COVID-19 Vaccine Booster Strategies for Omicron SARS-CoV-2 Variant: Effectiveness and Future Prospects
Abstract
:1. Introduction
2. The Increased Omicron-Neutralizing Activity of the Booster (Three Doses) Vaccination
3. The Increased Effectiveness of the Booster Vaccination (Three Doses) against the Omicron Variant
4. Fourth Dose–What Is the Evidence So Far?
5. Conclusions
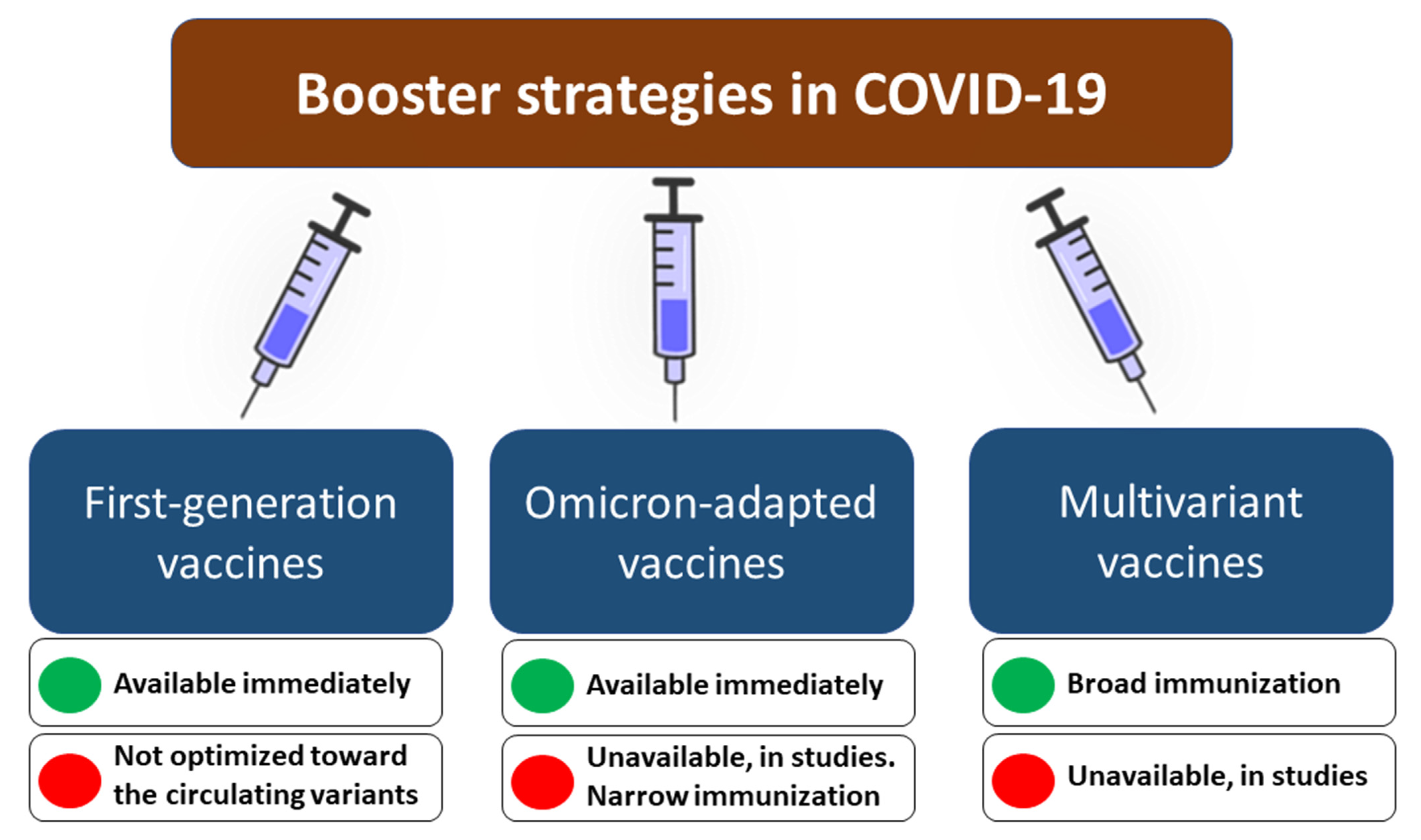
- Offering an additional (second) booster dose of the first generation before the expected wave. This approach has potential limitations in the long-term effectiveness since first-generation vaccines are not adapted to a heavily mutated spike protein of the Omicron variant, while novel sublineages of this variant (i.e., BA4, BA5), with high immune evasion, are emerging [71]. The necessity for repeated vaccinations may be met with increasing unwillingness and hesitancy–the share of individuals vaccinated with subsequent doses may gradually decrease.
- Development and use of Omicron-adapted booster dose before the expected wave. This approach would likely increase the specificity of the responses against the Omicron lineage but also does not come without challenges. Firstly, the Omicron variant continues to evolve, and its novel sublineages, characterized by enhanced transmissibility, are characterized by some unique mutations increasing antibody evasion [79,80]. The question remains which variant of spike protein should be selected as an antigen for such a booster vaccine. Furthermore, studies show that despite the Omicron dominance, other SARS-CoV-2 variants, including Delta, remain in cryptic circulation [81]. If one considers the asymmetric cross-immunization in which a person with a history of Omicron infection is four-fold less protected from Delta infection than protection from Omicron in a Delta-immunized individual [82,83], basing booster strategy on the vaccine adapted only to the Omicron variant could bring the potential risk of contracting other SARS-CoV-2 variants.
- Development and use of multiple antigen-based (multivariant-adapted) booster dose before the expected wave. This approach would possibly allow inducing a broad immunity against various variants, including Delta, Omicron, Beta, and others. This approach is used against influenza, with trivalent and quadrivalent vaccines targeting three and four strains of the virus, respectively [84]. However, it also comes with some shortcomings. Firstly, the chemical inactivation of SARS-CoV-2 has been shown to induce a transformation of prefusion conformation of spike protein to form resembling postfusion conformation, which is less immunogenic [85]. This challenge can be overcome by developing multivalent subunit vaccines, but their production is longer and more expensive [86]. A more cost- and time-efficient approach would involve the development of multivariant mRNA vaccines. However, these vaccines would require using more than one mRNA molecule to encode different versions of the spike protein. Whether using multiple mRNA molecules in a single-dose vaccine would affect translation efficiency, immunogenicity, and efficacy remain to be understood. There is, however, some evidence that such an approach may provide a broad neutralizing immunity against different SARS-CoV-2 variants, as shown in vivo for mRNA-1273.211 comprising a 1:1 mix of mRNA-1273 (present in the first-generation vaccine developed by Moderna, USA) and mRNA-1273.351 (adapted to Beta variant) [87,88]. More publicly available data is required to understand whether mRNA vaccines adapted to different SARS-CoV-2 variants, including Omicron, are providing efficient protection.
- Development of vaccines providing broad immune responses with enhanced durability. The main challenge of currently available COVID-19 vaccines is related to a gradual decrease of antibody levels observed within a few months from dose administration [16]. Although a booster dose temporarily restores antibody levels and strengthens cellular responses [89], the provided protection from different outcomes (including infections and hospital admission) of Omicron infection starts to wane after three-four months from administration [44,48,66]. It becomes more and more evident that vaccine strategies that would increase the durability of protection are necessary. This requires more studies to understand which amino acid substitutions could extend the half-life of antibodies but not decrease their neutralization activities and then design an antigen that would trigger their production. Moreover, some promise is also brought with vaccine candidates based on self-amplifying RNAs (saRNA), which enhance antigen presentation and may therefore mount a robust adaptive immune response against SARS-CoV-2 [90,91]. Further studies are required to understand whether saRNA can enhance the durability of protection.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid Epidemic Expansion of the SARS-CoV-2 Omicron Variant in Southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Lan, W.; Wu, X.; Zhao, T.; Duan, B.; Yang, P.; Ren, Y.; Quan, L.; Zhao, W.; Seto, D.; et al. Tracking SARS-CoV-2 Omicron Diverse Spike Gene Mutations Identifies Multiple Inter-Variant Recombination Events. Signal Transduct. Target. Ther. 2022, 7, 138. [Google Scholar] [CrossRef]
- Araf, Y.; Akter, F.; Tang, Y.-D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron Variant of SARS-CoV-2: Genomics, Transmissibility, and Responses to Current COVID-19 Vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- McCallum, M.; Czudnochowski, N.; Rosen, L.E.; Zepeda, S.K.; Bowen, J.E.; Walls, A.C.; Hauser, K.; Joshi, A.; Stewart, C.; Dillen, J.R.; et al. Structural Basis of SARS-CoV-2 Omicron Immune Evasion and Receptor Engagement. Science 2022, 375, 864–868. [Google Scholar] [CrossRef]
- Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 26 June 2022).
- Puhach, O.; Adea, K.; Hulo, N.; Sattonnet, P.; Genecand, C.; Iten, A.; Bausch, F.J.; Kaiser, L.; Vetter, P.; Eckerle, I.; et al. Infectious Viral Load in Unvaccinated and Vaccinated Patients Infected with SARS-CoV-2 WT, Delta and Omicron. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, L.; Mo, M.; Liu, T.; Wu, C.; Gong, C.; Lu, K.; Gong, L.; Zhu, W.; Xu, Z. SARS-CoV-2 Omicron RBD Shows Weaker Binding Affinity than the Currently Dominant Delta Variant to Human ACE2. Signal Transduct. Target. Ther. 2022, 7, 8. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, L.; Rocha, C.; Sidarovich, A.; Kempf, A.; Schulz, S.; Cossmann, A.; Manger, B.; Baier, E.; Tampe, B.; et al. Comparable Neutralisation Evasion of SARS-CoV-2 Omicron Subvariants BA.1, BA.2, and BA.3. Lancet Infect. Dis. 2022, 22, 766–767. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Mohamed, K.; Rzymski, P.; Islam, M.S.; Makuku, R.; Mushtaq, A.; Khan, A.; Ivanovska, M.; Makka, S.A.; Hashem, F.; Marquez, L.; et al. COVID-19 Vaccinations: The Unknowns, Challenges, and Hopes. J. Med. Virol. 2022, 94, 1336–1349. [Google Scholar] [CrossRef]
- Sikora, D.; Rzymski, P. COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2. Vaccines 2022, 10, 437. [Google Scholar] [CrossRef]
- Grannis, S.J.; Rowley, E.A.; Ong, T.C.; Stenehjem, E.; Klein, N.P.; DeSilva, M.B.; Naleway, A.L.; Natarajan, K.; Thompson, M.G. VISION Network Interim Estimates of COVID-19 Vaccine Effectiveness against COVID-19-Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations among Adults during SARS-CoV-2 B.1.617.2 (Delta) Variant Predominance—Nine States, June-August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1291–1293. [Google Scholar] [CrossRef]
- Nasreen, S.; Chung, H.; He, S.; Brown, K.A.; Gubbay, J.B.; Buchan, S.A.; Fell, D.B.; Austin, P.C.; Schwartz, K.L.; Sundaram, M.E.; et al. Effectiveness of COVID-19 Vaccines against Symptomatic SARS-CoV-2 Infection and Severe Outcomes with Variants of Concern in Ontario. Nat. Microbiol. 2022, 7, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 Booster Vaccines against COVID-19-Related Symptoms, Hospitalization and Death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Camargo, C.A., Jr.; Fal, A.; Flisiak, R.; Gwenzi, W.; Kelishadi, R.; Leemans, A.; Nieto, J.J.; Ozen, A.; Perc, M.; et al. COVID-19 Vaccine Boosters: The Good, the Bad, and the Ugly. Vaccines 2021, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Yasuhara, A.; Ito, M.; Akasaka, O.; Nakamura, M.; Nakachi, I.; Koga, M.; Mitamura, K.; Yagi, K.; Maeda, K.; et al. Antibody Titers against SARS-CoV-2 Decline, but Do Not Disappear for Several Months. EClinicalMedicine 2021, 32, 100734. [Google Scholar] [CrossRef]
- Rzymski, P.; Pazgan-Simon, M.; Kamerys, J.; Moniuszko-Malinowska, A.; Sikorska, K.; Wernik, J.; Zarębska-Michaluk, D.; Supronowicz, Ł.; Sobala-Szczygieł, B.; Skrzat-Klapaczyńska, A.; et al. Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines 2022, 10, 557. [Google Scholar] [CrossRef]
- A Study to Evaluate the Immunogenicity and Safety of Omicron Variant Vaccines in Comparison With MRNA-1273 Booster Vaccine for COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT05249829 (accessed on 26 June 2022).
- Board Members Pfizer and BioNTech Initiate Study to Evaluate Omicron-Based COVID-19 Vaccine in Adults 18 to 55 Years of Age. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-evaluate-omicron-based (accessed on 26 June 2022).
- SINOVAC: Supply Vaccines to Eliminate Human Diseases. Available online: http://www.sinovac.com/news/shownews.php?id=1448&lang=en (accessed on 26 June 2022).
- De Marco, L.; D’Orso, S.; Pirronello, M.; Verdiani, A.; Termine, A.; Fabrizio, C.; Capone, A.; Sabatini, A.; Guerrera, G.; Placido, R.; et al. Assessment of T-Cell Reactivity to the SARS-CoV-2 Omicron Variant by Immunized Individuals. JAMA Netw. Open 2022, 5, e2210871. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. MRNA-Based COVID-19 Vaccine Boosters Induce Neutralizing Immunity against SARS-CoV-2 Omicron Variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef]
- Ariën, K.K.; Heyndrickx, L.; Michiels, J.; Vereecken, K.; Van Lent, K.; Coppens, S.; Willems, B.; Pannus, P.; Martens, G.A.; Van Esbroeck, M.; et al. Three Doses of BNT162b2 Vaccine Confer Neutralising Antibody Capacity against the SARS-CoV-2 Omicron Variant. NPJ Vaccines 2022, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Pollett, S.D.; Neerukonda, S.N.; Wang, W.; Wang, R.; Vassell, R.; Epsi, N.J.; Fries, A.C.; Agan, B.K.; Lindholm, D.A.; et al. SARS-CoV-2 BA.1 Variant Is Neutralized by Vaccine Booster-Elicited Serum but Evades Most Convalescent Serum and Therapeutic Antibodies. Sci. Transl. Med. 2022, 14, eabn8543. [Google Scholar] [CrossRef] [PubMed]
- Hastert, F.D.; Hein, S.; von Rhein, C.; Benz, N.I.; Husria, Y.; Oberle, D.; Maier, T.J.; Hildt, E.; Schnierle, B.S. The SARS-CoV-2 Variant Omicron Is Able to Escape Vaccine-Induced Humoral Immune Responses, but Is Counteracted by Booster Vaccination. Vaccines 2022, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- van Gils, M.J.; Lavell, A.; van der Straten, K.; Appelman, B.; Bontjer, I.; Poniman, M.; Burger, J.A.; Oomen, M.; Bouhuijs, J.H.; van Vught, L.A.; et al. Antibody Responses against SARS-CoV-2 Variants Induced by Four Different SARS-CoV-2 Vaccines in Health Care Workers in the Netherlands: A Prospective Cohort Study. PLoS Med. 2022, 19, e1003991. [Google Scholar] [CrossRef] [PubMed]
- Belik, M.; Jalkanen, P.; Lundberg, R.; Reinholm, A.; Laine, L.; Väisänen, E.; Skön, M.; Tähtinen, P.A.; Ivaska, L.; Pakkanen, S.H.; et al. Comparative Analysis of COVID-19 Vaccine Responses and Third Booster Dose-Induced Neutralizing Antibodies against Delta and Omicron Variants. Nat. Commun. 2022, 13, 2476. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Collier, A.-R.Y.; Rowe, M.; Mardas, F.; Ventura, J.D.; Wan, H.; Miller, J.; Powers, O.; Chung, B.; Siamatu, M.; et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 Variants. N. Engl. J. Med. 2022, 386, 1579–1580. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.M.; Bang, L.L.; Madsen, L.W.; Sydenham, T.V.; Johansen, I.S.; Jensen, T.G.; Justesen, U.S.; Andersen, T.E. Serum Neutralization of SARS-CoV-2 Omicron BA.1 and BA.2 after BNT162b2 Booster Vaccination. Emerg. Infect. Dis. 2022, 28, 1274–1275. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef]
- Alidjinou, E.K.; Demaret, J.; Corroyer-Simovic, B.; Labreuche, J.; Goffard, A.; Trauet, J.; Lupau, D.; Miczek, S.; Vuotto, F.; Dendooven, A.; et al. Immunogenicity of BNT162b2 Vaccine Booster against SARS-CoV-2 Delta and Omicron Variants in Nursing Home Residents: A Prospective Observational Study in Older Adults Aged from 68 to 98 Years. Lancet Reg. Health Eur. 2022, 17, 100385. [Google Scholar] [CrossRef]
- Canaday, D.H.; Oyebanji, O.A.; White, E.; Keresztesy, D.; Payne, M.; Wilk, D.; Carias, L.; Aung, H.; St Denis, K.; Sheehan, M.L.; et al. COVID-19 Vaccine Booster Dose Needed to Achieve Omicron-Specific Neutralisation in Nursing Home Residents. EBioMedicine 2022, 80, 104066. [Google Scholar] [CrossRef]
- Pajon, R.; Doria-Rose, N.A.; Shen, X.; Schmidt, S.D.; O’Dell, S.; McDanal, C.; Feng, W.; Tong, J.; Eaton, A.; Maglinao, M.; et al. SARS-CoV-2 Omicron Variant Neutralization after MRNA-1273 Booster Vaccination. N. Engl. J. Med. 2022, 386, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Yin, S.; Tao, Y.; Zhu, L.; Tong, X.; Mao, M.; Li, M.; Wan, Y.; Ni, J.; et al. The Third Dose of CoronVac Vaccination Induces Broad and Potent Adaptive Immune Responses That Recognize SARS-CoV-2 Delta and Omicron Variants. Emerg. Microbes Infect. 2022, 11, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-M.; Chang, S.-C.; Cheng, H.-Y.; Shih, S.-R.; Lien, C.E. Durability and Immunogenicity of Neutralizing Antibodies Response against Omicron Variants after Three Doses of Subunit SARS-CoV-2 Vaccine MVC-COV1901: An Extension to an Open-Label, Dose-Escalation Phase 1 Study. Infect. Dis. Ther. 2022, 11, 1493–1504. [Google Scholar] [CrossRef]
- Gagne, M.; Moliva, J.I.; Foulds, K.E.; Andrew, S.F.; Flynn, B.J.; Werner, A.P.; Wagner, D.A.; Teng, I.-T.; Lin, B.C.; Moore, C.; et al. MRNA-1273 or MRNA-Omicron Boost in Vaccinated Macaques Elicits Similar B Cell Expansion, Neutralizing Responses, and Protection from Omicron. Cell 2022, 185, 1556–1571.e18. [Google Scholar] [CrossRef] [PubMed]
- Poh, X.Y.; Tan, C.W.; Lee, I.R.; Chavatte, J.-M.; Fong, S.-W.; Prince, T.; Hartley, C.; Yeoh, A.Y.-Y.; Rao, S.; Chia, P.Y.; et al. Antibody Response of Heterologous vs Homologous MRNA Vaccine Boosters against the SARS-CoV-2 Omicron Variant: Interim Results from the PRIBIVAC Study, A Randomized Clinical Trial. Clin. Infect. Dis. 2022, 345. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Song, J.; Wu, J.; Zhu, Y.; Li, M.; Cui, Y.; Chen, Y.; Yang, L.; Liu, J.; et al. Homologous or Heterologous Booster of Inactivated Vaccine Reduces SARS-CoV-2 Omicron Variant Escape from Neutralizing Antibodies. Emerg. Microbes Infect. 2022, 11, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus Homologous COVID-19 Booster Vaccination in Previous Recipients of Two Doses of CoronaVac COVID-19 Vaccine in Brazil (RHH-001): A Phase 4, Non-Inferiority, Single Blind, Randomised Study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Zuo, F.; Abolhassani, H.; Du, L.; Piralla, A.; Bertoglio, F.; de Campos-Mata, L.; Wan, H.; Schubert, M.; Cassaniti, I.; Wang, Y.; et al. Heterologous Immunization with Inactivated Vaccine Followed by MRNA-Booster Elicits Strong Immunity against SARS-CoV-2 Omicron Variant. Nat. Commun. 2022, 13, 2670. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.M.S.; Mok, C.K.P.; Leung, Y.W.Y.; Ng, S.S.; Chan, K.C.K.; Ko, F.W.; Chen, C.; Yiu, K.; Lam, B.H.S.; Lau, E.H.Y.; et al. Neutralizing Antibodies against the SARS-CoV-2 Omicron Variant BA.1 Following Homologous and Heterologous CoronaVac or BNT162b2 Vaccination. Nat. Med. 2022, 28, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity of, and Effectiveness of MRNA Vaccines against, COVID-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Xie, F.; Ackerson, B.K.; Valluri, S.R.; Jodar, L.; McLaughlin, J.M. Durability of BNT162b2 Vaccine against Hospital and Emergency Department Admissions Due to the Omicron and Delta Variants in a Large Health System in the USA: A Test-Negative Case-Control Study. Lancet Respir. Med. 2022, 10, 689–699. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of MRNA-1273 against SARS-CoV-2 Omicron and Delta Variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Šmíd, M.; Berec, L.; Přibylová, L.; Májek, O.; Pavlík, T.; Jarkovský, J.; Weiner, J.; Barusová, T.; Trnka, J. Protection by Vaccines and Previous Infection against the Omicron Variant of SARS-CoV-2. J. Infect. Dis. 2022, 161. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning 2-Dose and 3-Dose Effectiveness of MRNA Vaccines against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021-January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Talisa, V.B.; Shaikh, O.S.; Omer, S.B.; Mayr, F.B. Relative Vaccine Effectiveness of a SARS-CoV-2 MRNA Vaccine Booster Dose against the Omicron Variant. Clin. Infect. Dis. 2022, 328. [Google Scholar] [CrossRef]
- Modes, M.E.; Directo, M.P.; Melgar, M.; Johnson, L.R.; Yang, H.; Chaudhary, P.; Bartolini, S.; Kho, N.; Noble, P.W.; Isonaka, S.; et al. Clinical Characteristics and Outcomes among Adults Hospitalized with Laboratory-Confirmed SARS-CoV-2 Infection during Periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) Variant Predominance—One Hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 217–223. [Google Scholar] [CrossRef]
- Plumb, I.D.; Feldstein, L.R.; Barkley, E.; Posner, A.B.; Bregman, H.S.; Hagen, M.B.; Gerhart, J.L. Effectiveness of COVID-19 MRNA Vaccination in Preventing COVID-19-Associated Hospitalization among Adults with Previous SARS-CoV-2 Infection—United States, June 2021-February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 549–555. [Google Scholar] [CrossRef]
- Rzymski, P.; Sikora, D.; Zeyland, J.; Poniedziałek, B.; Kiedik, D.; Falfushynska, H.; Fal, A. Frequency and Nuisance Level of Adverse Events in Individuals Receiving Homologous and Heterologous COVID-19 Booster Vaccine. Vaccines 2022, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Mayr, F.B.; Talisa, V.B.; Shaikh, O.; Yende, S.; Butt, A.A. Effectiveness of Homologous or Heterologous COVID-19 Boosters in Veterans. N. Engl. J. Med. 2022, 386, 1375–1377. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Quach, T.H.T.; Tran, T.M.; Phuoc, H.N.; Nguyen, H.T.; Vo, T.K.; Van Vo, G. Reactogenicity and Immunogenicity of Heterologous Prime-Boost Immunization with COVID-19 Vaccine. Biomed. Pharmacother. 2022, 147, 112650. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, E.K.; Britton, A.; Shang, N.; Fleming-Dutra, K.E.; Link-Gelles, R.; Smith, Z.R.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Effectiveness of Homologous and Heterologous COVID-19 Boosters against Omicron. N. Engl. J. Med. 2022, 386, 2433–2435. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of MRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med. 2022, 386, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Suah, J.L.; Tng, B.H.; Tok, P.S.K.; Husin, M.; Thevananthan, T.; Peariasamy, K.M.; Sivasampu, S. Real-World Effectiveness of Homologous and Heterologous BNT162b2, CoronaVac, and AZD1222 Booster Vaccination against Delta and Omicron SARS-CoV-2 Infection. Emerg. Microbes Infect. 2022, 11, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Prasad, N.; Dascomb, K.; Irving, S.A.; Yang, D.-H.; Gaglani, M.; Klein, N.P.; DeSilva, M.B.; Ong, T.C.; Grannis, S.J.; et al. Effectiveness of Homologous and Heterologous COVID-19 Booster Doses Following 1 Ad.26.COV2.S (Janssen [Johnson & Johnson]) Vaccine Dose against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults—VISION Network, 10 States, December 2021-March 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Bajema, K.L.; Dahl, R.M.; Prill, M.M.; Meites, E.; Rodriguez-Barradas, M.C.; Marconi, V.C.; Beenhouwer, D.O.; Brown, S.T.; Holodniy, M.; Lucero-Obusan, C.; et al. Effectiveness of COVID-19 MRNA Vaccines against COVID-19-Associated Hospitalization—Five Veterans Affairs Medical Centers, United States, February 1-August 6, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1294–1299. [Google Scholar] [CrossRef]
- Higdon, M.M.; Baidya, A.; Walter, K.K.; Patel, M.K.; Issa, H.; Espié, E.; Feikin, D.R.; Knoll, M.D. Duration of Effectiveness of Vaccination against COVID-19 Caused by the Omicron Variant. Lancet Infect. Dis. 2022, 22, 1114–1116. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Xie, F.; Ackerson, B.K.; Valluri, S.R.; Jodar, L.; McLaughlin, J.M. Immunocompromise and Durability of BNT162b2 Vaccine against Severe Outcomes Due to Omicron and Delta Variants. Lancet Respir. Med. 2022, 10, e61–e62. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of COVID-19 MRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022, 386, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Burki, T.K. Fourth Dose of COVID-19 Vaccines in Israel. Lancet Respir. Med. 2022, 10, e19. [Google Scholar] [CrossRef]
- Magen, O.; Waxman, J.G.; Makov-Assif, M.; Vered, R.; Dicker, D.; Hernán, M.A.; Lipsitch, M.; Reis, B.Y.; Balicer, R.D.; Dagan, N. Fourth Dose of BNT162b2 MRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2022, 386, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Faraone, J.; Evans, J.P.; Zou, X.; Zheng, Y.-M.; Carlin, C.; Bednash, J.S.; Lozanski, G.; Mallampalli, R.K.; Saif, L.J.; et al. Neutralization of the SARS-CoV-2 Omicron BA.4/5 and BA.2.12.1 Subvariants. N. Engl. J. Med. 2022, 386, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.P.; Flaxman, S.; Gallinat, A.S.; Kinosian, S.P.; Stemkovski, M.; Unwin, H.J.T.; Watson, O.J.; Whittaker, C.; Cattarino, L.; Dorigatti, I.; et al. Temperature and Population Density Influence SARS-CoV-2 Transmission in the Absence of Nonpharmaceutical Interventions. Proc. Natl. Acad. Sci. USA 2021, 118, e2019284118. [Google Scholar] [CrossRef]
- Gavenčiak, T.; Monrad, J.T.; Leech, G.; Sharma, M.; Mindermann, S.; Brauner, J.M.; Bhatt, S.; Kulveit, J. Seasonal Variation in SARS-CoV-2 Transmission in Temperate Climates. bioRxiv 2021. [Google Scholar] [CrossRef]
- Yeh, T.-Y.; Contreras, G.P. Full Vaccination against COVID-19 Suppresses SARS-CoV-2 Delta Variant and Spike Gene Mutation Frequencies and Generates Purifying Selection Pressure. bioRxiv 2021. [Google Scholar] [CrossRef]
- Rzymski, P.; Szuster-Ciesielska, A. The COVID-19 Vaccination Still Matters: Omicron Variant Is a Final Wake-up Call for the Rich to Help the Poor. Vaccines 2022, 10, 1070. [Google Scholar] [CrossRef]
- Ganesan, S.; Al Ketbi, L.M.B.; Al Kaabi, N.; Al Mansoori, M.; Al Maskari, N.N.; Al Shamsi, M.S.; Alderei, A.S.; El Eissaee, H.N.; Al Ketbi, R.M.; Al Shamsi, N.S.; et al. Vaccine Side Effects Following COVID-19 Vaccination among the Residents of the UAE-an Observational Study. Front. Public Health 2022, 10, 876336. [Google Scholar] [CrossRef]
- Rzymski, P.; Perek, B.; Flisiak, R. Thrombotic Thrombocytopenia after COVID-19 Vaccination: In Search of the Underlying Mechanism. Vaccines 2021, 9, 559. [Google Scholar] [CrossRef]
- Mushtaq, H.A.; Khedr, A.; Koritala, T.; Bartlett, B.N.; Jain, N.K.; Khan, S.A. A Review of Adverse Effects of COVID-19 Vaccines. Infez. Med. 2022, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Iketani, S.; Li, Z.; Mohri, H.; Wang, M.; Yu, J.; Bowen, A.D.; Chang, J.Y.; Shah, J.G.; et al. SARS-CoV-2 Omicron BA.2.12.1, BA.4, and BA.5 Subvariants Evolved to Extend Antibody Evasion. bioRxiv 2022. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 Escape Antibodies Elicited by Omicron Infection. Nature 2022. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, K.; Ozer, E.; Shagan, M.; Paitan, Y.; Granek, R.; Kushmaro, A. Managing an Evolving Pandemic: Cryptic Circulation of the Delta Variant during the Omicron Rise. Sci. Total Environ. 2022, 836, 155599. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Chen, I.P.; Ma, T.; Syed, A.M.; Brazer, N.; Saldhi, P.; Simoneau, C.R.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; et al. Limited Cross-Variant Immunity after Infection with the SARS-CoV-2 Omicron Variant without Vaccination. medRxiv 2022. [Google Scholar] [CrossRef]
- Laurie, M.T.; Liu, J.; Sunshine, S.; Peng, J.; Black, D.; Mitchell, A.M.; Mann, S.A.; Pilarowski, G.; Zorn, K.C.; Rubio, L.; et al. SARS-CoV-2 Variant Exposures Elicit Antibody Responses with Differential Cross-Neutralization of Established and Emerging Strains Including Delta and Omicron. J. Infect. Dis. 2022, 225, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, L.; Kiseleva, I.; Krutikova, E.; Stepanova, E.; Rekstin, A.; Donina, S.; Pisareva, M.; Grigorieva, E.; Kryshen, K.; Muzhikyan, A.; et al. Rationale for Vaccination with Trivalent or Quadrivalent Live Attenuated Influenza Vaccines: Protective Vaccine Efficacy in the Ferret Model. PLoS ONE 2018, 13, e0208028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Mendonça, L.; Yang, Y.; Gao, Y.; Shen, C.; Liu, J.; Ni, T.; Ju, B.; Liu, C.; Tang, X.; et al. The Architecture of Inactivated SARS-CoV-2 with Postfusion Spikes Revealed by Cryo-EM and Cryo-ET. Structure 2020, 28, 1218–1224.e4. [Google Scholar] [CrossRef]
- Carpenter, C.F.; Aljassem, A.; Stassinopoulos, J.; Pisacreta, G.; Hutton, D. A Cost-Effectiveness Analysis of an Adjuvanted Subunit Vaccine for the Prevention of Herpes Zoster and Post-Herpetic Neuralgia. Open Forum Infect. Dis. 2019, 6, ofz219. [Google Scholar] [CrossRef]
- Wu, K.; Choi, A.; Koch, M.; Elbashir, S.; Ma, L.; Lee, D.; Woods, A.; Henry, C.; Palandjian, C.; Hill, A.; et al. Variant SARS-CoV-2 MRNA Vaccines Confer Broad Neutralization as Primary or Booster Series in Mice. Vaccine 2021, 39, 7394–7400. [Google Scholar] [CrossRef]
- Ying, B.; Whitener, B.; VanBlargan, L.A.; Hassan, A.O.; Shrihari, S.; Liang, C.-Y.; Karl, C.E.; Mackin, S.; Chen, R.E.; Kafai, N.M.; et al. Protective Activity of MRNA Vaccines against Ancestral and Variant SARS-CoV-2 Strains. Sci. Transl. Med. 2022, 14, eabm3302. [Google Scholar] [CrossRef]
- Busà, R.; Sorrentino, M.C.; Russelli, G.; Amico, G.; Miceli, V.; Miele, M.; Di Bella, M.; Timoneri, F.; Gallo, A.; Zito, G.; et al. Specific Anti-SARS-CoV-2 Humoral and Cellular Immune Responses after Booster Dose of BNT162b2 Pfizer-BioNTech MRNA-Based Vaccine: Integrated Study of Adaptive Immune System Components. Front. Immunol. 2022, 13, 856657. [Google Scholar] [CrossRef]
- Maruggi, G.; Mallett, C.P.; Westerbeck, J.W.; Chen, T.; Lofano, G.; Friedrich, K.; Qu, L.; Sun, J.T.; McAuliffe, J.; Kanitkar, A.; et al. A Self-Amplifying MRNA SARS-CoV-2 Vaccine Candidate Induces Safe and Robust Protective Immunity in Preclinical Models. Mol. Ther. 2022, 30, 1897–1912. [Google Scholar] [CrossRef]
- Rappaport, A.R.; Hong, S.-J.; Scallan, C.D.; Gitlin, L.; Akoopie, A.; Boucher, G.R.; Egorova, M.; Espinosa, J.A.; Fidanza, M.; Kachura, M.A.; et al. Low-Dose Self-Amplifying MRNA COVID-19 Vaccine Drives Strong Protective Immunity in Non-Human Primates against SARS-CoV-2 Infection. Nat. Commun. 2022, 13, 3289. [Google Scholar] [CrossRef]
| Reference | Design | Findings |
|---|---|---|
| [33] |
|
|
| [34] |
|
|
| [35] |
|
|
| [38] |
|
|
| [39] |
|
|
| [40] |
|
|
| Reference | Design | Findings |
|---|---|---|
| [43] |
|
|
| [44] |
|
|
| [45] |
|
|
| [46] |
|
|
| [47] |
|
|
| [48] |
|
|
| [49] |
|
|
| [56] |
|
|
| [57] |
|
|
| [58] |
|
|
| [59] |
|
|
| Reference | Design | Findings |
|---|---|---|
| [67] | Prospective, open-label, non-randomized study Participants–healthcare workers ≥ 18Arms treatment arm
| Immunogenicity and efficacy
|
| [68] | Retrospective real-world population-based study Participants ≥ 60 yrs
| Effectiveness
|
| [70] | Retrospective real-world population-based study Participants ≥ 60 yrs
| Effectiveness–protection assessed 7–30 days and 14–30 days after 4th dose against
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarębska-Michaluk, D.; Hu, C.; Brzdęk, M.; Flisiak, R.; Rzymski, P. COVID-19 Vaccine Booster Strategies for Omicron SARS-CoV-2 Variant: Effectiveness and Future Prospects. Vaccines 2022, 10, 1223. https://doi.org/10.3390/vaccines10081223
Zarębska-Michaluk D, Hu C, Brzdęk M, Flisiak R, Rzymski P. COVID-19 Vaccine Booster Strategies for Omicron SARS-CoV-2 Variant: Effectiveness and Future Prospects. Vaccines. 2022; 10(8):1223. https://doi.org/10.3390/vaccines10081223
Chicago/Turabian StyleZarębska-Michaluk, Dorota, Chenlin Hu, Michał Brzdęk, Robert Flisiak, and Piotr Rzymski. 2022. "COVID-19 Vaccine Booster Strategies for Omicron SARS-CoV-2 Variant: Effectiveness and Future Prospects" Vaccines 10, no. 8: 1223. https://doi.org/10.3390/vaccines10081223
APA StyleZarębska-Michaluk, D., Hu, C., Brzdęk, M., Flisiak, R., & Rzymski, P. (2022). COVID-19 Vaccine Booster Strategies for Omicron SARS-CoV-2 Variant: Effectiveness and Future Prospects. Vaccines, 10(8), 1223. https://doi.org/10.3390/vaccines10081223











