HPV Vaccination Coverage Rate in a Rural Area: An Observational, Retrospective, and Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
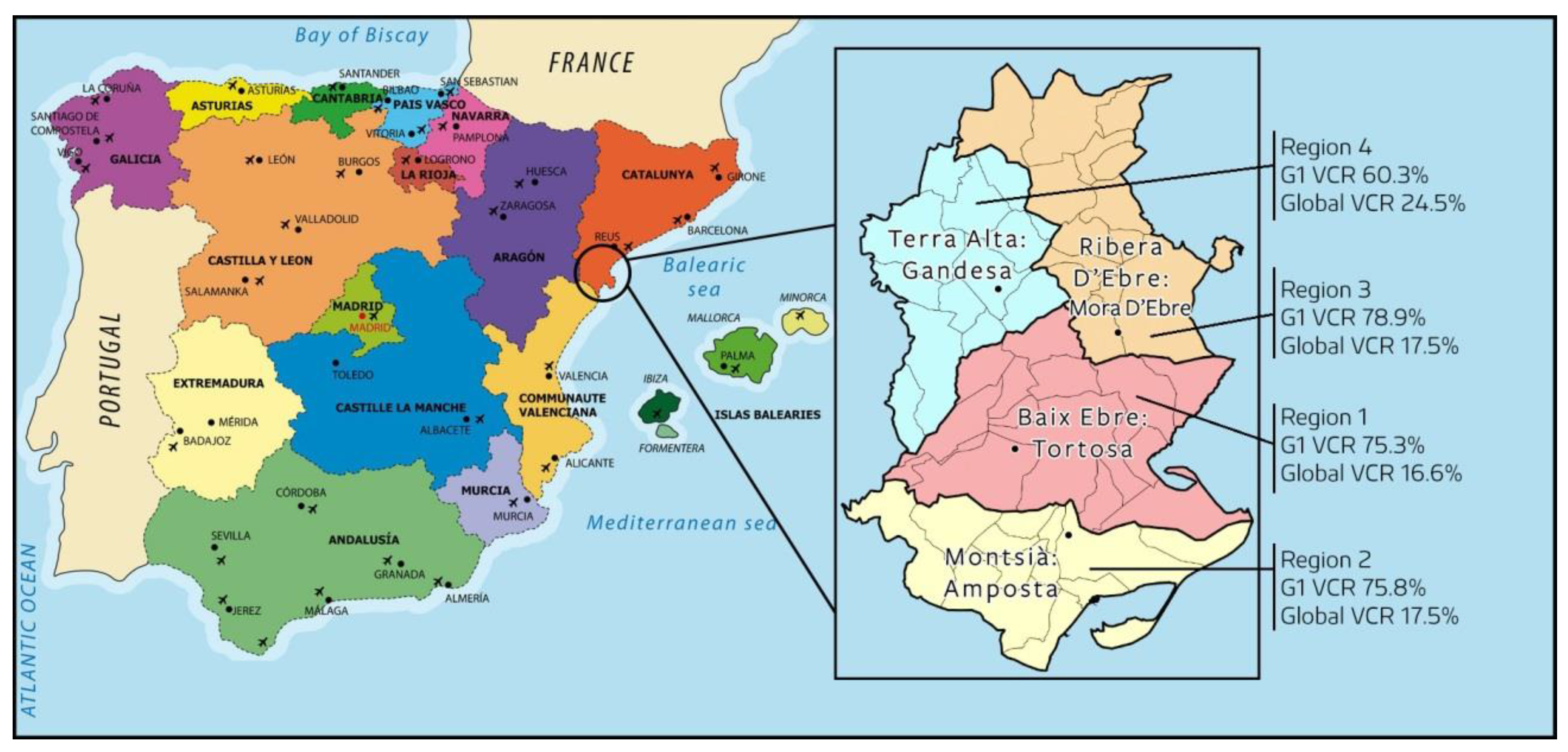
2.2. Study Scope
2.3. Patients/Subjects of Study
2.4. Observation Period
2.5. Inclusion Criteria
- Active medical history at any of the clinical centres of the territory (HC3);
- Residents for ≥5 years in the study territory;
- Accessibility to registered variables and clinical data recorded.
2.6. Exclusion Criteria
- Patients without an active clinical history and/or not enough recorded data or inaccessibility to clinical data;
- Disease with a vital prognosis <1 year.
2.7. Variables
2.7.1. Dependent Variable: HPV Vaccination Status
2.7.2. Independent Variables
- (1)
- Sociodemographic variables: Age, region of residence, assigned primary care team (EAP), aging index (people ≥65 years old per 100 people ≤15 years old), and annual per capita average income as the relative percentage of each region vs. Catalonia (100%).
- (2)
- Clinical variables: Number of administered doses, type of vaccine, date of administration, HPV vaccination (G1, public vaccination program funded for target population; G2, funded vaccination according to risk criteria; G3, nonfunded vaccination), cervical screening test and cytology-registered, HPV polymerase chain reaction test (PCR)-registered, and HPV-positive PCR diagnosis. These variables were described by vaccination status. All presented results have been stratified by age.
2.8. Data Collection and Information Sources
- The 11 primary care teams all managed by the Catalonian Health Institute (Governmental agency) shared clinical information database for all general practice (E-cap, HC3) and hospital (E-sap) interactions, including clinical data, symptoms, investigations, diagnoses, comorbidities, prescribed medication, referrals to secondary and tertiary care, and status (alive/death) of 97.7% of resident people as of 31 December 2021. Pharmacological variables were collected from the SIRE (Catalan acronym for Integrated Electronic Prescription System).
- The HC3 Shared Clinical Record in Catalonia (CatSalut, Health Department), i.e., the Patient Episode Dataset for Catalonia, which includes demographic and clinical data on all inpatient and outpatient daily admissions in Catalonian hospitals.
- The Institute of Statistics of Catalonia for each region of the territory, including percentage gross disposable household income/inhabitant vs. the Catalonian average (100%), inhabitant density/km2, and aging index vs. Catalonia (100%) [19,31,34]. Data on these factors were collected automatically when possible, or manually otherwise.
2.9. Statistical Analysis
3. Results
3.1. Vaccination Coverage Rate Obtained in the Target Population
3.1.1. Description of the Sample
3.1.2. Vaccination Coverage and Screening Test Results
3.2. Identification of Risk Groups
3.3. Barriers to Vaccine Accessibility
4. Discussion
- Significant differences in vaccination coverage between neighbouring regions;
- Women aged ≥25 years old (excluded by age from funded vaccination) do not have subsequent differentiated screening or opportunistic monitoring for HPV clinical guidance in primary care;
- Guidance of clinical practice toward the systematic screening of high-risk candidates for HPV vaccination in primary care;
- Avoidable conditions restricting access to funded HPV vaccination (e.g., age, income, temporal residence, or low accessibility to gynaecologist professionals);
- Monitoring public policies according to homogeneous criteria about the vaccination coverage achieved and sharing of corrective decisions;
- Men’s coverage, especially high-risk candidates for HPV vaccination as primary or secondary prevention.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Brotons Aguiló, M.; Lubrano Rosales, A.; Alba Menéndex, A.; Guarch Troyas, R.; Serrano Velasco, M.; De la Fuente Valero, J. AEPCC-Guía: Condilomas Acuminados, 1st ed.; Publicaciones AEPCC: Madrid, Spain, 2015. [Google Scholar]
- Hartwig, S.; Baldauf, J.-J.; Dominiak-Felden, G.; Simondon, F.; Alemany, L.; de Sanjosé, S.; Castellsagué, X. Estimation of the epidemiological burden of HPV-related anogenital cancers, precancerous lesions, and genital warts in women and men in Europe: Potential additional benefit of a nine-valent second generation HPV vaccine compared to first generation HPV vaccines. Papillomavirus Res. 2015, 1, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Syrjänen, K.J. Human papillomavirus (HPV) infections of the female genital tract and their associations with intraepithelial neoplasia and squamous cell carcinoma. Pathol. Annu. 1986, 21 Pt 1, 53–89. Available online: https://pubmed.ncbi.nlm.nih.gov/3001622/ (accessed on 9 January 2022).
- Al Bitar, S.; Ballouz, T.; Doughan, S.; Gali-Muhtasib, H.; Rizk, N. Potential role of micro ribonucleic acids in screening for anal cancer in human papilloma virus and human immunodeficiency virus related malignancies. World J. Gastrointest. Pathophysiol. 2021, 12, 59–83. [Google Scholar] [CrossRef]
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.E.; Gershman, S.T.; Kim, J.J.; Tamimi, R.M.; Klevens, R.M.; Holmes, M.D. Trends of two HPV-associated cancers in Massachusetts: Cervical and oropharyngeal cancer. Cancer Causes Control 2018, 29, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Preventable and Treatable Mortality Statistics—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Preventable_and_treatable_mortality_statistics (accessed on 16 January 2022).
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 23 February 2022).
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. J. Oncol. 2019, 2019, 3257939. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Okoye, J.O.; Chukwukelu, C.F.; Okekpa, S.I.; Ogenyi, S.I.; Onyekachi-Umah, I.N.; Ngokere, A.A. Racial Disparities Associated with the Prevalence of Vaccine and Non-Vaccine HPV Types and Multiple HPV Infections between Asia and Africa: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2021, 22, 2729–2741. [Google Scholar] [CrossRef]
- Rasmussen, C.L.; Thomsen, L.T.; Aalborg, G.L.; Kjaer, S.K. Incidence of vulvar high-grade precancerous lesions and cancer in Denmark before and after introduction of HPV vaccination. Gynecol. Oncol. 2020, 157, 664–670. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Andujar, M.; Roura, E.; Torres, A.; Vega, B.; Pavcovich, M.; Sanchez, M.A.; Lubrano, A.; Trujillo, J.L.; Almeida, L.; Santana, M.; et al. Prevalence and genotype distribution of cervical human papilomavirus infection in the pre-vaccination era: A population-based study in the Canary Islands. BMJ Open 2020, 10, e037402. [Google Scholar] [CrossRef]
- Castro, F.L.; Rubio, V.O. Mortalidad innecesariamente prematura y sanitariamente evitable en Costa Rica. Rev. Esp. Salud Pública 2010, 84, 771–787. [Google Scholar] [CrossRef] [Green Version]
- Eyes on Europe. Nobody Lives Here! Rural Depopulation in the EU and Citizen Engagement in “Emptied Spain”. Available online: https://www.eyes-on-europe.eu/nobody-lives-here-rural-depopulation-in-the-eu-and-citizen-engagement-in-emptied-spain/ (accessed on 12 May 2021).
- Rodríguez-Salés, V.; Roura, E.; Ibáñez, R.; Peris, M.; Bosch, F.X.; Coma, E.E.; de Sanjosé, S. Cobertura del cribado de cáncer de cuello uterino en Cataluña (2008–2011) [Coverage of cervical cancer screening in Catalonia, Spain (2008–2011)]. Gac. Sanit. 2014, 28, 7–13. (In Spanish) [Google Scholar] [CrossRef]
- Rudolph, C.E.S.; Katalinic, A. Basisinzidenz HPV-assoziierter invasiver und in situ Karzinome in Deutschland vor Eintreten möglicher Impfeffekte. Das. Gesundh. 2018, 81, 993–1000. [Google Scholar] [CrossRef]
- Robles, C.; Bruni, L.; Acera, A.; Riera, J.C.; Prats, L.; Poljak, M.; Mlakar, J.; Valenčak, A.O.; Eriksson, T.; Lehtinen, M.; et al. Determinants of Human Papillomavirus Vaccine Uptake by Adult Women Attending Cervical Cancer Screening in 9 European Countries. Am. J. Prev. Med. 2020, 60, 478–487. [Google Scholar] [CrossRef]
- Duncan, J.; Harris, M.; Skyers, N.; Bailey, A.; Figueroa, J.P. A Call for Low- and Middle-Income Countries to Commit to the Elimination of Cervical Cancer. Lancet Reg. Health Am. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Vorsters, A.; Arbyn, M.; Baay, M.; Bosch, X.; de Sanjosé, S.; Hanley, S.; Karafillakis, E.; Lopalco, P.L.; Pollock, K.G.; Yarwood, J.; et al. Overcoming barriers in HPV vaccination and screening programs. Papillomavirus Res. 2017, 4, 45–53. [Google Scholar] [CrossRef]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2020, 144, 106399. [Google Scholar] [CrossRef]
- Orumaa, M.; Kjaer, S.K.; Dehlendorff, C.; Munk, C.; Olsen, A.O.; Hansen, B.T.; Campbell, S.; Nygård, M. The impact of HPV multi-cohort vaccination: Real-world evidence of faster control of HPV-related morbidity. Vaccine 2020, 38, 1345–1351. [Google Scholar] [CrossRef]
- Saulle, R.; Sinopoli, A.; De Paula Baer, A.; Mannocci, A.; Marino, M.; De Belvis, A.G.; Federici, A.; La Torre, G. The PRECEDE-PROCEED model as a tool in Public Health screening: A systematic review. Clin Ter. 2020, 171, e167–e177. [Google Scholar] [CrossRef]
- Cereda, D.; Federici, A.; Guarino, A.; Serantoni, G.; Gruppo PRECEDE-PROCEED; Coppola, L.; Lemma, P.; Rossi, P.G. Development and first application of an audit system for screening programs based on the PRECEDE-PROCEED model: An experience with breast cancer screening in the region of Lombardy (Italy). BMC Public Health. 2020, 20, 1778. [Google Scholar] [CrossRef]
- Della Polla, G.; Pelullo, C.P.; Napolitano, F.; Angelillo, I.F. HPV vaccine hesitancy among parents in Italy: A cross-sectional study. Hum Vaccin Immunother. 2020, 16, 2744–2751. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Mascaro, V.; Zucco, R.; Pavia, M. Parent perspectives on childhood vaccination: How to deal with vaccine hesitancy and refusal? Vaccine 2019, 37, 984–990. [Google Scholar] [CrossRef]
- Dubé, È.; Farrands, A.; Lemaitre, T.; Boulianne, N.; Sauvageau, C.; Boucher, F.D.; Tapiero, B.; Quach, C.; Ouakki, M.; Gosselin, V.; et al. Overview of knowledge, attitudes, beliefs, vaccine hesitancy and vaccine acceptance among mothers of infants in Quebec, Canada. Hum. Vaccines Immunother. 2019, 15, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Idescat. Anuario Estadístico de Cataluña. Densidad de Población. Comarcas y Aran, Ámbitos y Provincias. Available online: https://www.idescat.cat/pub/?id=aec&n=249&lang=es (accessed on 25 February 2022).
- Idescat. Indicadors Demogràfics i de Territori. Estructura Per Edats, Envelliment i Dependència. Comarques i Aran. Available online: http://www.idescat.cat/pub/?id=inddt&n=915&by=com (accessed on 25 February 2022).
- Agència de Qualitat i Avaluació Sanitàries de de Catalunya, Generalitat de Catalunya. Què Destaquem De La Regió Sanitària De Camp De Tarragona? Observatori Del Sistema De Salut De Catalunya. Generalitat De Catalunya. Available online: https://observatorisalut.gencat.cat/web/.content/minisite/observatorisalut/ossc_central_resultats/Presentacions/Presentacio-RSTarragona-i-RSTE_def.pdf (accessed on 23 January 2022).
- Generalitat de Catalunya. Projeccions de Població Principals Resultats 2013–2051. 2008. Available online: https://www.idescat.cat/serveis/biblioteca/docs/cat/pp2021-2041pr.pdf (accessed on 23 January 2022).
- Idescat. Anuari Estadístic De Catalunya. Renda Familiar Disponible Bruta. Índex. Comarques i Aran, i Àmbits. Available online: http://www.idescat.cat/pub/?id=aec&n=941 (accessed on 25 February 2022).
- World Health Organization. Strategic Advisory Group of Experts on Immunization. Available online: https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization (accessed on 10 April 2022).
- Pan American Health Organization. One-dose Human Papillomavirus (HPV) Vaccine Offers Solid Protection against Cervical Cancer. Available online: https://www.paho.org/en/news/11-4-2022-one-dose-human-papillomavirus-hpv-vaccine-offers-solid-protection-against-cervical (accessed on 10 April 2022).
- Departament de Salut. Manual De Vacunacions De Catalunya; Agència de Salut Pública de Catalunya: Barcelona, Spain, 2020. [Google Scholar]
- Campins, M.T.A.; Alemany, L.; Bayas, J.M.; Borruel, N.; Castellsagué, X.; Curran, A.; Diaz Heredia, C.; Martinez, X.; Moraga llop, F.A. Aepcc-Guía: Vacunación Selectiva Frente Al Virus Del Papiloma Humano En Poblaciones De Riesgo Elevado; Publicaciones AEPCC: Madrid, Spain, 2016; pp. 1–46. [Google Scholar]
- Sankaranarayanan, R.; Joshi, S.; Muwonge, R.; Esmy, P.O.; Basu, P.; Prabhu, P.; Bhatla, N.; Nene, B.M.; Shaw, J.; Poli, U.; et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine 2018, 36, 4783–4791. [Google Scholar] [CrossRef]
- Ramírez, M.; de la Fuente, J.; Andía, D.; Hernández, J.J.; Fiol, G.; Torné, A. HPV vaccination coverage in women between 15-55 years in Spain. Temporal trend during the period 2007–2020. Int. J. Gynecol. Obstet. 2021. [Google Scholar] [CrossRef]
- Human Papillomavirus and Related Diseases Report WORLD. Available online: www.hpvcentre.net (accessed on 25 February 2022).
- Sanidad de Valencia Dice que Las dos Niñas con Efectos Adversos a la Vacuna del VPH no Tenían Patología de Base Previa. Available online: https://www.europapress.es/ciencia/noticia-sanidad-valencia-dice-dos-ninas-efectos-adversos-vacuna-vph-no-tenian-patologia-base-previa-20090210145652.html (accessed on 6 March 2022).
- Calendario Común de Vacunación a lo Largo de Toda la Vida. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromo- (accessed on 26 October 2020).
- Li, K.; Li, Q.; Song, L.; Wang, D.; Yin, R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer 2019, 125, 1030–1037. [Google Scholar] [CrossRef]
- Aimagambetova, G.; Azizan, A. Epidemiology of HPV Infection and HPV-Related Cancers in Kazakhstan: A Review. Asian Pac. J. Cancer Prev. 2018, 19, 1175–1180. [Google Scholar] [CrossRef]
- Spayne, J.; Hesketh, T. Estimate of global human papillomavirus vaccination coverage: Analysis of country-level indicators. BMJ Open 2021, 11, e052016. [Google Scholar] [CrossRef]
- Nota informativa de la AEMPS Sobre la Seguridad de Las Vacunas Frente al Virus Del Papiloma Humano: Conclusiones Del Comité de Expertos—Agencia Española de Medicamentos y Productos Sanitarios. Available online: https://www.aemps.gob.es/informa/notasinformativas/medicamentosusohumano-3/seguridad-1/2009/ni_2009-06_segvacupapilomahumano/ (accessed on 27 February 2022).
- Simms, K.T.; Steinberg, J.; Caruana, M.; Smith, M.A.; Lew, J.-B.; Soerjomataram, I.; Castle, P.E.; Bray, F.; Canfell, K. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–2099: A modelling study. Lancet Oncol. 2019, 20, 394–407. [Google Scholar] [CrossRef]
- Amdisen, L.; Kristensen, M.L.; Rytter, D.; Mølbak, K.; Valentiner-Branth, P. Identification of determinants associated with uptake of the first dose of the human papillomavirus vaccine in Denmark. Vaccine 2018, 36, 5747–5753. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Navaro, M.; Vezzosi, L.; Santagati, G.; Angelillo, I.F. Primary care pediatricians’ attitudes and practice towards HPV vaccination: A nationwide survey in Italy. PLoS ONE 2018, 13, e0194920. [Google Scholar] [CrossRef] [PubMed]
- Nowak, G.J.; Cacciatore, M.A. Parents’ confidence in recommended childhood vaccinations: Extending the assessment, expanding the context. Hum. Vaccines Immunother. 2017, 13, 687–700. [Google Scholar] [CrossRef] [Green Version]
- Claeys, A.P.; Anttila, P.; Bonnanni, A.; Finn, D.; Lévy-Bruhl; Soldan, K. Guidance for the introduction of HPV vaccines in EU countries. Guid. Rep. 2008, 58. [Google Scholar]
- Jit, M.; Brisson, M.; Portnoy, A.; Hutubessy, R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: A PRIME modelling study. Lancet Glob. Health 2014, 2, e406–e414. [Google Scholar] [CrossRef] [Green Version]
- Human Papillomavirus in Young People Epidemiological Research 2 (HYPER2)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03000933?term=NCT03000933&draw=2&rank=1 (accessed on 27 February 2022).
- Natural History of HPV Infection in Men: The HIM Study—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00786760?term=NCT00786760&draw=2&rank=1 (accessed on 27 February 2022).
- The HPV 9-10 Trial: Early Initiation of HPV Vaccination—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04722822?term=NCT04722822&draw=2&rank=1 (accessed on 27 February 2022).
| Region 1 | Region 2 | Region 3 | Region 4 | All Regions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | N | (VCR%) 1 | N | (VCR%) 1 | N | (VCR%) 1 | N | (VCR%) 1 | N | (VCR%) 1 |
| 15–19 | 1756 | 55.5 | 1613 | 57.5 | 449 | 58.6 | 241 | 56.9 | 4059 | 56.7 |
| 20–24 | 1765 | 17.6 | 1484 | 18.6 | 462 | 20.2 | 196 | 22.4 | 3907 | 18.5 |
| 25–29 | 1728 | 9.8 | 1494 | 12.3 | 484 | 11.9 | 224 | 24.5 | 3930 | 11.8 |
| 30–34 | 2015 | 6.2 | 1773 | 6.1 | 515 | 3.9 | 241 | 16.6 | 4544 | 6.4 |
| 35–40 | 3020 | 4.2 | 2678 | 3.4 | 678 | 2.5 | 320 | 7.5 | 6696 | 3.8 |
| All | 10,284 | 16.6 | 9042 | 17.5 | 2588 | 17.4 | 1222 | 24.5 | 23,136 | 17.4 |
| Average age (years (SD)) 2 | ||||||||||
| G1 3 | 19.2 (2.9) | 19.7 (2.9) | 20.3 (5.3) | 19.7 (3.3) | 19.2 (2.9) | |||||
| G2 4 | 30.4 (6.8) | 29.9 (6.4) | 29.7 (5.6) | 27.5 (7.5) | 29.4 (6.6) | |||||
| G3 5 | 28.6 (7.6) | 28.6 (7.7) | 28.3 (7.5) | 28.2 (7.6) | 28.4 (7.6) | |||||
| Non-vaccinated | 31.8 (6.0) | 32.1 (5.9) | 31.6 (5.9) | 32.1 (5.8) | 31.9 (5.9) | |||||
| Vaccinated | 21.0 (5.4) | 20.7 (5.2) | 20.6 (4.7) | 22.1 (5.9) | 21.0 (5.4) | |||||
| All | 26.4 (5.7) | 26.7 (5.6) | 26.1 (5.3) | 27.1 (5.9) | 26.6 (5.6) | |||||
| Aging index | 153.5 | 151.4 | 200.7 | 247.9 | 162.7 | |||||
| Average income/inhabitant | 79.1 | 73 | 84.3 | 79.3 | 77.4 | |||||
| Inhabitant density/km2 | 78.5 | 93 | 26.4 | 15.3 | 54.5 | |||||
| Region 1 | Region 2 | Region 3 | Region 4 | All Regions | |
|---|---|---|---|---|---|
| Complete Vaccination (%) According to Group | |||||
| G1 1 | 75.3 | 75.8 | 78.9 | 60.3 | 74.8 |
| G2 2 | 9.2 | 8.7 | 4.6 | 11.0 | 8.7 |
| G3 3 | 15.4 | 15.4 | 16.4 | 28.6 | 16.4 |
| Cervical screening test and cytology (%) according to vaccine provider registration | |||||
| Average age | |||||
| (years (SD) 4) | 33.4 (4.7) | 33.3 (4.9) | 33.2 (4.5) | 33.5 (4.4) | 33.4 (4.7) |
| Not vaccinated | 42.8 | 31.1 | 32.5 | 44.4 | 37.1 |
| Vaccinated | 11.9 | 9.4 | 3.5 | 13.6 | 10.1 6 |
| All | 33.3 | 24.2 | 23.4 | 31.6 | 28.5 |
| HPV screening PCR 5 (%) according to vaccine provider registration | |||||
| Average age | |||||
| (years (SD) 4) | 34.2 (4.5) | 34.5 (4.7) | 31.8 (5.4) | 35.3 (4.1) | 34.6 (4.4) |
| Not vaccinated | 6.7 | 4 | 2.8 | 6.5 | 5.2 |
| Vaccinated | 4.7 | 3 | 1.7 | 3.3 | 3.6 6 |
| All | 6.1 | 3.7 | 2.5 | 5.2 | 4.7 |
| HPV-positive PCR 5 (%) according to vaccine provider registration | |||||
| Average age | |||||
| (years (SD) 4) | 32.5 (4.8) | 33.3 (5.2) | 27.2 (4.3) | 34.6 (5.4) | 32.4 (5.2) |
| Not vaccinated | 0.6 | 0.4 | 0.5 | 1.1 | 0.5 |
| Vaccinated | 2 | 1.9 | 1.3 | 0.9 | 1.8 6 |
| All | 1 | 0.9 | 0.8 | 0.9 | 0.9 |
| Age Groups | 15–19 | 20–24 | 25–29 | 30–34 | 35–40 | Total |
|---|---|---|---|---|---|---|
| Complete HPV Vaccination/Total Vaccinable Group 1 | ||||||
| G1 | 2295 | 692 | 187 | 0 | 0 | 3174 |
| (56.4%) | (17.6%) | (4.7%) | (0.0%) | (0.0%) | (26.6%) | |
| G2 + G3 | 10 | 32 | 281 | 295 | 260 | 878 |
| (0.2%) | (0.8%) | (7.1%) | (6.4%) | (3.8%) | (7.7%) | |
| Cervical Screening Test and Cytology/Total for Age Group | ||||||
| Average Age | 33.3 (4.8) | |||||
| (years, (SD)) | ||||||
| N | 7 | 253 | 1279 | 2008 | 3083 | 6630 |
| (%) | (0.2) | (6.5) | (32.4) | (44.0) | (45.8) | (28.5) |
| HPV Screening PCR Test/Total for Age Group | ||||||
| Average Age | 34.2 (4.6) | |||||
| (years, (SD)) | ||||||
| N | 1 | 34 | 155 | 324 | 583 | 1097 |
| (%) | (0.0) | (0.9) | (3.9) | (7.1) | (8.7) | (4.7) |
| Positive Result HPV Screening PCR Test/Total HPV Screening PCR Test | ||||||
| Average Age | ||||||
| (years, (SD)) | 32.4 (5.2) | |||||
| N | 0 | 20 | 47 | 65 | 92 | 224 |
| (%) | (0.0) | (58.8) | (30.3) | (20.1) | (18.9) | (20.4) |
| Region 1 | Region 2 | Region 3 | Region 4 | All Regions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | Number of Doses Administered | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) |
| 15–19 | 0 | 272 | 15.4 | 210 | 13 | 75 | 16.7 | 29 | 12 | 586 | 14.4 |
| 1 | 508 | 28.9 | 476 | 29.5 | 111 | 24.7 | 75 | 31.1 | 1170 | 28.8 | |
| ≥2 | 976 | 55.5 | 927 | 57.4 | 263 | 58.5 | 137 | 56.8 | 2303 | 56.7 | |
| 20–24 | 0 | 582 | 32.9 | 445 | 29.9 | 133 | 28.7 | 36 | 18.3 | 1196 | 30.6 |
| 1 | 873 | 49.4 | 763 | 51.4 | 236 | 51 | 116 | 59.1 | 1988 | 50.8 | |
| ≥2 | 310 | 17.5 | 276 | 18.6 | 93 | 20.1 | 44 | 22.4 | 723 | 18.5 | |
| 25–29 | 0 | 1523 | 88.1 | 1280 | 85.6 | 417 | 86.1 | 160 | 71.4 | 3380 | 86 |
| 1 | 35 | 2 | 31.0 | 2 | 9 | 1.8 | 9 | 4 | 84 | 2.1 | |
| ≥2 | 170 | 9.8 | 183 | 12.2 | 58 | 11.9 | 55 | 24.5 | 466 | 11.8 | |
| 30–34 | 0 | 1862 | 92.4 | 1650 | 93 | 494 | 95.9 | 198 | 82.1 | 4204 | 92.5 |
| 1 | 29.0 | 1.4 | 14 | 0.7 | 1 | 0.1 | 3 | 1.2 | 47 | 1 | |
| ≥2 | 124 | 6.1 | 109 | 6.1 | 20 | 3.8 | 40 | 16.6 | 293 | 6.4 | |
| 35–40 | 0 | 2873 | 95.1 | 2571 | 96 | 658 | 97 | 293 | 91.5 | 6395 | 95.5 |
| 1 | 20 | 0.6 | 16 | 0.6 | 3 | 0.4 | 3 | 0.9 | 42 | 0.6 | |
| ≥2 | 127 | 4.2 | 91 | 3.4 | 17 | 2.5 | 24 | 7.5 | 259 | 3.8 | |
| All incompletely or not vaccinated | 8577 | 83.4 | 7456 | 82.4 | 2137 | 82.5 | 922 | 75.4 | 19.092 | 82.5 | |
| All completely vaccinated | 1707 | 16.6 | 1586 | 17.5 | 451 | 17.4 | 300 | 24.5 | 4044 | 17.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomé-Ceballos, L.; Clua-Espuny, J.L.; Fernández-Sáez, J.; Ceballos-García, C.; Andrés-Cubells, N.; Pla-Farnós, M.J. HPV Vaccination Coverage Rate in a Rural Area: An Observational, Retrospective, and Cohort Study. Vaccines 2022, 10, 1274. https://doi.org/10.3390/vaccines10081274
Colomé-Ceballos L, Clua-Espuny JL, Fernández-Sáez J, Ceballos-García C, Andrés-Cubells N, Pla-Farnós MJ. HPV Vaccination Coverage Rate in a Rural Area: An Observational, Retrospective, and Cohort Study. Vaccines. 2022; 10(8):1274. https://doi.org/10.3390/vaccines10081274
Chicago/Turabian StyleColomé-Ceballos, Lara, Josep Lluís Clua-Espuny, José Fernández-Sáez, Concepción Ceballos-García, Natàlia Andrés-Cubells, and Maria Jesús Pla-Farnós. 2022. "HPV Vaccination Coverage Rate in a Rural Area: An Observational, Retrospective, and Cohort Study" Vaccines 10, no. 8: 1274. https://doi.org/10.3390/vaccines10081274
APA StyleColomé-Ceballos, L., Clua-Espuny, J. L., Fernández-Sáez, J., Ceballos-García, C., Andrés-Cubells, N., & Pla-Farnós, M. J. (2022). HPV Vaccination Coverage Rate in a Rural Area: An Observational, Retrospective, and Cohort Study. Vaccines, 10(8), 1274. https://doi.org/10.3390/vaccines10081274






