Efficacy Studies of a Trivalent Vaccine Containing PCV-2a, PCV-2b Genotypes and Mycoplasma hyopneumoniae When Administered at 3 Days of Age and 3 Weeks Later against Porcine Circovirus 2 (PCV-2) Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Preclinical Studies
2.1.1. PCV-2a Challenge Study
2.1.2. PCV-2b Challenge Study
2.1.3. PCV-2 Challenge Strains
2.2. Field Trials
2.2.1. Farm Selection
2.2.2. Study Design
2.2.3. PCV-2 Genotyping
2.3. Laboratory Methods of Preclinical and Field Studies
2.3.1. DNA Extraction and PCV-2 qPCR
2.3.2. Serology to Detect PCV-2 Antibodies
2.3.3. Histopathology and PCV-2 IHC
- Presence of at least one of the following clinical signs: wasting, weight loss, paleness of the skin, dyspnoea, diarrhoea, jaundice and/or inguinal superficial lymphadenopathy (only applicable to PCV-2-SD cases).
- LD and/or HR of lymphoid tissues (PCV-2-SI: LD and HR ≤ 1; PCV-2-SD: LD and HR > 1).
- PCV-2 in lymphoid tissues (PCV-2-SI: IHC ≤ 1; PCV-2-SD: IHC > 1).
2.4. Statistical Analyses
3. Results
3.1. Preclinical Studies
3.1.1. PCV-2a Challenge Study
Clinical Evaluation
PCV-2 Antibody Detection
PCV-2 Viraemia and Faecal Shedding
PCV-2 Detection in Lymphoid Tissues and Microscopic Lymphoid Lesions
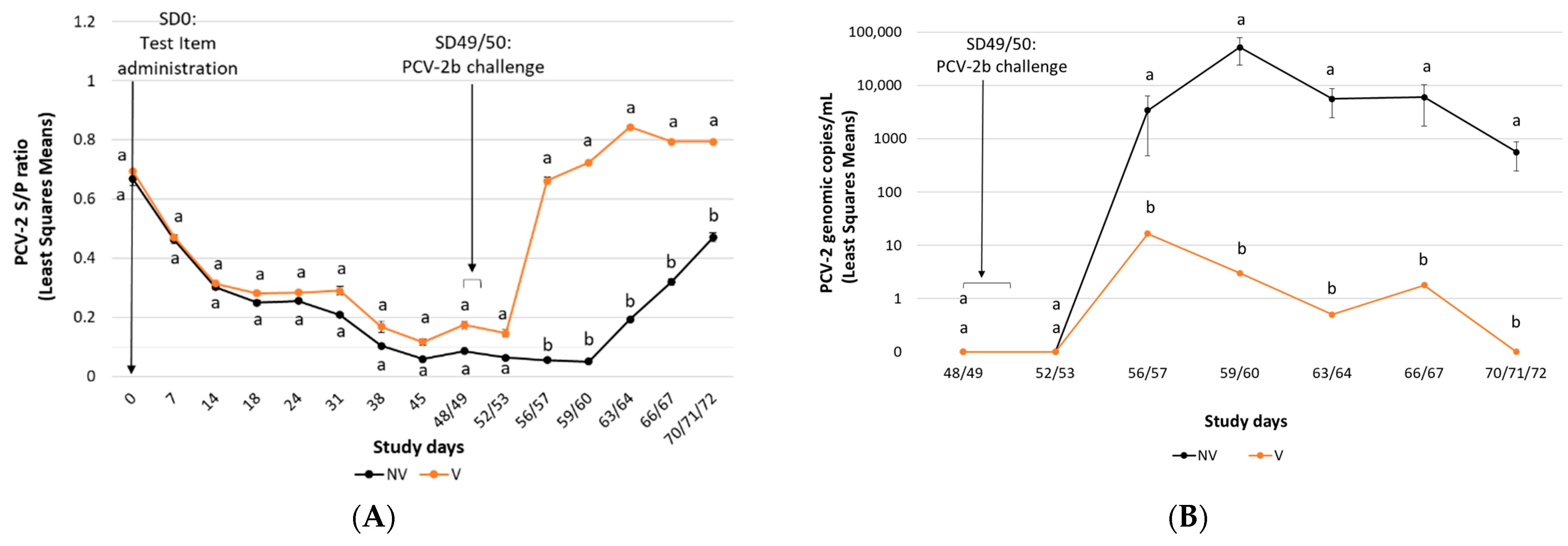
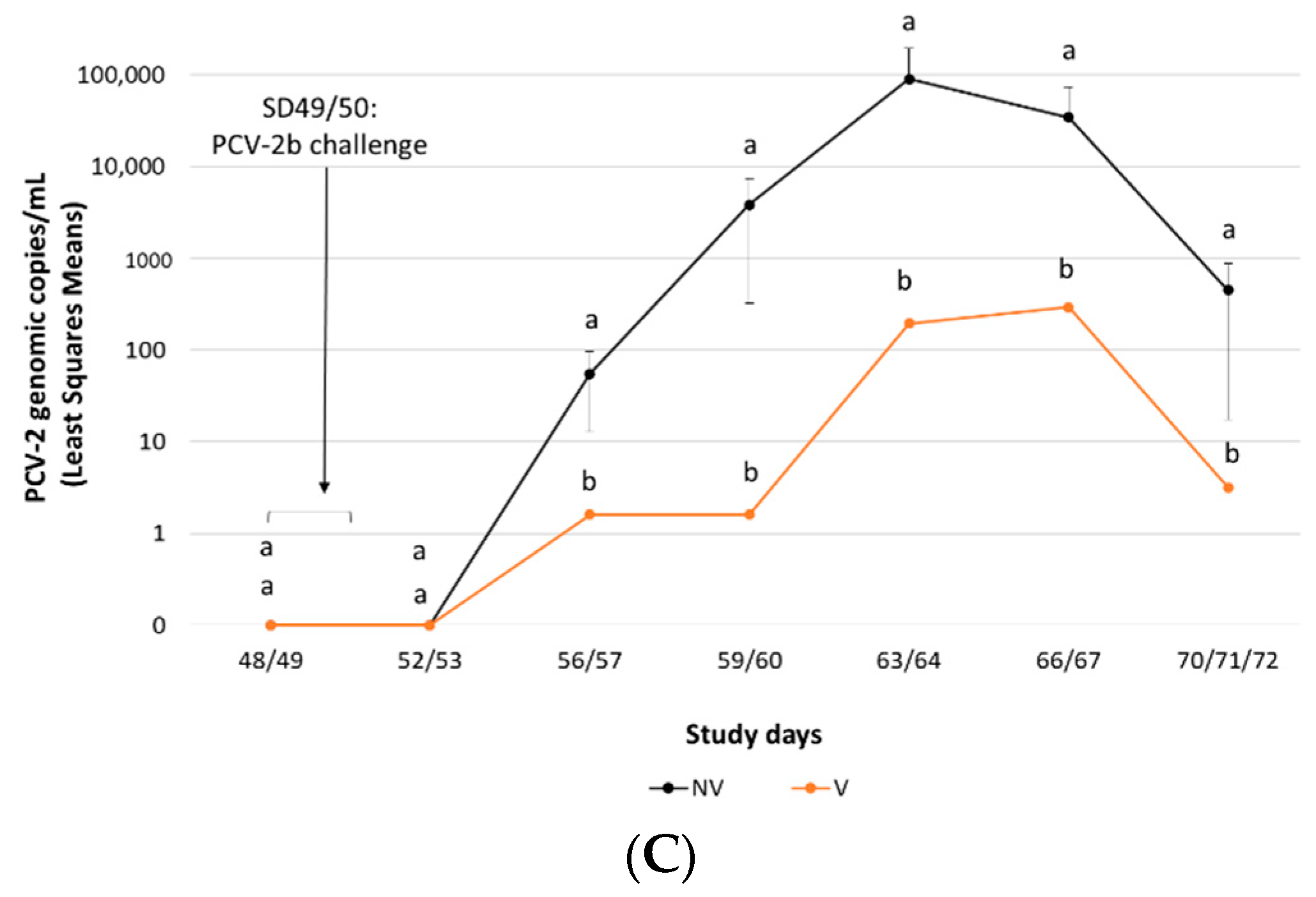
3.1.2. PCV-2b Challenge Study
Clinical Evaluation
PCV-2 Antibody Detection
Viraemia and Faecal Shedding
PCV-2 Detection in Lymphoid Tissues and Microscopic Lymphoid Lesions
3.2. Field Trials A and B
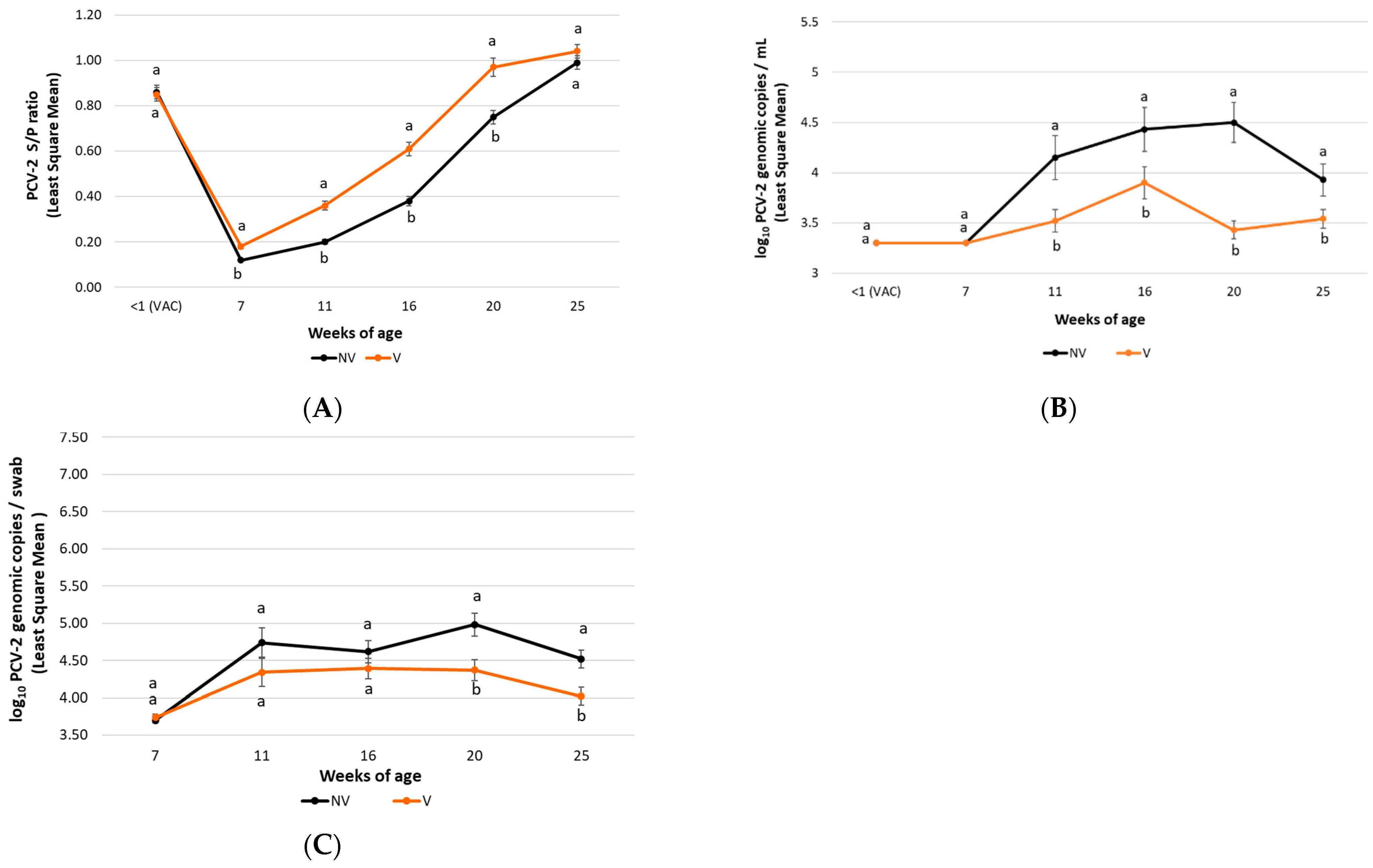
3.2.1. Clinical Evaluation
3.2.2. PCV-2 Viraemia
3.2.3. PCV-2 Faecal Shedding
3.2.4. PCV-2 Genotyping
3.2.5. PCV-2 Antibody Detection
3.2.6. Histopathology and PCV-2 IHC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segalés, J.; Allan, G.M.; Domingo, M. Porcine circovirus diseases. Anim. Health Res. Rev. 2005, 6, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J. Porcine circovirus type 2 (PCV-2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chung, H.K.; Chae, C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet. J. 2003, 166, 251–256. [Google Scholar] [CrossRef]
- Kim, J.; Ha, Y.; Jung, K.; Choi, C.; Chae, C. Enteritis associated with porcine circovirus 2 in pigs. Can. J. Vet. Res. 2004, 68, 218–221. [Google Scholar] [PubMed]
- Baró, J.; Segalés, J.; Martínez, J. Porcine circovirus type 2 (PCV2) enteric disease: An independent condition or part of the systemic disease? Vet. Microbiol. 2015, 176, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ticó, G.; Segalés, J.; Martínez, J. The blurred border between porcine circovirus type 2-systemic disease and porcine respiratory disease complex. Vet. Microbiol. 2013, 163, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Segalés, J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS ONE 2018, 13, e0208585. [Google Scholar] [CrossRef]
- Wang, Y.; Noll, L.; Lu, N.; Porter, E.; Stoy, C.; Zheng, W.; Liu, X.; Peddireddi, L.; Niederwerder, M.; Bai, J. Genetic diversity and prevalence of porcine circovirus type 3 (PCV3) and type 2 (PCV2) in the Midwest of the USA during 2016–2018. Transbound. Emerg. Dis. 2020, 67, 1284–1294. [Google Scholar] [CrossRef]
- Franzo, G.; Segalés, J. Porcine Circovirus 2 Genotypes, Immunity and Vaccines: Multiple Genotypes but One Single Serotype. Pathogens 2020, 9, 1049. [Google Scholar] [CrossRef]
- Opriessnig, T.; Castro, A.M.M.G.; Karuppanan, A.K.; Gauger, P.C.; Halbur, P.G.; Matzinger, S.R.; Meng, X.J. A Porcine circovirus type 2b (PCV2b)-based experimental vaccine is effective in the PCV2b-Mycoplasma hyopneumoniae coinfection pig model. Vaccine 2019, 37, 6688–6695. [Google Scholar] [CrossRef]
- Sibila, M.; Rocco, C.; Franzo, G.; Huerta, E.; Domingo, M.; Núñez, J.I.; Segalés, J. Genotyping of Porcine Circovirus 2 (PCV-2) in Vaccinated Pigs Suffering from PCV-2-Systemic Disease between 2009 and 2020 in Spain. Pathogens 2021, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Karuppannan, A.K.; Castro, A.M.M.G.; Xiao, C.T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Res. 2020, 286, 198044. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J. Best practice and future challenges for vaccination against porcine circovirus type 2. Expert Rev. Vaccines 2015, 14, 473–487. [Google Scholar] [CrossRef]
- Fort, M.; Sibila, M.; Allepuz, A.; Mateu, E.; Roerink, F.; Segalés, J. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine 2008, 26, 1063–1071. [Google Scholar] [CrossRef]
- Sibila, M.; Guevara, G.; Cuadrado, R.; Pleguezuelos, P.; Pérez, D.; Pérez de Rozas, A.; Huerta, E.; Llorens, A.; Valero, O.; Pérez, M.; et al. Comparison of Mycoplasma hyopneumoniae and porcine circovirus 2 commercial vaccines efficacy when applied separate or combined under experimental conditions. Porc. Health Manag. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Urniza, A.; Alegre, A.; Bru, T.; Crisci, E.; Nofrarías, M.; López-Soria, S.; Balasch, M.; Sibila, M.; Xu, Z.; et al. A genetically engineered chimeric vaccine against porcine circovirus type 2 (PCV2) improves clinical, pathological and virological outcomes in postweaning multisystemic wasting syndrome affected farms. Vaccine 2009, 27, 7313–7321. [Google Scholar] [CrossRef] [PubMed]
- Fachinger, V.; Bischoff, R.; Jedidia, S.B.; Saalmuller, A.; Elbers, K. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine 2008, 26, 1488–1499. [Google Scholar] [CrossRef]
- Cline, G.; Wilt, V.; Diaz, E.; Edler, R. Efficacy of immunising pigs against porcine circovirus type 2 at three or six weeks of age. Vet. Rec. 2008, 163, 737–740. [Google Scholar] [PubMed]
- Horlen, K.P.; Dritz, S.S.; Nietfeld, J.C.; Henry, S.C.; Hesse, R.A.; Oberst, R.; Hays, M.; Anderson, J.; Rowland, R.R. A field evaluation of mortality rate and growth performance in pigs vaccinated against porcine circovirus type 2. J. Am. Vet. Med. Assoc. 2008, 232, 906–912. [Google Scholar] [CrossRef]
- Kixmoller, M.; Ritzmann, M.; Eddicks, M.; Saalmuller, A.; Elbers, K.; Fachinger, V. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine 2008, 26, 3443–3451. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, R.; Clark, E.; Tremblay, D.; Tremblay, R.; Polson, D. Use of a one-dose subunit vaccine to prevent losses associated with porcine circovirus type 2. J. Swine Health Prod. 2009, 17, 148–154. [Google Scholar]
- Young, M.G.; Cunningham, G.L.; Sanford, S.E. Circovirus vaccination in pigs with subclinical porcine circovirus type 2 infection complicated by ileitis. J. Swine Health Prod. 2011, 19, 175–180. [Google Scholar]
- Kekarainen, T.; McCullough, K.; Fort, M.; Fossum, C.; Segalés, J.; Allan, G.M. Immune responses and vaccine-induced immunity against Porcine circovirus type 2. Vet. Immunol. Immunopathol. 2010, 136, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Haake, M.; Palzer, A.; Rist, B.; Weissenbacher-Lang, C.; Fachinger, V.; Eggen, A.; Ritzmann, M.; Eddicks, M. Influence of age on the effectiveness of PCV2 vaccination in piglets with high levels of maternally derived antibodies. Vet. Microbiol. 2014, 168, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Segalés, J.; Fraile, L.; López-Soria, S.; Sibila, M. Effect of high and low levels of maternally derived antibodies on porcine circovirus type 2 (PCV2) infection dynamics and production parameters in PCV2 vaccinated pigs under field conditions. Vaccine 2016, 34, 3044–3050. [Google Scholar] [CrossRef]
- Poulsen Nautrup, B.; Van Vlaenderen, I.; Mah, C.; Angulo, J. Do High Levels of Maternally Derived Antibodies Interfere with the Vaccination of Piglets against Porcine Circovirus Type 2? A Literature Review and Data Analysis. Vaccines 2021, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Patterson, A.R.; Elsener, J.; Meng, X.J.; Halbur, P.G. Influence of maternal antibodies on efficacy of porcine circovirus type 2 (PCV2) vaccination to protect pigs from experimental infection with PCV2. Clin. Vaccine Immunol. 2008, 15, 397–401. [Google Scholar] [CrossRef]
- Fort, M.; Sibila, M.; Pérez-Martín, E.; Nofrarías, M.; Mateu, E.; Segalés, J. One dose of a porcine circovirus 2 (PCV-2) sub-unit vaccine administered to 3-weekold conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viraemia in an experimental model. Vaccine 2009, 27, 4031–4037. [Google Scholar] [CrossRef]
- Fraile, L.; Grau-Roma, L.; Sarasola, P.; Sinovas, N.; Nofrarías, M.; López-Jimenez, R.; López-Soria, S.; Sibila, M.; Segalés, J. Inactivated PCV2 one shot vaccine applied in 3-week-old piglets: Improvement of production parameters and interaction with maternally derived immunity. Vaccine 2012, 30, 1986–1992. [Google Scholar] [CrossRef]
- Martelli, P.; Ferrari, L.; Morganti, M.; De Angelis, E.; Bonilauri, P.; Guazzetti, S.; Caleffi, A.; Borghetti, P. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet. Microbiol. 2011, 149, 339–351. [Google Scholar] [CrossRef]
- EMEA. European Agency for the evaluation of Medicinal Products. In Guidelines on Good Clinical Practices (CVMP/VICH/595/1998); EMEA: London, UK, 2000; Volume 155, pp. 254–258. [Google Scholar]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet. Res. 2016, 47, 121. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Mancera Gracia, J.C.; Smutzer, M.; Taylor, L.; Balasch, M.; Bandrick, M. One Dose of a Novel Vaccine Containing Two Genotypes of Porcine Circovirus (PCV2a and PCV2b) and Mycoplasma hyopneumoniae Conferred a Duration of Immunity of 23 Weeks. Vaccines 2021, 9, 834. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Exploratory field study on the effect of Porcine circovirus 2 (PCV2) sow vaccination on serological, virological and reproductive parameters in a PCV2 subclinically infected sow herd. BMC Vet. Res. 2018, 14, 130. [Google Scholar] [CrossRef]
- Nawagitgul, P.; Harms, P.A.; Morozov, I.; Thacker, B.J.; Sorden, S.D.; Lekcharoensuk, C.; Paul, P.S. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin. Lab Immunol. 2002, 9, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rosell, C.; Segalés, J.; Plana-Durán, J.; Balasch, M.; Rodríguez-Arrioja, G.M.; Kennedy, S.; Allan, G.M.; McNeilly, F.; Latimer, K.S.; Domingo, M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 1999, 120, 59–78. [Google Scholar] [CrossRef]
- Opriessnig, T.; Gerber, P.F.; Xiao, C.T.; Halbur, P.G.; Matzinger, S.R.; Meng, X.J. Commercial PCV2a-based vaccines are effective in protecting naturally PCV2b-infected finisher pigs against experimental challenge with a 2012 mutant PCV2. Vaccine 2014, 32, 4342–4348. [Google Scholar] [CrossRef] [PubMed]
- Karuppannan, A.K.; Opriessnig, T. Porcine Circovirus Type 2 (PCV2) Vaccines in the Context of Current Molecular Epidemiology. Viruses 2017, 9, 99. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Cecchinato, M.; Drigo, M. Porcine circovirus type 2 (PCV2) evolution before and after the vaccination introduction: A large scale epidemiological study. Sci. Rep. 2016, 6, 39458. [Google Scholar] [CrossRef] [PubMed]
- Bandrick, M.; Balasch, M.; Heinz, A.; Taylor, L.; King, V.; Toepfer, J.; Foss, D. A bivalent porcine circovirus type 2 (PCV2), PCV2a-PCV2b, vaccine offers biologically superior protection compared to monovalent PCV2 vaccines. Vet. Res. 2022, 53, 12. [Google Scholar] [CrossRef] [PubMed]
- Bandrick, M.; Gutiérrez, A.H.; Desai, P.; Rincon, G.; Martin, W.D.; Terry, F.E.; De Groot, A.S.; Foss, D.L. T cell epitope content comparison (EpiCC) analysis demonstrates a bivalent PCV2 vaccine has greater T cell epitope overlap with field strains than monovalent PCV2 vaccines. Vet. Immunol. Immunopathol. 2020, 223, 110034. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Rushton, J.; Wieland, B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England—an economic disease model. Prev. Vet. Med. 2013, 110, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Fraile, L.; Sibila, M.; Nofrarías, M.; López-Jimenez, R.; Huerta, E.; Llorens, A.; López-Soria, S.; Pérez, D.; Segalés, J. Effect of sow and piglet porcine circovirus type 2 (PCV2) vaccination on piglet mortality, viraemia, antibody titre and production parameters. Vet. Microbiol. 2012, 161, 229–234. [Google Scholar] [CrossRef]
- Nielsen, G.B.; Haugegaard, J.; Jolie, R. Field evaluation of a ready-to-use combined Porcine circovirus type 2 and Mycoplasma hyopneumoniae vaccine in Denmark—A historical comparison of productivity parameters in 20 nursery and 23 finishing herds. Porc. Health Manag. 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Park, C.; Choi, K.; Chae, C. A new single-dose bivalent vaccine of porcine circovirus type 2 and Mycoplasma hyopneumoniae elicits protective immunity and improves growth performance under field conditions. Vet. Microbiol. 2016, 182, 178–186. [Google Scholar] [CrossRef]
- Park, C.; Jeong, J.; Choi, K.; Chae, C. Efficacy of a new bivalent vaccine of porcine circovirus type 2 and Mycoplasma hyopneumoniae (Fostera™ PCV MH) under experimental conditions. Vaccine 2016, 34, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, M.; Opriessnig, T.; Halbur, P.G.; Elvinger, F.; Meng, X.J. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J. Virol. 2004, 78, 6297–6303. [Google Scholar] [CrossRef]
- Fenaux, M.; Opriessnig, T.; Halbur, P.G.; Meng, X.J. Immunogenicity and pathogenicity of chimeric infectious DNA clones of pathogenic porcine circovirus type 2 (PCV2) and nonpathogenic PCV1 in weanling pigs. J. Virol. 2003, 77, 11232–11243. [Google Scholar] [CrossRef]
- Ahn, Y.; Yang, S.; Oh, T.; Park, K.H.; Cho, H.; Suh, J.; Chae, C. Efficacy Evaluation of a Bivalent Vaccine Containing Porcine Circovirus Type 2b and Mycoplasma hyopneumoniae Against an Experimental Dual Challenge. Front. Vet. Sci. 2021, 8, 652313. [Google Scholar] [CrossRef] [PubMed]
- Witvliet, M.; Holtslag, H.; Nell, T.; Segers, R.; Fachinger, V. Efficacy and safety of a combined porcine Circovirus and Mycoplasma hyopneumoniae vaccine in finishing pigs. Trials Vaccinol. 2015, 4, 43–49. [Google Scholar] [CrossRef][Green Version]
- O’Neill, K.C.; Shen, H.G.; Lin, K.; Hemann, M.; Beach, N.M.; Meng, X.J.; Halbur, P.G.; Opriessnig, T. Studies on porcine circovirus type 2 vaccination of 5-day-old piglets. Clin. Vaccine Immunol. 2011, 18, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Patterson, A.R.; Madson, D.M.; Pal, N.; Ramamoorthy, S.; Meng, X.J.; Halbur, P.G. Comparison of the effectiveness of passive (dam) versus active (piglet) immunization against porcine circovirus type 2 (PCV2) and impact of passively derived PCV2 vaccine-induced immunity on vaccination. Vet. Microbiol. 2010, 142, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Seo, H.W.; Han, K.; Park, C.; Chae, C. Protective effect of the maternally derived porcine circovirus type 2 (PCV2)-specific cellular immune response in piglets by dam vaccination against PCV2 challenge. J. Gen. Virol. 2012, 93, 1556–1562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oh, Y.; Seo, H.W.; Park, C.; Chae, C. Comparison of sow and/or piglet vaccination of 3 commercial porcine circovirus type 2 (PCV2) single-dose vaccines on pigs under experimental PCV2 challenge. Vet. Microbiol. 2014, 172, 371–380. [Google Scholar] [CrossRef]
- Lin, C.N.; Ke, N.J.; Chiou, M.T. Cross-Sectional Study on the Sero- and Viral Dynamics of Porcine Circovirus Type 2 in the Field. Vaccines 2020, 8, 339. [Google Scholar] [CrossRef]
- Figueras-Gourgues, S.; Fraile, L.; Segalés, J.; Hernández-Caravaca, I.; López-Úbeda, R.; García-Vázquez, F.A.; Gomez-Duran, O.; Grosse-Liesner, B. Effect of Porcine circovirus 2 (PCV-2) maternally derived antibodies on performance and PCV-2 viremia in vaccinated piglets under field conditions. Porc. Health Manag. 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Tassis, P.D.; Tsakmakidis, I.; Papatsiros, V.G.; Koulialis, D.; Nell, T.; Brellou, G.; Tzika, E.D. A randomized controlled study on the efficacy of a novel combination vaccine against enzootic pneumonia (Mycoplasma hyopneumoniae) and porcine Circovirus type 2 (PCV2) in the presence of strong maternally derived PCV2 immunity in pigs. BMC Vet. Res. 2017, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Tzika, E.D.; Tassis, P.D.; Koulialis, D.; Papatsiros, V.G.; Nell, T.; Brellou, G.; Tsakmakidis, I. Field efficacy study of a novel ready-to-use vaccine against mycoplasma hyopneumoniae and porcine circovirus type 2 in a Greek farm. Porc. Health Manag. 2015, 1, 15. [Google Scholar] [CrossRef]
- Saporiti, V.; Huerta, E.; Correa-Fiz, F.; Grosse Liesner, B.; Duran, O.; Segalés, J.; Sibila, M. Detection and genotyping of Porcine circovirus 2 (PCV-2) and detection of Porcine circovirus 3 (PCV-3) in sera from fattening pigs of different European countries. Transbound. Emerg. Dis. 2020, 67, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Correa-Fiz, F.; Franzo, G.; Llorens, A.; Segalés, J.; Kekarainen, T. Porcine circovirus 2 (PCV-2) genetic variability under natural infection scenario reveals a complex network of viral quasispecies. Sci. Rep. 2018, 8, 15469. [Google Scholar] [CrossRef] [PubMed]
- Hesse, R.; Kerrigan, M.; Rowland, R.R. Evidence for recombination between PCV2a and PCV2b in the field. Virus Res. 2008, 132, 201–207. [Google Scholar] [CrossRef]
- Park, K.H.; Oh, T.; Yang, S.; Cho, H.; Kang, I.; Chae, C. Evaluation of a Porcine circovirus type 2a (PCV2a) vaccine efficacy against experimental PCV2a, PCV2b, and PCV2d challenge. Vet. Microbiol. 2019, 231, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Xiao, C.T.; Halbur, P.G.; Gerber, P.F.; Matzinger, S.R.; Meng, X.J. A commercial Porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viraemia and shedding and prevents PCV2d transmission to naive pigs under experimental conditions. Vaccine 2017, 35, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.; Andraud, M.; Bigault, L.; Jestin, A.; Grasland, B. A commercial PCV2a-based vaccine significantly reduces PCV2b transmission in experimental conditions. Vaccine 2016, 34, 3738–3745. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Grau-Roma, L.; Cortey, M.; Fort, M.; Rodríguez, F.; Sibila, M.; Segalés, J. Pigs naturally exposed to porcine circovirus type 2 (PCV2) generate antibody responses capable to neutralise PCV2 isolates of different genotypes and geographic origins. Vet. Res. 2014, 45, 29. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of Porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; O’Neill, K.; Gerber, P.F.; de Castro, A.M.; Gimenez-Lirola, L.G.; Beach, N.M.; Zhou, L.; Meng, X.J.; Wang, C.; Halbur, P.G. A PCV2 vaccine based on genotype 2b is more effective than a 2a-based vaccine to protect against PCV2b or combined PCV2a/2b viraemia in pigs with concurrent PCV2, PRRSV and PPV infection. Vaccine 2013, 31, 487–494. [Google Scholar] [CrossRef] [PubMed]



| Experimental Groups | Pre-Clinical Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCV-2a Challenge | PCV-2b Challenge | |||||||||
| PCV-2 MDA (Mean S/P Ratio ± SE) at SD0 * | N * | NV/V Administration | Challenge | Necropsy | PCV-2 MDA (Mean S/P Ratio ± SE) at SD0 | N ** | NV/V Administration | Challenge | Necropsy | |
| NV | 0.63 ± 0.01 (seropositive) | 45 | 3 and 24 days old | 8 weeks of age approx. (SD52) | 10–11 weeks of age (SD74) | 0.67 ± 0.02 (seropositive) | 34 | 3 and 24 days old | 7–8 weeks of age (SD49-50) | 10–11 weeks of age (SD70-72) |
| V | 0.64 ± 0.01 (seropositive) | 45 | 0.69 ± 0.02 (seropositive) | 35 | ||||||
| Field Trial | Farm | Treatment | Num. of Animals | Doses and Volume | Age at Vaccination |
|---|---|---|---|---|---|
| Field trial A | Farm A | V | 1017 | 2; 1 mL | 2–4 and 23–25 days of age |
| NV | 1021 | 2; 1 mL | |||
| Field trial B | Farm B | V | 966 | 2; 1 mL | 2–5 and 23–25 days of age |
| NV | 969 | 2; 1 mL |
| Group | PCV-2a Challenge Study | PCV-2b Challenge Study | ||
|---|---|---|---|---|
| Percentage of Ever Viraemic Pigs | Percentage of Ever Faecal Shedding Pigs | Percentage of Ever Viraemic Pigs | Percentage of Ever Faecal Shedding Pigs | |
| NV | 31/32 (96.9%) a | 30/32 (93.8%) a | 17/17 (100.0%) a | 17/17 (100.0%) a |
| V | 1/35 (2.9%) b | 19/35 (54.3%) b | 7/20 (35.0) b | 15/20 (75.0) b |
| Group | PCV-2a Challenge Study | PCV-2b Challenge Study | ||||
|---|---|---|---|---|---|---|
| HR | LD | IHC | HR | LD | IHC | |
| NV | 16/32 (50.0%) a | 20/32 (62.5%) a | 15/32 (46.9%) a | 13/17 (76.50%) a | 15/17 (88.2%) a | 12/17 (70.6%) a |
| V | 5/35 (14.3%) b | 9/35 (25.7%) b | 1/35 (2.9%) b | 6/20 (30.0) b | 12/20 (60.0%) a | 3/20 (1.5%) b |
| Study | Group | Body Weight (Kg ± SE) | ADWG (Kg/Day) | Mortality | |||||
|---|---|---|---|---|---|---|---|---|---|
| <1 WOA (Vac) | 16 WOA | 24–27 WOA | <1 WOA to 16 WOA | 16 WOA to 24–27 WOA | <1 WOA to 24–27 WOA | Each Treatment Group | Total | ||
| Field trial A | V | 2.2 ± 1.73 a | 56.4 ± 1.73 a | 114.3 ± 1.73 a | 0.47 a | 0.90 a | 0.63 a | 108/896 (12.1%) | 221/1801 (12.3%) |
| NV | 2.1 ± 1.74 a | 55.0 ± 1.74 b | 112.2 ± 1.73 b | 0.46 a | 0.89 a | 0.62 b | 113/905 (12.5%) | ||
| Field trial B | V | 1.5 ± 0.52 a | 45.6 ± 0.48 a | 103.4 ± 0.47 a | 0.39 a | 0.72 a | 0.53 a | 259/806 (32.1%) | 565/1652 (34.2%) |
| NV | 1.5 ± 0.48 a | 44.7 ± 0.45 a | 102.4 ± 0.45 a | 0.39 a | 0.72 a | 0.53 a | 306/846 (36.2%) | ||
| Study | Group | Proportion (%) of Pigs Detected Viraemic Per Sampling Point | Total Proportion (%) of Ever Viraemic Pigs * | |||||
|---|---|---|---|---|---|---|---|---|
| <1 WOA (Vac) | 7 WOA | 11 WOA | 16 WOA | 20 WOA | 25 WOA | |||
| Field trial A | V | 0/47 (0.0%) a | 0/42 (0.0%) a | 7/44 (15.9%) a | 22/44 (50.0%) a | 12/42 (28.6%) a | 3/39 (7.7%) a | 30/43 (69.8%) a |
| NV | 0/50 (0.0%) a | 0/44 (0.0%) a | 13/43 (30.2%) a | 23/41 (56.1%) a | 27/39 (69.2%) b | 21/40 (52.5%) b | 39/43 (90.7%) b | |
| Field trial B | V | 0/43 (0.0%) a | 0/30 (0.0%) a | 14/42 (33.3%) a | 25/40 (62.5%) a | 17/41 (41.5%) a | 5/48 (10.4%) a | 33/52 (63.5%) a |
| NV | 0/48 (0.0%) a | 0/31 (0.0%) a | 15/46 (32.6%) a | 42/42 (100%) a | 27/37 (73.0%) a | 20/53 (37.7%) a | 51/65 (78.5%) b | |
| Study | Group | HR | LD | IHC |
|---|---|---|---|---|
| Field trial A | V | 6/81 (7.4%) a | 13/81 (16.0%) a | 8/81 (9.9%) a |
| NV | 10/91 (11.0%) a | 16/91 (17.6%) a | 22/91 (24.2%) b | |
| Field trial B* | V | 0/172 (0.0%) a | 24/171 (14.0%) a | 3/192 (1.6%) a |
| NV | 1/220 (1.0%) a | 55/221 (24.9%) b | 4/241 (1.7%) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleguezuelos, P.; Sibila, M.; Cuadrado-Matías, R.; López-Jiménez, R.; Pérez, D.; Huerta, E.; Pérez, M.; Correa-Fiz, F.; Mancera-Gracia, J.C.; Taylor, L.P.; et al. Efficacy Studies of a Trivalent Vaccine Containing PCV-2a, PCV-2b Genotypes and Mycoplasma hyopneumoniae When Administered at 3 Days of Age and 3 Weeks Later against Porcine Circovirus 2 (PCV-2) Infection. Vaccines 2022, 10, 1234. https://doi.org/10.3390/vaccines10081234
Pleguezuelos P, Sibila M, Cuadrado-Matías R, López-Jiménez R, Pérez D, Huerta E, Pérez M, Correa-Fiz F, Mancera-Gracia JC, Taylor LP, et al. Efficacy Studies of a Trivalent Vaccine Containing PCV-2a, PCV-2b Genotypes and Mycoplasma hyopneumoniae When Administered at 3 Days of Age and 3 Weeks Later against Porcine Circovirus 2 (PCV-2) Infection. Vaccines. 2022; 10(8):1234. https://doi.org/10.3390/vaccines10081234
Chicago/Turabian StylePleguezuelos, Patricia, Marina Sibila, Raúl Cuadrado-Matías, Rosa López-Jiménez, Diego Pérez, Eva Huerta, Mónica Pérez, Florencia Correa-Fiz, José Carlos Mancera-Gracia, Lucas P. Taylor, and et al. 2022. "Efficacy Studies of a Trivalent Vaccine Containing PCV-2a, PCV-2b Genotypes and Mycoplasma hyopneumoniae When Administered at 3 Days of Age and 3 Weeks Later against Porcine Circovirus 2 (PCV-2) Infection" Vaccines 10, no. 8: 1234. https://doi.org/10.3390/vaccines10081234
APA StylePleguezuelos, P., Sibila, M., Cuadrado-Matías, R., López-Jiménez, R., Pérez, D., Huerta, E., Pérez, M., Correa-Fiz, F., Mancera-Gracia, J. C., Taylor, L. P., Borowski, S., Saunders, G., Segalés, J., López-Soria, S., & Balasch, M. (2022). Efficacy Studies of a Trivalent Vaccine Containing PCV-2a, PCV-2b Genotypes and Mycoplasma hyopneumoniae When Administered at 3 Days of Age and 3 Weeks Later against Porcine Circovirus 2 (PCV-2) Infection. Vaccines, 10(8), 1234. https://doi.org/10.3390/vaccines10081234







