yeh, H.-T.; et al. Association between Stroke Risk and Influenza Vaccination in Patients with Gout: A Nationwide Population-Based Study. Vaccines 2022, 10, 1278. https://doi.org/10.3390/vaccines10081278
Chen C-C, Chou K-T, Liu J-C, Chiu C-C, Yang T-Y, Lin C-H, Fang Y-A, Jian W, Lei M-H, Yeh H-T, et al. Association between Stroke Risk and Influenza Vaccination in Patients with Gout: A Nationwide Population-Based Study. Vaccines. 2022; 10(8):1278. https://doi.org/10.3390/vaccines10081278
Chicago/Turabian StyleChen, Chun-Chao, Kuan-Ting Chou, Ju-Chi Liu, Chun-Chih Chiu, Tsung-Yeh Yang, Cheng-Hsin Lin, Yu-Ann Fang, William Jian, Meng-Huan Lei, Hsien-Tang Yeh, and et al. 2022. "Association between Stroke Risk and Influenza Vaccination in Patients with Gout: A Nationwide Population-Based Study" Vaccines 10, no. 8: 1278. https://doi.org/10.3390/vaccines10081278
APA StyleChen, C.-C., Chou, K.-T., Liu, J.-C., Chiu, C.-C., Yang, T.-Y., Lin, C.-H., Fang, Y.-A., Jian, W., Lei, M.-H., Yeh, H.-T., Hsu, M.-H., & Hao, W.-R. (2022). Association between Stroke Risk and Influenza Vaccination in Patients with Gout: A Nationwide Population-Based Study. Vaccines, 10(8), 1278. https://doi.org/10.3390/vaccines10081278










