Abstract
Human lymphatic filariae have evolved numerous immune evasion strategies to secure their long-term survival in a host. These strategies include regulation of pattern recognition receptors, mimicry with host glycans and immune molecules, manipulation of innate and adaptive immune cells, induction of apoptosis in effector immune cells, and neutralization of free radicals. This creates an anti-inflammatory and immunoregulatory milieu in the host: a modified Th2 immune response. Therefore, targeting filarial immunomodulators and manipulating the filariae-driven immune system against the filariae can be a potential therapeutic and prophylactic strategy. Filariae-derived immunosuppression can also be exploited to treat other inflammatory diseases and immunopathologic states of parasitic diseases, such as cerebral malaria, and to prevent leishmaniasis. This paper reviews immunomodulatory mechanisms acquired by these filariae for their own survival and their potential application in the development of novel therapeutic approaches against parasitic and inflammatory diseases. Insight into the intricate network of host immune-parasite interactions would aid in the development of effective immune-therapeutic options for both infectious and immune-pathological diseases.
1. Introduction
Lymphatic filariasis is an infection in humans caused by filarial parasites: Wuchereria bancrofti, Brugia malayi, and B. timori. To ensure effective transmission, these parasites evolved with multiple hosts, including a human as a definitive host and the mosquito as an intermediate host. Adult worms can live for up to 7 years in a human, and their microfilarial (Mf) stage can circulate in the bloodstream for up to 9 months. While biting an infected human, mosquitoes ingest microfilariae. Here, microfilariae molt twice and grow into the infective larval (L3) stage. When infected mosquitoes bite another human host, they deposit L3 filariae on the skin, which travel through the cutaneous tissues to the local lymphatics, rapidly develop into the L4 stage, and then migrate to the central lymphatic system, where they develop into sexually mature adults. After mating with male adults, female worms produce approximately 10,000 microfilariae per day, continuing the transmission cycle. A constant co-evolutionary race between the parasite and its host leads to mutual co-existence without causing harm to the host. Their success implies masterful immune evasion strategies. To achieve long-term survival in different anatomical compartments of the host, parasites release a variety of products that are stage- and gender-specific, reflecting specific developmental processes and diverse strategies for evasion of host immune responses [1]. Evaluation of these products will pave the way for a better understanding of how these filarial parasites orchestrate immune evasion.
In 1994, the WHO/United Nations Development Programme/World Bank sponsored the Filarial Genome Project (FGP) to study and map the B. malayi genome (the first parasitic nematode that was sequenced) [2]. Comparative genomic and proteomic analyses of B. malayi with other parasitic and free-living nematodes have led scientists to understand the genetic and molecular basis of the host–parasite relationship, including the parasite’s immune avoidance tactics [1,3,4]. As a result of these analyses, new potential immunomodulatory and therapeutic targets were identified and prioritized for further in vivo and clinical studies. Many recent reviews have shed light on the immunoregulatory role of helminth-derived fractions and individual products. In this review, we begin with immunomodulatory strategies acquired by human–tropic lymphatic filarial parasites to establish parasitism. Furthermore, we highlight the potential of these filariae and their immunomodulatory molecules in the development of novel therapeutic approaches against various parasitic and inflammatory diseases. A better comprehension of the relationship between the host immune system, filarial infection, and other co-infections can provide new insights into effective immunological treatments.
2. Immunomodulation Strategies
Within 3 h of exposure to large and migrating larvae, the host initiates an early acute inflammatory response that elicits a Th1 cytokine response (IFN-γ, TNF-α, IL-1α, IL-8, and granulocyte-macrophage colony-stimulating factor) within 24 h [5,6]. However, this may also cause significant and undesirable tissue damage. Therefore, to maintain homeostasis, the host switches to a different immune profile against these long-lived filariae 7 days post-infection. This immune-regulatory response is of the IL-10-dominated modified Th2-type, characterized by dysfunction of antigen-presenting cells (APC); increased levels of anti-inflammatory cytokines (IL-4, IL-10, and TGF-β), regulatory T cells, and alternatively activated macrophages (AAM); and induction of immune cell apoptosis. Unlike Th1- or Th17-type immune responses induced by other microbial infections, the modified Th2-type response controls inflammation, repairs tissues injured during infection and migration, and restores homeostasis in an infected host [7].
Filariae manipulate the host’s defense system by producing and releasing a variety of bioactive chemicals and extracellular vesicles (helminth-derived particles (HDPs)) that attack the host’s intracellular and extracellular immune apparatus. Certainly, comprehensive efforts to characterize the full immunomodulatory abilities of filariae and their HDPs are still in their infancy, making this a large and prospective area of research.
2.1. Infective L3 Mute Cutaneous Innate Cells
In response to microbial infection, damaged cells produce alarmins such as thymic stromal lymphopoietin (TSLP), IL-25, and IL-33. These cytokines activate type 2 innate lymphoid cells (ILC2), a major contributor of anti-helminthic immunity that triggers and amplifies type 2 inflammation [8]. However, L3 filariae masterfully evade the immune response in the cutaneous tissue. Although the presence of ILC2 and alarmins, IL-25 and IL-33, has not been studied directly at the site of infection (skin), previous studies have demonstrated that L3 filariae do not induce ILC2 stimulating cytokines IL-18 and TSLP [9,10]. The spatial difference between ILCs (found mainly in the upper dermal layer) and Langerhans cells (LCs, found mainly in the epidermal layer) in the skin [11] can be one reason for the failure of cutaneous ILC activation. LCs and dermal dendritic cells (DCs) remain quiescent [10] and fail to initiate an ILC2-dependent inflammatory response to L3 filariae. Filarial molecules responsible for muting this cutaneous immune response remain unexplored and warrant further investigation. Strikingly, W. bancrofti infected Mf+ patients showed increased cKit+ ILCs in the peripheral blood that drives Th17 immune response [12], which may be involved in immune-pathologic consequences [5,13]. However, significant increases in IL-4 alone or in both IL-4 and IL-10 producing CD4+ cells, IL-10-producing adaptive T regulatory cells (aTreg/Tr1), and natural T regulatory cells (nTregs) in Mf+ patients at homeostasis are likely to downregulate the immune response [13]. Overall, this suggests a heterogeneous ILC response to different filarial stages and anatomical locations in the host.
2.2. Filariae Regulate PRR Signaling
Innate immune cells are equipped with pattern recognition receptors (PRRs) that recognize specific pathogen-associated molecular patterns (PAMPs) and trigger intracellular downstream pathways that evoke pro-inflammatory responses. Most PRRs can be classified into Toll-like receptors (TLRs), nucleotide oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), C-type lectin receptors (CLRs), and absent in melanoma-2 (AIM2)-like receptors (ALRs) [14].
During immunomodulation, mostly glycan conjugated products (glycoproteins and glycolipids) target TLRs and CLRs. This strategy of parasitic survival, called “glycan gimmickry”, not only alters TLR expression, but also masterly manipulates its intracellular signaling [15,16]. For instance, B. malayi and W. bancrofti secrete glycoproteins, such as leucyl aminopeptidase [17,18,19], a homolog of a phosphorylcholine (PC)-carrying glycoprotein ES-62 of Acanthocheilonema viteae [20]. In monocytes, ES-62 forms a complex with TLR4. Subsequent internalization of ES-62–TLR4 complexes drives caveolae/lipid raft-dependent sequestration and autophagolysosomal degradation of protein kinase C-α, a molecule essential for mast cell activation [21] (Figure 1A). In DCs, ES-62 downregulates TLR4-associated protein kinase C-δ (PKC-δ), upregulates and sequesters p62 and LC3 (components of an autophagy machinery), induces their autophagolysosomal degradation, and suppresses the LPS-driven release of IL-6, IL-12p70, and TNF-α [22] (Figure 1B). ES-62-induced degradation of PKC-δ is supposed to hamper DC development and motility, IL-12p40/p70 expression, MHC II-Ag presentation, and Th1 polarization. Although the effect of purified B. malayi homologs of ES-62 has not been studied yet, B. malayi microfilariae were found to significantly downregulate the expression of TLR4, downstream molecules (MyD88 protein and the binding ability of p50 and p65), and pro-inflammatory IL-12p40 in LPS activated DCs [23]. In contrast, phosphorylcholine-binding W. bancrofti sheath antigen induces DC maturation and pro-inflammatory cytokine secretion via the TLR4-dependent pathway that drives Th1 and regulatory T cell responses [24]. TLR4-dependent downstream signaling in DC subsets with differing cellular responses invites additional mechanistic research regarding the role of these glycans in immune response modulation. Setaria cervi thioredoxin reductase (TrxR) shows 100% sequence identity with B. malayi TrxR isoform C. In addition to its antioxidant activity, TrxR possesses anti-inflammatory activity in macrophages with inhibition of the TLR4/NF-κB axis, down-regulation of the inflammasome pathway, and activation of AAM [25] (Figure 1C).
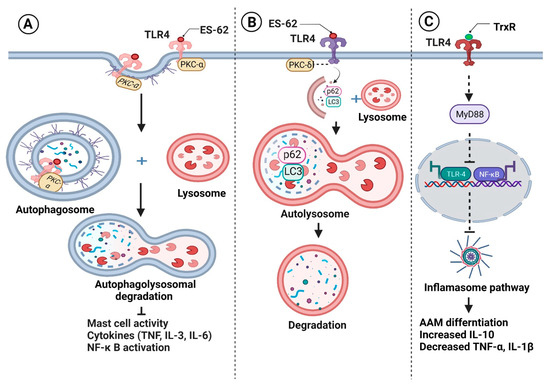
Figure 1.
Filariae regulate pattern recognition receptors signaling for their survival. Filarial glycoproteins such as ES-62 (A) form complexes with Toll-like receptor 4 (TLR) on monocytes, followed by their internalization and sequestration with protein kinase C-α and (B) down-regulate TLR4-associated protein kinase C-δ (PKC-δ) in dendritic cells, followed by sequestration of p62 and LC3. As a result, sequestered components are degraded by autophagolysosomes, inhibiting the production of pro-inflammatory cytokines and inflammatory cell polarization. (C) Thioredoxin reductase (TrxR) inhibits the TLR4/NF-κB pathway, prevents inflammasome activation, and induces alternatively activated macrophages (AAM) activation, which is characterized by an increase in anti-inflammatory IL-10 synthesis and a decrease in pro-inflammatory cytokines. Figure created using Biorender.com.
2.3. HDPs Mimic Host Glycans and Immune Molecules
Another approach used by parasites is molecular mimicry, in which filariae express host-like glycan antigens on their surface to evade recognition by the host’s immune system. Ludin et al. (2011) provided a list of molecular mimicry candidates from human parasites, including B. malayi [26]. Microfilarial and adult stages of B. malayi express TGH-2, a human TGF-β homolog, and activate immunosuppressive mechanisms through TGF-βR signaling [27,28]. Another example is the B. malayi protein BmHsp12.6, which has an IL-10-like function in addition to chaperone activities [29]. Microfilariae of W. bancrofti and B. malayi secrete prostaglandins, mainly PGE2 [30]. PGE2 plays a diverse role in immune regulation [31], including induction of FOXP3+ Treg cells [32,33], vasodilation, and inhibition of platelet aggregation [30,34]. Similar to human macrophage migration inhibitory factor (MIF), B. malayi MIF-1 and -2 activate human monocytes to produce pro-inflammatory cytokines IL-8, TNF-α, and endogenous MIF in vitro [35]. However, in the presence of IL-4, B. malayi MIF promotes AAM differentiation, implying that Bm-MIF plays a different role on macrophages depending on the prevailing cytokine environment [36]. Moreover, the sex specific fucosylation of MIF-1 is believed to enhance its immune-suppressive function, demanding further investigation of its glycosylated state [37].
2.4. Filariae Manipulate B and T Cell Response
Among several immune pathways, the effect of filarial parasites on Tregs with concomitant secretion of immunosuppressive cytokines (IL-10 and TGF-β) is a primary mechanism of controlling inflammation (Figure 2). For instance, W. bancrofti sheath antigen induces DC activation through TLR4 signaling, followed by Th1 and Treg cell elicitation [24]. Strikingly, in another study, a W. bancrofti-infected population showed distinct IL-10-producing regulatory B and T cell subsets, which is helpful for the parasite’s survival [38]. At the time of infection, B. malayi larvae secrete abundant larval transcript (Bm-ALT) protein, which is associated with upregulation of GATA-3 transcription factor in macrophages, inducing a Th2 immune response. Moreover, Bm-ALT induces SOCS-1, inhibits IFN-γR associated JAK kinase in macrophages, and interferes with signals required for the development of pro-inflammatory Th1 cells [39]. BmK1 protein from B. malayi selectively blocks voltage-gated potassium (Kv) 1.3 channels and suppresses IFN-γ production in CCR7- effector memory T cells, but not in naïve or central memory T cells [40].
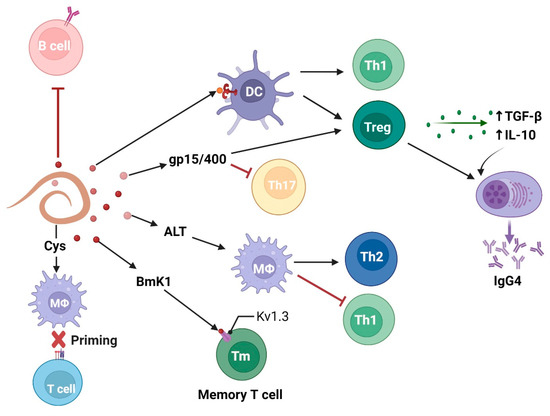
Figure 2.
Filarial parasites modulate adaptive immune cell response characterized by T regulatory and anti-inflammatory Th2 cells with concomitant release of immunosuppressive cytokines (IL-10 and TGF-β). Figure created using Biorender.com.
Researchers have shown that PC alone, PC-BSA, and PC carrying ES-62 directly inhibit polyclonal activation of B cells in a PKC-dependent manner, implying that PC-containing molecules in B. malayi can imitate immunosuppressive action [41]. Notably, PC is not identified on the ES-62 homolog, leucyl aminopeptidase (LAP), in B. malayi, but on another secretory protein called N-acetylglucosaminyltransferase [42]. It is known that Treg cells and IL-10 induce IgG4 production by B cells. Immunosuppressive IgG4 is neither able to activate the complement system nor induce antibody-dependent cell-mediated cytotoxicity (ADCC) after binding to CD16 on neutrophils and eosinophils [43].
Some filarial molecules exploit the immune response in a receptor-independent manner. For example, apart from receptor-mediated modulation of immune cells [44], B. malayi cystatins (BmCys) inhibit host cysteine proteases and asparaginyl endopeptidase, impair antigen presentation on APCs, and reduce T cell priming [45]. Another atypical filarial immunomodulator is a B. malayi polyprotein “ladder”, gp15/400, that exploits the immune response in a metabolite-dependent manner. It is suggested that this retinoid binding protein enhances vitamin A uptake by host tissues [46]. In the presence of retinoic acid (a vitamin A metabolite), TGF-β inhibits IL-6-driven TH17 cell proliferation and enhances FOXP3+ Treg cell differentiation [47].
2.5. Filarial Parasites Induce Immune Cell Apoptosis and Autophagy
Filarial parasites prolong their infection by inducting apoptosis to lower the immune cell population. B. malayi L3 filariae activate NK cells to produce IFN-γ and TNF-α, which facilitates cell death via the caspase-dependent pathway [48]. B. malayi Mf affect human DCs in two ways: (1) by modifying their function and (2) by triggering cell death, resulting in an antigen-specific T cell hypo-response. Microfilariae interact with human DCs to form cell–parasite aggregates, trigger DC apoptosis in a TRAIL- and TNF-alpha-dependent manner, and impair their ability to produce IL-12, limiting CD4+ T cell activation and proliferation [23,49]. Apart from apoptosis, microfilariae trigger autophagy in DCs by inhibiting phosphorylation of mTOR and its downstream proteins (p70S6K1 and 4E-BP1), upregulating Beclin 1 phosphorylation, inducing LC3II, and degrading p62 [50]. Recently, researchers demonstrated that microfilariae release extracellular vesicles that DCs quickly internalize. These extracellular vesicles are rich in unique miRNAs that target the mTOR signaling pathway [51]. W. bancrofti has been shown to trigger apoptosis of CD4+ T cells via FasL-expressing B-1 cells (induced by elevated IL-10 levels), resulting in a hypo-immune response in infected patients [52].
2.6. Non-Cellular Immune Evasion by Filarial Parasites
Filarial parasites are armed with high levels of antioxidant enzymes and non-enzymatic anti-oxidants—glutathione peroxidase, superoxide dismutases, glutathione-s-transferase (GST), thioredoxin peroxidase, TrxR, glutathione (GSH), ascorbic acid, translationally controlled tumor protein, and α-tocopherol—that play an important role in protection against free radicals generated during host immune cell attack [1,46,53,54,55]. In addition, B. malayi secretes acetylcholinesterases, which may prevent fluid accumulation in the gut and inhibit parasite clearance [46]. Another B. malayi protein, calreticulin, binds to human C1q and blocks further classical complement pathways [56].
These are not the only mechanisms used by filarial parasites to ensure their own survival and prevent extensive damage to the host’s body. A variety of unexplored mechanisms and complex molecules facilitate immune evasion. The evidence for human lymphatic filariae, however, is limited.
3. Filarial Immunomodulatory Strategy as a Treatment against Diseases
3.1. Lymphatic Filariasis
In 1997, the World Health Assembly set the goal of eliminating lymphatic filariasis globally by 2020 through mass drug administration (MDA). During MDA, all individuals living in endemic areas received one of these single-dose two-drug combinations: albendazole (ALB) + diethylcarbamazine (DEC) citrate; ALB + ivermectin (IVM) in areas co-endemic for onchocerciasis; or ALB, preferably twice a year, in areas co-endemic for loiasis. However, numerous obstacles stand in the way of successful implementation. These drugs are only effective against microfilariae and not against adult and larval parasites. By the end of 2020, MDA had not yet been delivered to ten endemic countries [57], which raised concerns about the recurrence of filarial infections in countries or areas that were previously declared free of LF infection [58]. One reason for this concern is human migration from endemic to LF-free areas [59,60,61,62]. The majority of migrants were from rural endemic areas, which had poor sanitation, rice fields, and inadequate mosquito control. Moreover, climate change and delays in MDA due to COVID-19 are likely to further sabotage eradication efforts [63,64]. According to the WHO’s 2021 report, 859 million people in 50 countries are at risk of lymphatic filariasis, which requires preventive treatment. As a result, the WHO revised the target date to 2030, using a triple-drug MDA combination of IVM, DEC citrate, and ALB (IDA-MDA), which may result in patient non-compliance [65,66,67,68]. This evidence demands the development of effective vaccines and novel therapeutics.
Strikingly, current antifilarial drugs target the immunomodulatory arsenal of filariae; they alter the host-parasite interface, unmasking the host immune system to access the parasite. The widely used drug DEC is believed to block PGI2 and PGE2 production in both microfilariae and endothelial cells. The resulting vasoconstriction enhances endothelial adhesion and microfilariae immobilization as well as destruction by host platelets and granulocytes [69]. IVM prevents protein release from microfilarial extracellular vesicles by blocking the GluCl channel. These proteins are indispensable for evading the host immune system [70]. Maclean et al. (2021) recently investigated the effects of DEC and IVM treatment on the B. malayi gene expression that may be responsible for filarial clearance from blood circulation [71]. For example, treatment with either IVM or DEC downregulated galectin expression in adults. Galectins, among many other immunomodulatory effects, impede lymphocyte trafficking [72], stimulate alternative macrophage activation [73], and cause T cell apoptosis [74]. Since oxidative and xenobiotic detoxification mediated by antioxidants is a fundamental survival strategy for filariae, we synthesized and studied the library of sulphonamide chalcones that affect filarial GSH status, produce oxidative stress, and lead to apoptosis [75,76].
Indeed, drugs can heal existing infections, but they will not prevent infections unless they, or their active metabolites, are removed slowly from the host system, and remain in circulation for a lengthy period. Given that filariae orchestrate the host’s immune system for their own growth and survival, manipulating the host’s defense system against LF could be a viable prophylactic option. Several potential vaccine candidates have been identified and tested for their potential against LF [77]. Many antigens are non-homologous to human and immunomodulatory proteins that subvert the host’s immune response against the parasite.
Table 1 summarizes immune-regulatory proteins that have been evaluated as vaccine candidates. B. malayi immunomodulatory proteins such as heat shock protein 12.6 (BmHsp12.6αc), abundant larval transcript-2 (Bm-ALT-2), and tetraspanin large extracellular loop (Bm-TSP LEL), showed maximum protection in mouse challenge experiments [29,78,79]. To improve the protective efficacy of monovalent vaccines, these best vaccine candidates were fused to prepare a single multivalent vaccine, rBmHAT (BmHsp12.6 + BmALT-2 + BmTSPLEL). Strikingly, it showed >95% protection against B. malayi infection in mice when AL007 or AL019 was used as an adjuvant [80]. However, when administered with alum in non-human primates, rBmHAT provided ~35% protection [81], hinting at a need to change the adjuvant and/or multivalent formulation before using this vaccine in human clinical trials. Adding another immunomodulatory antigen, thioredoxin peroxide (BmTPX-2), to rBmHAT showed >88% protection against the challenge infection [82]. This tetravalent rBmHAXT confers approximately 57% protection against challenge infections in a primate model, which meets the WHO requirement, and hence offers great potential for using this vaccine in human clinical trials [83].

Table 1.
Filarial immune-regulatory proteins that have been evaluated as vaccine candidates.
3.2. Malaria
Co-infections are common in endemic regions. Control of intracellular pathogens, such as Plasmodium species that cause malaria, Leishmania donovani, Mycobacterium tuberculosis (Mtb), and human immunodeficiency virus (HIV), requires pro-inflammatory Th1 (IL-12, IFN-γ, and TNF-α) and Th17 (IL-17A and IL-23) responses. Increasing evidence suggests that filariae-driven Th2 and Treg immunity can negatively affect the host’s ability to combat these pathogens.
The effect of filarial co-infection on Plasmodium spp. has already been discussed in detail [94]. Human and animal studies on LF/malaria co-infection have provided conflicting results, with some demonstrating more severe malaria in the presence of filarial co-infections and others suggesting filariae-induced protection against malaria, depending on the infection severity and parasite type [95,96,97,98,99,100]. A strong Th1 immune response plays a major role in controlling primary malaria infection. However, the filariae-induced IL-10-dependent Th2 immune response modulates inflammatory IL-12p70/ IFN-γ pathways and increases resistance to malaria [98]. Moreover, pre-existing filarial infection can impair the immunogenicity of anti-Plasmodium vaccination, as evidenced by decreases in plasmodium antigen-specific CD8+ T cells, IFN-γ, and TNF-α production, resulting in reduced cytotoxicity and protection against malarial infection [101]. A simple solution to filarial interference with vaccination efficacy is deworming before vaccination [102]. However, there are several obstacles to drug-induced abolition of filarial infection in endemic locations. These include (1) the lack of an adulticidal or adult-sterilizing drug or vaccine, (2) the time it takes to return to a normal immune response, and (3) the risk of re-infection during the recovery period. Therefore, it is desirable to optimize appropriate vaccination regimes that elicit a multifaceted and potent immune response in filariae-infected individuals [101,102].
Unlike acute malaria, cerebral malaria and malarial sepsis are triggered due to exaggerated pro-inflammatory responses; filariae-derived immunosuppression can protect against this severe immunopathology [95,99]. However, maintaining a filarial infection to avoid an inflammatory exacerbation is not a smart option. In-depth study is required to strike a delicate balance between permissive filarial infection that does not progress to lymphatic filariasis and appropriate immunosuppression that does not lead to severe complications of malaria. Therapies that imitate filariae-derived immunosuppression may be investigated for the treatment of cerebral malaria.
3.3. Leishmaniasis
Leishmaniasis, the third most common vector-borne disease after malaria and lymphatic filariasis, is caused by the protozoan Leishmania parasite. Visceral leishmaniasis, also known as kala-azar, is caused by L. donovani and L. infantum throughout Asia, North Africa, Latin America, and Southern Europe. Every year, 700,000 to 1 million new cases are reported. The WHO actively encourages research into effective leishmaniasis control [57]. Fractions derived from B. malayi were found to cross-react with sera from hamsters infected with L. donovani, suggesting that these filarial cross-reactive molecules can contribute to the development of anti-leishmanial prophylactics [103]. In vivo studies in hamsters demonstrated that B. malayi L3/adult worms or immunization with a fraction of the adult parasite extract (BmAFII) inhibited the progression of both filarial and L. donovani infections [104,105]. Recently, studies have shown that heat shock protein 60 (BmHSP60) shares several antigenic regions of B- and T-cell epitopes of leishmania counterparts and protects against leishmanial infection via Th1-mediated immune responses and NO production [103,106]. In contrast, a fraction of L. donovani (Ld1) that cross-reacted with sera of B. malayi infected animals facilitated filarial infection. Ld1 consists of eight proteins, including HSPs [107]. Therefore, more comprehensive and in-depth investigations are needed to optimize and develop prophylactics based on cross-reactive rationale in co-endemic regions.
The prevalence of filarial and leishmanial co-infections has been reported in some parts of the world [108]. In the co-infected mouse model, local immune responses to filarial and leishmanial infections were polarized and compartmentalized [109]. These findings contradict acute malarial findings in which microfilariae and Plasmodium share the same niche—blood. In popliteal lymph nodes (which drain the L. major infection site) and thoracic lymph nodes (which drain the L. sigmodontis infection site) immune responses were IFN-γ- and IL-4-dominant, respectively. Moreover, pre-existing helminth infection delayed IFN-γ production and L. major-induced lesion progression [109]. Notably, unlike the leishmanial co-infection model, which confines parasites to the thoracic cavity, the presence of human lymphatic microfilariae in the bloodstream may provide a different immune outcome. Appropriate filarial animal and human population studies are needed to assess whether the immune response to LF/leishmaniasis co-infection is defensive or progressive; such assessment will aid in the development of appropriate and specific immune modulation therapies.
3.4. Inflammatory Diseases
In developed societies, large-scale deworming programs, reduced exposure to infection due to vaccination, and improved sanitation are associated with an increase in the occurrence of inflammatory and metabolic disorders, supporting the hygiene hypothesis [110,111,112]. The ability of parasitic worms to shift the immune response from Th1 to Th2/Treg has sparked interest in employing live worms as immunotherapy. However, rather than reintroducing an infection, one approach to reducing the incidence of inflammatory and autoimmune disorders is to employ substitutes for these infections that retain their protective benefits.
Many studies regarding the therapeutic potential of helminthic proteins in inflammatory diseases have recently been discussed [113,114]. The use of non-human helminthic proteins may be one reason for unsuccessful clinical trials. Human filariae have co-evolved with the human immune system, suggesting that it is more suitable to use human filarial proteins over other helminthic proteins. There is strong evidence in mouse models that human filarial therapy, excretory-secretory components, and their recombinant molecules can treat and/or prevent inflammatory diseases such as inflammatory bowel disease (IBD), type-1-diabetes (T1D), and rheumatoid arthritis (RA) (Table 2). However, potential filarial proteins have only been tested in the laboratory and have not been tested in clinical trials. Effective coordination can reduce duplication of work, as many proteins have the same mode of action in different inflammatory diseases. For example, rBmALT-2 has been found to reduce the severity of T1D and IBD by downregulating IFN-γ and upregulating IL-10 and IgG1/IgG2a [115,116]. Although promising results have been achieved with human lymphatic filarial therapy, many questions, such as those regarding optimal dose, treatment duration, immunization route, safety profile, and cellular mode of action, remain unanswered. There is considerable scope for research in this area. For instance, site-directed administration of filarial immunomodulatory proteins using anti-colitic probiotics can provide effective IBD prevention/cure therapy [117]. A series of research studies, ranging from basic to clinical, is essential to evaluate the efficacy, safety, tolerability, and ethical implications of genetically modified immunobiotics.

Table 2.
Human lymphatic filariae-derived molecules as a therapy against inflammatory diseases.
4. Conclusions and Future Prospects
A millennia-long co-evolutionary struggle between the filarial parasite and its host has resulted in a mutually advantageous coexistence; the parasite’s sophisticated immune evasion strategies do not perceptibly harm the host. Filariae manipulate the host’s intracellular and extracellular machinery to create an immunomodulatory milieu to suppress inflammation by producing and releasing various bioactive chemicals and extracellular vesicles. Future research should delve into the involvement of filarial proteins with unknown functions in filariae’s intriguing regulatory effects on the host’s immune system.
Manipulation of the filariae-induced anti-inflammatory immune response can be a rational treatment approach not only against filariae, but also against intracellular parasites such as Plasmodium spp. and L. donovani in LF co-infected regions. The presence of LF in co-endemic regions either negatively affects the inflammatory protective response against microbes or positively prevents immunopathologies produced by an exacerbated host response to chronic microbial infection. Therefore, the contribution of filarial infection should be considered when conducting vaccine trials against non-helminthic infections in co-endemic areas. Compared with existing targeted medicines, helminths have proven to be safer and more controllable immunotherapies for acute and chronic inflammatory diseases due to their ability to activate immunoregulatory circuits and reduce inflammation. However, to date no helminth therapy has demonstrated promising results in human trials, suggesting the need for more in-depth work to identify and test more specific molecules as therapeutic candidates.
Indeed, comprehensive efforts to characterize human filarial immunomodulators against inflammatory diseases are still in their infancy; however, their significant translational potential must be explored. The emerging immunosuppressive role of filarial parasites in sepsis, inflammaging, and organ transplantation warrants further investigation. As filarial parasites and tumors both influence the host’s immune response through immunosuppression and immunological blinding, a multidisciplinary approach will advance research by encouraging crosstalk among diverse fields of science. Finally, deciphering the mechanisms of immune-modulation by filarial proteins provides information that can be used to design new strategies, not only for the treatment of filariasis and other infections, but also for the treatment of immunopathological diseases.
Author Contributions
Conceptualization, P.B., N.T. and V.K.; validation, K.G.; writing—original draft preparation, P.B. and N.T.; writing—review and editing, K.G. and V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any commercial or not-for-profit funding agency.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moreno, Y.; Geary, T.G. Stage- and Gender-Specific Proteomic Analysis of Brugia malayi Excretory-Secretory Products. PLoS Negl. Trop. Dis. 2008, 2, e326. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.A.; Lizotte-Waniewski, M.R.; Foster, J.; Guiliano, D.; Daub, J.; Scott, A.L.; Slatko, B.; Blaxter, M.L. The Filarial Genome Project: Analysis of the Nuclear, Mitochondrial and Endosymbiont Genomes of Brugia malayi. Int. J. Parasitol. 2000, 30, 411–419. [Google Scholar] [CrossRef]
- Scott, A.L.; Ghedin, E. The Genome of Brugia malayi—All Worms Are Not Created Equal. Parasitol. Int. 2009, 58, 6–11. [Google Scholar] [CrossRef] [PubMed]
- International Helminth Genomes Consortium. Comparative genomics of the major parasitic worms. Nat. Genet. 2019, 51, 163–174. [Google Scholar] [CrossRef]
- Babu, S.; Nutman, T.B. Proinflammatory Cytokines Dominate the Early Immune Response to Filarial Parasites. J. Immunol. 2003, 171, 6723–6732. [Google Scholar] [CrossRef]
- Porthouse, K.H.; Chirgwin, S.R.; Coleman, S.U.; Taylor, H.W.; Klei, T.R. Inflammatory Responses to Migrating Brugia pahangi Third-Stage Larvae. Infect. Immun. 2006, 74, 2366–2372. [Google Scholar] [CrossRef]
- Allen, J.E.; Maizels, R.M. Diversity and Dialogue in Immunity to Helminths. Nat. Rev. Immunol. 2011, 11, 375–388. [Google Scholar] [CrossRef]
- Herbert, D.; Douglas, B.; Zullo, K. Group 2 Innate Lymphoid Cells (ILC2): Type 2 Immunity and Helminth Immunity. Int. J. Mol. Sci. 2019, 20, 2276. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Bennuru, S.; Wang, Y.; Sanprasert, V.; Law, M.; Chaussabel, D.; Nutman, T.B.; Semnani, R.T. Quiescent Innate Response to Infective Filariae by Human Langerhans Cells Suggests a Strategy of Immune Evasion. Infect. Immun. 2013, 81, 1420–1429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cotton, R.N.; McDonald-Fleming, R.; Boyd, A.; Spates, K.; Nutman, T.B.; Tolouei Semnani, R. Brugia malayi Infective Larvae Fail to Activate Langerhans Cells and Dermal Dendritic Cells in Human Skin. Parasite Immunol. 2015, 37, 79–91. [Google Scholar] [CrossRef]
- Bonne-Année, S.; Nutman, T.B. Human Innate Lymphoid Cells (ILCs) in Filarial Infections. Parasite Immunol. 2018, 40, e12442. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Ribeiro, J.M.C.; Nutman, T.B. Human CD117 (CKit)+ Innate Lymphoid Cells Have a Discrete Transcriptional Profile at Homeostasis and Are Expanded during Filarial Infection. PLoS ONE 2014, 9, e108649. [Google Scholar] [CrossRef]
- Metenou, S.; Dembele, B.; Konate, S.; Dolo, H.; Coulibaly, S.Y.; Coulibaly, Y.I.; Diallo, A.A.; Soumaoro, L.; Coulibaly, M.E.; Sanogo, D.; et al. At Homeostasis Filarial Infections Have Expanded Adaptive T Regulatory but Not Classical Th2 Cells. J. Immunol. 2010, 184, 5375–5382. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Tawill, S.; le Goff, L.; Ali, F.; Blaxter, M.; Allen, J.E. Both Free-Living and Parasitic Nematodes Induce a Characteristic Th2 Response That Is Dependent on the Presence of Intact Glycans. Infect. Immun. 2004, 72, 398–407. [Google Scholar] [CrossRef]
- van Die, I.; Cummings, R.D. Glycan Gimmickry by Parasitic Helminths: A Strategy for Modulating the Host Immune Response? Glycobiology 2010, 20, 2–12. [Google Scholar] [CrossRef]
- Forsyth, K.P.; Spark, R.; Kazura, J.; Brown, G.V.; Peters, P.; Heywood, P.; Dissanayake, S.; Mitchell, G.F. A Monoclonal Antibody-Based Immunoradiometric Assay for Detection of Circulating Antigen in Bancroftian Filariasis. J. Immunol. 1985, 134, 1172–1177. [Google Scholar]
- Maizels, R.M.; Burke, J.; Denham, D.A. Phosphorylcholine-Bearing Antigens in Filarial Nematode Parasites: Analysis of Somatic Extracts, in-Vitro Secretions and Infection Sera from Brugia malayi and B. pahangi. Parasite Immunol. 1987, 9, 49–66. [Google Scholar] [CrossRef]
- Harnett, W.; Deehan, M.R.; Houston, K.M.; Harnett, M.M. Immunomodulatory Properties of a Phosphorylcholine-Containing Secreted Filarial Glycoprotein. Parasite Immunol. 1999, 21, 601–608. [Google Scholar] [CrossRef]
- Bunte, M.J.M.; Schots, A.; Kammenga, J.E.; Wilbers, R.H.P. Helminth Glycans at the Host-Parasite Interface and Their Potential for Developing Novel Therapeutics. Front. Mol. Biosci. 2022, 8, 807821. [Google Scholar] [CrossRef]
- Melendez, A.J.; Harnett, M.M.; Pushparaj, P.N.; Wong, W.S.F.; Tay, H.K.; McSharry, C.P.; Harnett, W. Inhibition of Fc Epsilon RI-Mediated Mast Cell Responses by ES-62, a Product of Parasitic Filarial Nematodes. Nat. Med. 2007, 13, 1375–1381. [Google Scholar] [CrossRef]
- Eason, R.J.; Bell, K.S.; Marshall, F.A.; Rodgers, D.T.; Pineda, M.A.; Steiger, C.N.; Al-Riyami, L.; Harnett, W.; Harnett, M.M. The Helminth Product, ES-62 Modulates Dendritic Cell Responses by Inducing the Selective Autophagolysosomal Degradation of TLR-Transducers, as Exemplified by PKCδ. Sci. Rep. 2016, 6, 37276. [Google Scholar] [CrossRef] [PubMed]
- Semnani, R.T.; Venugopal, P.G.; Leifer, C.A.; Mostböck, S.; Sabzevari, H.; Nutman, T.B. Inhibition of TLR3 and TLR4 Function and Expression in Human Dendritic Cells by Helminth Parasites. Blood 2008, 112, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Karnam, A.; Das, M.; Babu, S.P.S.; Bayry, J. Wuchereria bancrofti Filaria Activates Human Dendritic Cells and Polarizes T Helper 1 and Regulatory T Cells via Toll-like Receptor 4. Commun. Biol. 2019, 2, 169. [Google Scholar] [CrossRef] [PubMed]
- Joardar, N.; Bhattacharya, R.; Halder, S.; Sen, A.; Biswas, S.R.; Jana, K.; Babu, S.P.S. Filarial Thioredoxin Reductase Exerts Anti-Inflammatory Effects upon Lipopolysaccharide Induced Inflammation in Macrophages. Int. J. Biol. Macromol. 2021, 193, 1379–1390. [Google Scholar] [CrossRef]
- Ludin, P.; Nilsson, D.; Mäser, P. Genome-Wide Identification of Molecular Mimicry Candidates in Parasites. PLoS ONE 2011, 6, e17546. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escobar, N.; Gregory, W.F.; Maizels, R.M. Identification of Tgh-2, a Filarial Nematode Homolog of Caenorhabditis Elegans Daf-7 and Human Transforming Growth Factor β, Expressed in Microfilarial and Adult Stages of Brugia malayi. Infect. Immun. 2000, 68, 6402–6410. [Google Scholar] [CrossRef]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef]
- Dakshinamoorthy, G.; Samykutty, A.K.; Munirathinam, G.; Shinde, G.B.; Nutman, T.; Reddy, M.V.; Kalyanasundaram, R. Biochemical Characterization and Evaluation of a Brugia malayi Small Heat Shock Protein as a Vaccine against Lymphatic Filariasis. PLoS ONE 2012, 7, e34077. [Google Scholar] [CrossRef]
- Liu, L.X.; Weller, P.F. Intravascular Filarial Parasites Inhibit Platelet Aggregation. Role of Parasite-Derived Prostanoids. J. Clin. Investig. 1992, 89, 1113–1120. [Google Scholar] [CrossRef][Green Version]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E 2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- Baratelli, F.; Lin, Y.; Zhu, L.; Yang, S.-C.; Heuzé-Vourc’h, N.; Zeng, G.; Reckamp, K.; Dohadwala, M.; Sharma, S.; Dubinett, S.M. Prostaglandin E 2 Induces FOXP3 Gene Expression and T Regulatory Cell Function in Human CD4+ T Cells. J. Immunol. 2005, 175, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Harcus, Y.M.; Murray, J.; Taylor, M.D.; Maizels, R.M. Expansion of Foxp3+ Regulatory T Cells in Mice Infected with the Filarial Parasite Brugia malayi. J. Immunol. 2008, 181, 6456–6466. [Google Scholar] [CrossRef]
- Oyesola, O.O.; Tait Wojno, E.D. Prostaglandin Regulation of Type 2 Inflammation: From Basic Biology to Therapeutic Interventions. Eur. J. Immunol. 2021, 51, 2399–2416. [Google Scholar] [CrossRef]
- Zang, X.; Taylor, P.; Wang, J.M.; Meyer, D.J.; Scott, A.L.; Walkinshaw, M.D.; Maizels, R.M. Homologues of Human Macrophage Migration Inhibitory Factor from a Parasitic Nematode. J. Biol. Chem. 2002, 277, 44261–44267. [Google Scholar] [CrossRef]
- Prieto-Lafuente, L.; Gregory, W.F.; Allen, J.E.; Maizels, R.M. MIF Homologues from a Filarial Nematode Parasite Synergize with IL-4 to Induce Alternative Activation of Host Macrophages. J. Leukoc. Biol. 2009, 85, 844–854. [Google Scholar] [CrossRef]
- Koussa, J.; Vitrinel, B.; Whitney, P.; Kasper, B.T.; Mahal, L.K.; Vogel, C.; Lustigman, S.; Salehi-Ashtiani, K.; Ghedin, E. Sex-Specific Glycosylation of Secreted Immunomodulatory Proteins in the Filarial Nematode Brugia malayi. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ritter, M.; Osei-Mensah, J.; Debrah, L.B.; Kwarteng, A.; Mubarik, Y.; Debrah, A.Y.; Pfarr, K.; Hoerauf, A.; Layland, L.E. Wuchereria Bancrofti-Infected Individuals Harbor Distinct IL-10-Producing Regulatory B and T Cell Subsets Which Are Affected by Anti-Filarial Treatment. PLoS Negl. Trop. Dis. 2019, 13, e0007436. [Google Scholar] [CrossRef]
- Gomez-Escobar, N.; Bennett, C.; Prieto-Lafuente, L.; Aebischer, T.; Blackburn, C.C.; Maizels, R.M. Heterologous Expression of the Filarial Nematode Alt Gene Products Reveals Their Potential to Inhibit Immune Function. BMC Biol. 2005, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Chang, S.C.; Nguyen, H.M.; Huq, R.; Tanner, M.R.; Londono, L.M.; Estrada, R.; Dhawan, V.; Chauhan, S.; Upadhyay, S.K.; et al. Kv1.3 Channel-blocking Immunomodulatory Peptides from Parasitic Worms: Implications for Autoimmune Diseases. FASEB J. 2014, 28, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- Harnett, W.; Harnett, M.M. Inhibition of Murine B Cell Proliferation and Down-Regulation of Protein Kinase C Levels by a Phosphorylcholine-Containing Filarial Excretory-Secretory Product. J. Immunol. 1993, 151, 4829–4837. [Google Scholar]
- Hewitson, J.P.; Harcus, Y.M.; Curwen, R.S.; Dowle, A.A.; Atmadja, A.K.; Ashton, P.D.; Wilson, A.; Maizels, R.M. The Secretome of the Filarial Parasite, Brugia malayi: Proteomic Profile of Adult Excretory–Secretory Products. Mol. Biochem. Parasitol. 2008, 160, 8–21. [Google Scholar] [CrossRef]
- Adjobimey, T.; Hoerauf, A. Induction of Immunoglobulin G4 in Human Filariasis: An Indicator of Immunoregulation. Ann. Trop. Med. Parasitol. 2010, 104, 455–464. [Google Scholar] [CrossRef]
- Khatri, V.; Chauhan, N.; Kalyanasundaram, R. Parasite Cystatin: Immunomodulatory Molecule with Therapeutic Activity against Immune Mediated Disorders. Pathogens 2020, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Manoury, B.; Gregory, W.F.; Maizels, R.M.; Watts, C. Bm-CPI-2, a Cystatin Homolog Secreted by the Filarial Parasite Brugia malayi, Inhibits Class II MHC-Restricted Antigen Processing. Curr. Biol. 2001, 11, 447–451. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth Immunoregulation: The Role of Parasite Secreted Proteins in Modulating Host Immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef]
- Babu, S.; Blauvelt, C.P.; Nutman, T.B. Filarial Parasites Induce NK Cell Activation, Type 1 and Type 2 Cytokine Secretion, and Subsequent Apoptotic Cell Death. J. Immunol. 2007, 179, 2445–2456. [Google Scholar] [CrossRef]
- Semnani, R.T.; Liu, A.Y.; Sabzevari, H.; Kubofcik, J.; Zhou, J.; Gilden, J.K.; Nutman, T.B. Brugia malayi Microfilariae Induce Cell Death in Human Dendritic Cells, Inhibit Their Ability to Make IL-12 and IL-10, and Reduce Their Capacity to Activate CD4 + T Cells. J. Immunol. 2003, 171, 1950–1960. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Bennuru, S.; Meng, Z.; Cotton, R.N.; Elliott, K.R.; Ganesan, S.; McDonald-Fleming, R.; Veenstra, T.D.; Nutman, T.B.; Tolouei Semnani, R. Microfilariae of Brugia malayi Inhibit the MTOR Pathway and Induce Autophagy in Human Dendritic Cells. Infect. Immun. 2016, 84, 2463–2472. [Google Scholar] [CrossRef]
- Ricciardi, A.; Bennuru, S.; Tariq, S.; Kaur, S.; Wu, W.; Elkahloun, A.G.; Arakelyan, A.; Shaik, J.; Dorward, D.W.; Nutman, T.B.; et al. Extracellular Vesicles Released from the Filarial Parasite Brugia malayi Downregulate the Host MTOR Pathway. PLoS Negl. Trop. Dis. 2021, 15, e0008884. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, S.K.; Sahoo, P.K.; Bal, M.S.; Satapathy, A.K. Increased Fas Ligand Expression of Peripheral B-1 Cells Correlated with CD4 + T-Cell Apoptosis in Filarial-Infected Patients. Parasite Immunol. 2017, 39, e12421. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Eisinger, S.W.; Raghavan, N.; Scott, A.L. Thioredoxin Peroxidases from Brugia malayi. Mol. Biochem. Parasitol. 1998, 91, 207–220. [Google Scholar] [CrossRef]
- Gnanasekar, M.; Rao, K.V.N.; Chen, L.; Narayanan, R.B.; Geetha, M.; Scott, A.L.; Ramaswamy, K.; Kaliraj, P. Molecular Characterization of a Calcium Binding Translationally Controlled Tumor Protein Homologue from the Filarial Parasites Brugia malayi and Wuchereria bancrofti. Mol. Biochem. Parasitol. 2002, 121, 107–118. [Google Scholar] [CrossRef]
- Bhargavi, R.; Vishwakarma, S.; Murty, U.S. Modeling Analysis of GST (Glutathione-S-Transferases) from Wuchereria bancrofti and Brugia malayi. Bioinformation 2005, 1, 25–27. [Google Scholar] [CrossRef][Green Version]
- Yadav, S.; Gupta, S.; Selvaraj, C.; Doharey, P.K.; Verma, A.; Singh, S.K.; Saxena, J.K. In Silico and In Vitro Studies on the Protein-Protein Interactions between Brugia malayi Immunomodulatory Protein Calreticulin and Human C1q. PLoS ONE 2014, 9, e106413. [Google Scholar] [CrossRef]
- World Health Organization Lymphatic Filariasis. 2022. Lymphatic Filariasis. Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 2 June 2022).
- Mallawarachchi, C.H.; Nilmini Chandrasena, T.G.A.; Premaratna, R.; Mallawarachchi, S.M.N.S.M.; de Silva, N.R. Human Infection with Sub-Periodic Brugia spp. in Gampaha District, Sri Lanka: A Threat to Filariasis Elimination Status? Parasites Vectors 2018, 11, 68. [Google Scholar] [CrossRef]
- George, S.; Joy, T.M.; Kumar, A.; Panicker, K.N.; George, L.S.; Raj, M.; Leelamoni, K.; Nair, P. Prevalence of Neglected Tropical Diseases (Leishmaniasis and Lymphatic Filariasis) and Malaria among a Migrant Labour Settlement in Kerala, India. J. Immigr. Minority Health 2019, 21, 563–569. [Google Scholar] [CrossRef]
- Zuchi, A.; Prust, L.T.; Rocha, A.; Araújo, J.; da Silva, P.S.; Fiorillo, K.; Brandão, E.; Ximenes, C.; Lopes, F.; Ponzi, C.C. Screening and Evaluation of Lymphatic Filariasis in Immigrants from Endemic Countries Residing in a Focus Where It Is Considered Eliminated in the Southern Region of Brazil: A Risk of Reemergence? Acta Trop. 2017, 176, 192–196. [Google Scholar] [CrossRef]
- Da Silva, E.F.; de Lacerda, M.V.G.; Fontes, G.; Mourão, M.P.G.; Martins, M. Wuchereria bancrofti Infection in Haitian Immigrants and the Risk of Re-Emergence of Lymphatic Filariasis in the Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2017, 50, 256–259. [Google Scholar] [CrossRef][Green Version]
- Xu, Z.; Lau, C.L.; Zhou, X.; Fuimaono, S.; Soares Magalhães, R.J.; Graves, P.M. The Extensive Networks of Frequent Population Mobility in the Samoan Islands and Their Implications for Infectious Disease Transmission. Sci. Rep. 2018, 8, 10136. [Google Scholar] [CrossRef]
- Prada, J.M.; Stolk, W.A.; Davis, E.L.; Touloupou, P.; Sharma, S.; Muñoz, J.; Caja Rivera, R.M.; Reimer, L.J.; Michael, E.; de Vlas, S.J.; et al. Delays in Lymphatic Filariasis Elimination Programmes Due to COVID-19, and Possible Mitigation Strategies. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 261–268. [Google Scholar] [CrossRef]
- Bizhani, N.; Hashemi Hafshejani, S.; Mohammadi, N.; Rezaei, M.; Rokni, M.B. Lymphatic Filariasis in Asia: A Systematic Review and Meta-Analysis. Parasitol. Res. 2021, 120, 411–422. [Google Scholar] [CrossRef]
- Hussain, M.A.; Sitha, A.K.; Swain, S.; Kadam, S.; Pati, S. Mass Drug Administration for Lymphatic Filariasis Elimination in a Coastal State of India: A Study on Barriers to Coverage and Compliance. Infect. Dis. Poverty 2014, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Ahorlu, C.S.K.; Koka, E.; Adu-Amankwah, S.; Otchere, J.; de Souza, D.K. Community Perspectives on Persistent Transmission of Lymphatic Filariasis in Three Hotspot Districts in Ghana after 15 Rounds of Mass Drug Administration: A Qualitative Assessment. BMC Public Health 2018, 18, 238. [Google Scholar] [CrossRef]
- Willis, G.A.; Mayfield, H.J.; Kearns, T.; Naseri, T.; Thomsen, R.; Gass, K.; Sheridan, S.; Graves, P.M.; Lau, C.L. A Community Survey of Coverage and Adverse Events Following Country-Wide Triple-Drug Mass Drug Administration for Lymphatic Filariasis Elimination, Samoa 2018. PLOS Negl. Trop. Dis. 2020, 14, e0008854. [Google Scholar] [CrossRef]
- de Souza, D.K.; Gass, K.; Otchere, J.; Htet, Y.M.; Asiedu, O.; Marfo, B.; Biritwum, N.-K.; Boakye, D.A.; Ahorlu, C.S. Review of MDA Registers for Lymphatic Filariasis: Findings, and Potential Uses in Addressing the Endgame Elimination Challenges. PLoS Negl. Trop. Dis. 2020, 14, e0008306. [Google Scholar] [CrossRef]
- Martin, R.J. Modes of Action of Anthelmintic Drugs. Vet. J. 1997, 154, 11–34. [Google Scholar] [CrossRef]
- Moreno, Y.; Nabhan, J.F.; Solomon, J.; Mackenzie, C.D.; Geary, T.G. Ivermectin Disrupts the Function of the Excretory-Secretory Apparatus in Microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. USA 2010, 107, 20120–20125. [Google Scholar] [CrossRef]
- Maclean, M.J.; Lorenz, W.W.; Dzimianski, M.T.; Anna, C.; Moorhead, A.R.; Reaves, B.J.; Wolstenholme, A.J. Effects of Diethylcarbamazine and Ivermectin Treatment on Brugia malayi Gene Expression in Infected Gerbils (Meriones unguiculatus). Parasitol. Open 2019, 5, e2. [Google Scholar] [CrossRef][Green Version]
- Norling, L.V.; Sampaio, A.L.F.; Cooper, D.; Perretti, M. Inhibitory Control of Endothelial Galectin-1 on in Vitro and in Vivo Lymphocyte Trafficking. FASEB J. 2008, 22, 682–690. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, A.C.; Farnworth, S.L.; Hodkinson, P.S.; Henderson, N.C.; Atkinson, K.M.; Leffler, H.; Nilsson, U.J.; Haslett, C.; Forbes, S.J.; Sethi, T. Regulation of Alternative Macrophage Activation by Galectin-3. J. Immunol. 2008, 180, 2650–2658. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Zhang, H.; Yuan, C.; Yan, R.; Song, X.; Xu, L.; Li, X. Galectin Hco-Gal-m from Haemonchus Contortus Modulates Goat Monocytes and T Cell Function in Different Patterns. Parasites Vectors 2014, 7, 342. [Google Scholar] [CrossRef]
- Bahekar, S.P.; Hande, S.V.; Agrawal, N.R.; Chandak, H.S.; Bhoj, P.S.; Goswami, K.; Reddy, M.V.R. Sulfonamide Chalcones: Synthesis and in Vitro Exploration for Therapeutic Potential against Brugia malayi. Eur. J. Med. Chem. 2016, 124, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Bhoj, P.S.; Bahekar, S.; Khatri, V.; Singh, N.; Togre, N.S.; Goswami, K.; Chandak, H.S.; Dash, D. Role of Glutathione in Chalcone Derivative Induced Apoptosis of Brugia malayi and Its Possible Therapeutic Implication. Acta Parasitol. 2021, 66, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, R.; Khatri, V.; Chauhan, N. Advances in Vaccine Development for Human Lymphatic Filariasis. Trends Parasitol. 2020, 36, 195–205. [Google Scholar] [CrossRef]
- Thirugnanam, S.; Pandiaraja, P.; Ramaswamy, K.; Murugan, V.; Gnanasekar, M.; Nandakumar, K.; Reddy, M.V.R.; Kaliraj, P. Brugia malayi: Comparison of Protective Immune Responses Induced by Bm-Alt-2 DNA, Recombinant Bm-ALT-2 Protein and Prime-Boost Vaccine Regimens in a Jird Model. Exp. Parasitol. 2007, 116, 483–491. [Google Scholar] [CrossRef]
- Dakshinamoorthy, G.; Munirathinam, G.; Stoicescu, K.; Reddy, M.V.; Kalyanasundaram, R. Large Extracellular Loop of Tetraspanin as a Potential Vaccine Candidate for Filariasis. PLoS ONE 2013, 8, e77394. [Google Scholar] [CrossRef]
- Dakshinamoorthy, G.; Kalyanasundaram, R. Evaluating the Efficacy of RBmHATαc as a Multivalent Vaccine against Lymphatic Filariasis in Experimental Animals and Optimizing the Adjuvant Formulation. Vaccine 2013, 32, 19–25. [Google Scholar] [CrossRef][Green Version]
- Dakshinamoorthy, G.; von Gegerfelt, A.; Andersen, H.; Lewis, M.; Kalyanasundaram, R. Evaluation of a Multivalent Vaccine against Lymphatic Filariasis in Rhesus Macaque Model. PLoS ONE 2014, 9, e112982. [Google Scholar] [CrossRef]
- Chauhan, N.; Khatri, V.; Banerjee, P.; Kalyanasundaram, R. Evaluating the Vaccine Potential of a Tetravalent Fusion Protein (RBmHAXT) Vaccine Antigen Against Lymphatic Filariasis in a Mouse Model. Front. Immunol. 2018, 9, 01520. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.; Chauhan, N.; Vishnoi, K.; von Gegerfelt, A.; Gittens, C.; Kalyanasundaram, R. Prospects of Developing a Prophylactic Vaccine against Human Lymphatic Filariasis—Evaluation of Protection in Non-Human Primates. Int. J. Parasitol. 2018, 48, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Atmadja, A.K.; Gray, P.; Allen, J.E.; Gray, C.A.; Lawrence, R.A.; Yazdanbakhsh, M.; Maizels, R.M. The Serpin Secreted by Brugia malayi Microfilariae, Bm-SPN-2, Elicits Strong, but Short-Lived, Immune Responses in Mice and Humans. J. Immunol. 2000, 165, 5161–5169. [Google Scholar] [CrossRef] [PubMed]
- Veerapathran, A.; Dakshinamoorthy, G.; Gnanasekar, M.; Reddy, M.V.R.; Kalyanasundaram, R. Evaluation of Wuchereria bancrofti GST as a Vaccine Candidate for Lymphatic Filariasis. PLoS Negl. Trop. Dis. 2009, 3, e457. [Google Scholar] [CrossRef] [PubMed]
- Andure, D.; Pote, K.; Khatri, V.; Amdare, N.; Padalkar, R.; Reddy, M.V.R. Immunization with Wuchereria bancrofti Glutathione-S-Transferase Elicits a Mixed Th1/Th2 Type of Protective Immune Response Against Filarial Infection in Mastomys. Indian J. Clin. Biochem. 2016, 31, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Dakshinamoorthy, G.; Samykutty, A.K.; Munirathinam, G.; Reddy, M.V.; Kalyanasundaram, R. Multivalent Fusion Protein Vaccine for Lymphatic Filariasis. Vaccine 2013, 31, 1616–1622. [Google Scholar] [CrossRef]
- Kushwaha, S.; Singh, P.K.; Rana, A.K.; Misra-Bhattacharya, S. Immunization of Mastomys Coucha with Brugia malayi Recombinant Trehalose-6-Phosphate Phosphatase Results in Significant Protection against Homologous Challenge Infection. PLoS ONE 2013, 8, e72585. [Google Scholar] [CrossRef]
- Prince, P.R.; Madhumathi, J.; Anugraha, G.; Jeyaprita, P.J.; Reddy, M.V.R.; Kaliraj, P. Tandem Antioxidant Enzymes Confer Synergistic Protective Responses in Experimental Filariasis. J. Helminthol. 2014, 88, 402–410. [Google Scholar] [CrossRef]
- Arumugam, S.; Wei, J.; Ward, D.; Abraham, D.; Lustigman, S.; Zhan, B.; Klei, T.R. Vaccination with a Genetically Modified Brugia malayi Cysteine Protease Inhibitor-2 Reduces Adult Parasite Numbers and Affects the Fertility of Female Worms Following a Subcutaneous Challenge of Mongolian Gerbils (Meriones unguiculatus) with B. malayi Infective Larvae. Int. J. Parasitol. 2014, 44, 675–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paul, R.; Ilamaran, M.; Khatri, V.; Amdare, N.; Reddy, M.V.R.; Kaliraj, P. Immunological Evaluation of Fusion Protein of Brugia malayi Abundant Larval Protein Transcript-2 (BmALT-2) and Tuftsin in Experimental Mice Model. Parasite Epidemiol. Control 2019, 4, e00092. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, P.; Sharma, A.; Ganga, L.; Saxena, J.K.; Srivastava, M. Immunization with Brugia malayi Calreticulin Protein Generates Robust Antiparasitic Immunity and Offers Protection during Experimental Lymphatic Filariasis. ACS Infect. Dis. 2021, 7, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.; Chauhan, N.; Kalyanasundaram, R. Fecundity of Adult Female Worms Were Affected When Brugia malayi Infected Mongolian Gerbils Were Immunized with a Multivalent Vaccine (RBmHAXT) against Human Lymphatic Filarial Parasite. Acta Trop. 2020, 208, 105487. [Google Scholar] [CrossRef]
- Metenou, S.; Babu, S.; Nutman, T.B. Impact of Filarial Infections on Coincident Intracellular Pathogens. Curr. Opin. HIV AIDS 2012, 7, 231–238. [Google Scholar] [CrossRef]
- Yan, Y.; Inuo, G.; Akao, N.; Tsukidate, S.; Fujita, K. Down-Regulation of Murine Susceptibility to Cerebral Malaria by Inoculation with Third-Stage Larvae of the Filarial Nematode Brugia pahangi. Parasitology 1997, 114, 333–338. [Google Scholar] [CrossRef]
- Graham, A.L.; Lamb, T.J.; Read, A.F.; Allen, J.E. Malaria-Filaria Coinfection in Mice Makes Malarial Disease More Severe Unless Filarial Infection Achieves Patency. J. Infect. Dis. 2005, 191, 410–421. [Google Scholar] [CrossRef]
- Fernández Ruiz, D.; Dubben, B.; Saeftel, M.; Endl, E.; Deininger, S.; Hoerauf, A.; Specht, S. Filarial Infection Induces Protection against P. berghei Liver Stages in Mice. Microbes Infect. 2009, 11, 172–180. [Google Scholar] [CrossRef]
- Metenou, S.; Dembélé, B.; Konate, S.; Dolo, H.; Coulibaly, S.Y.; Coulibaly, Y.I.; Diallo, A.A.; Soumaoro, L.; Coulibaly, M.E.; Sanogo, D.; et al. Patent Filarial Infection Modulates Malaria-Specific Type 1 Cytokine Responses in an IL-10-Dependent Manner in a Filaria/Malaria-Coinfected Population. J. Immunol. 2009, 183, 916–924. [Google Scholar] [CrossRef]
- Specht, S.; Ruiz, D.F.; Dubben, B.; Deininger, S.; Hoerauf, A. Filaria-Induced IL-10 Suppresses Murine Cerebral Malaria. Microbes Infect. 2010, 12, 635–642. [Google Scholar] [CrossRef]
- Panda, M.; Sahoo, P.K.; das Mohapatra, A.; kanti Dutta, S.; Thatoi, P.K.; Tripathy, R.; Das, B.K.; Satpathy, A.K.; Ravindran, B. Decreased Prevalence of Sepsis but Not Mild or Severe P. falciparum Malaria Is Associated with Pre-Existing Filarial Infection. Parasit Vectors 2013, 6, 203. [Google Scholar] [CrossRef]
- Kolbaum, J.; Tartz, S.; Hartmann, W.; Helm, S.; Nagel, A.; Heussler, V.; Sebo, P.; Fleischer, B.; Jacobs, T.; Breloer, M. Nematode-Induced Interference with the Anti-Plasmodium CD8+ T-Cell Response Can Be Overcome by Optimizing Antigen Administration. Eur. J. Immunol. 2012, 42, 890–900. [Google Scholar] [CrossRef]
- Noland, G.S.; Chowdhury, D.R.; Urban, J.F.; Zavala, F.; Kumar, N. Helminth Infection Impairs the Immunogenicity of a Plasmodium Falciparum DNA Vaccine, but Not Irradiated Sporozoites, in Mice. Vaccine 2010, 28, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Joseph, S.K.; Kushwaha, V.; Kumar, V.; Siddiqi, M.I.; Vishwakarma, P.; Shivahare, R.; Gupta, S.; Murthy, P.K. Cross Reactive Molecules of Human Lymphatic Filaria Brugia malayi Inhibit Leishmania Donovani Infection in Hamsters. Acta Trop. 2015, 152, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.K.; Dixit, S.; Gaur, R.L.; Kumar, R.; Sahoo, M.K.; Shakya, N.; Joseph, S.K.; Palne, S.; Gupta, S. Influence of Brugia malayi Life Stages and BmAFII Fraction on Experimental Leishmania Donovani Infection in Hamsters. Acta Trop. 2008, 106, 81–89. [Google Scholar] [CrossRef]
- Sahoo, M.K.; Sisodia, B.S.; Dixit, S.; Joseph, S.K.; Gaur, R.L.; Verma, S.K.; Verma, A.K.; Shasany, A.K.; Dowle, A.A.; Murthy, P.K. Immunization with Inflammatory Proteome of Brugia malayi Adult Worm Induces a Th1/Th2-Immune Response and Confers Protection against the Filarial Infection. Vaccine 2009, 27, 4263–4271. [Google Scholar] [CrossRef]
- Kushwaha, V.; Kaur, S. Cross-Protective Efficacy of Immuno-Stimulatory Recombinant Brugia malayi Protein HSP60 against the Leishmania Donovani in BALB/c Mice. Biologicals 2021, 72, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Kushwaha, V.; Pandey, S.; Thota, J.R.; Vishwakarma, P.; Parmar, N.; Yadav, P.K.; Tewari, P.; Kar, S.; Shukla, P.K.; et al. Leishmania Donovani Molecules Recognized by Sera of Filaria Infected Host Facilitate Filarial Infection. Parasitol. Res. 2018, 117, 2901–2912. [Google Scholar] [CrossRef]
- Sangare, M.B.; Coulibaly, Y.I.; Coulibaly, S.Y.; Coulibaly, M.E.; Traore, B.; Dicko, I.; Sissoko, I.M.; Samake, S.; Traore, S.F.; Nutman, T.B.; et al. A Cross-Sectional Study of the Filarial and Leishmania Co-Endemicity in Two Ecologically Distinct Settings in Mali. Parasit Vectors 2018, 11, 18. [Google Scholar] [CrossRef]
- Lamb, T.J.; Graham, A.L.; le Goff, L.; Allen, J.E. Co-Infected C57BL/6 Mice Mount Appropriately Polarized and Compartmentalized Cytokine Responses to Litomosoides Sigmodontis and Leishmania Major but Disease Progression Is Altered. Parasite Immunol. 2005, 27, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W. The Hygiene Hypothesis and the Increasing Prevalence of Chronic Inflammatory Disorders. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 1072–1074. [Google Scholar] [CrossRef]
- Caraballo, L. The Tropics, Helminth Infections and Hygiene Hypotheses. Expert Rev. Clin. Immunol. 2018, 14, 99–102. [Google Scholar] [CrossRef]
- Bach, J.-F. Revisiting the Hygiene Hypothesis in the Context of Autoimmunity. Front. Immunol. 2021, 11, 615192. [Google Scholar] [CrossRef]
- Smallwood, T.B.; Giacomin, P.R.; Loukas, A.; Mulvenna, J.P.; Clark, R.J.; Miles, J.J. Helminth Immunomodulation in Autoimmune Disease. Front. Immunol. 2017, 8, 00453. [Google Scholar] [CrossRef]
- Shi, W.; Xu, N.; Wang, X.; Vallée, I.; Liu, M.; Liu, X. Helminth Therapy for Immune-Mediated Inflammatory Diseases: Current and Future Perspectives. J. Inflamm. Res. 2022, 15, 475–491. [Google Scholar] [CrossRef]
- Khatri, V.; Amdare, N.; Yadav, R.S.; Tarnekar, A.; Goswami, K.; Reddy, M.V.R. Brugia malayi Abundant Larval Transcript 2 Protein Treatment Attenuates Experimentally-Induced Colitis in Mice. Indian J. Exp. Biol. 2015, 53, 732–739. [Google Scholar]
- Amdare, N.P.; Khatri, V.K.; Yadav, R.S.P.; Tarnekar, A.; Goswami, K.; Reddy, M.V.R. Therapeutic Potential of the Immunomodulatory Proteins Wuchereria bancrofti L2 and Brugia malayi Abundant Larval Transcript 2 against Streptozotocin-Induced Type 1 Diabetes in Mice. J. Helminthol. 2017, 91, 539–548. [Google Scholar] [CrossRef]
- Ou, B.; Yang, Y.; Tham, W.L.; Chen, L.; Guo, J.; Zhu, G. Genetic Engineering of Probiotic Escherichia Coli Nissle 1917 for Clinical Application. Appl. Microbiol. Biotechnol. 2016, 100, 8693–8699. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.; Amdare, N.; Tarnekar, A.; Goswami, K.; Reddy, M.V.R. Brugia malayi Cystatin Therapeutically Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice. J. Dig. Dis. 2015, 16, 585–594. [Google Scholar] [CrossRef]
- Bisht, N.; Khatri, V.; Chauhan, N.; Kalyanasundaram, R. Cystatin from Filarial Parasites Suppress the Clinical Symptoms and Pathology of Experimentally Induced Colitis in Mice by Inducing T-Regulatory Cells, B1-Cells, and Alternatively Activated Macrophages. Biomedicines 2019, 7, 85. [Google Scholar] [CrossRef]
- Yadav, R.S.P.; Khatri, V.; Amdare, N.; Goswami, K.; Shivkumar, V.B.; Gangane, N.; Reddy, M.V.R. Immuno-Modulatory Effect and Therapeutic Potential of Brugia malayi Cystatin in Experimentally Induced Arthritis. Indian J. Clin. Biochem. 2016, 31, 203–208. [Google Scholar] [CrossRef][Green Version]
- Yadav RS, P.; Khatri, V.; Amdare, N.; Goswami, K.; Shivkumar, V.B.; Gangane, N.; Reddy, M.V.R. Evaluation of Preventive Effect of Brugia malayi Recombinant Cystatin on MBSA-Induced Experimental Arthritis. Indian J. Exp. Biol. 2017, 55, 655–660. [Google Scholar]
- Khatri, V.; Chauhan, N.; Prasanna Kumar, S.B.; Kalyanasundaram, R. Peptide Fragments of Cystatin Protein from Filarial Parasite Has Potent Anti-Inflammatory Effect on DSS-Induced Colitis in Mouse. J. Immunol. 2020, 204, 237.28. [Google Scholar]
- Togre, N.; Bhoj, P.; Amdare, N.; Goswami, K.; Tarnekar, A.; Shende, M. Immunomodulatory Potential of Recombinant Filarial Protein, RWbL2, and Its Therapeutic Implication in Experimental Ulcerative Colitis in Mouse. Immunopharmacol. Immunotoxicol. 2018, 40, 483–490. [Google Scholar] [CrossRef]
- Togre, N.; Bhoj, P.; Goswami, K.; Tarnekar, A.; Patil, M.; Shende, M. Human Filarial Proteins Attenuate Chronic Colitis in an Experimental Mouse Model. Parasite Immunol. 2018, 40, e12511. [Google Scholar] [CrossRef] [PubMed]
- Amdare, N.; Khatri, V.; Yadav, R.S.P.; Tarnekar, A.; Goswami, K.; Reddy, M.V.R. Brugia malayi Soluble and Excretory-Secretory Proteins Attenuate Development of Streptozotocin-Induced Type 1 Diabetes in Mice. Parasite Immunol. 2015, 37, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Kron, M.A.; Metwali, A.; Vodanovic-Jankovic, S.; Elliott, D. Nematode Asparaginyl-TRNA Synthetase Resolves Intestinal Inflammation in Mice with T-Cell Transfer Colitis. Clin. Vaccine Immunol. 2013, 20, 276–281. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).