Safety and Efficacy of Influenza Vaccination in Patients Receiving Immune Checkpoint Inhibitors. Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search
2.5. Study Selection
2.6. Data Collection Process
2.7. Data Items
2.8. Risk of Bias in Individual Studies
2.9. Summary Measures
2.10. Synthesis of Results
2.11. Risk of Bias across Studies
2.12. Certainty Assessment
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Participants’ Characteristics
3.4. Risk of Bias within Studies
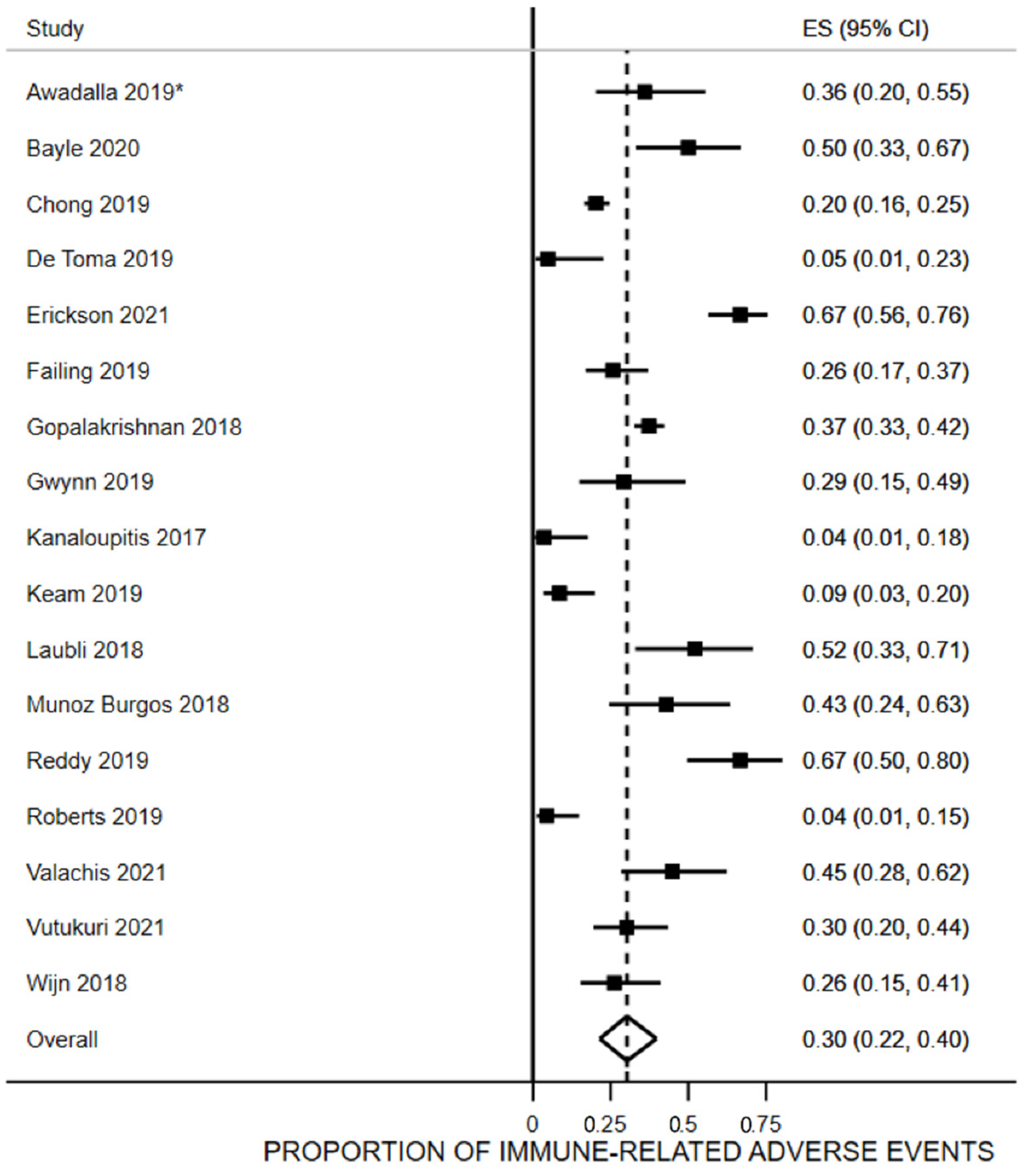
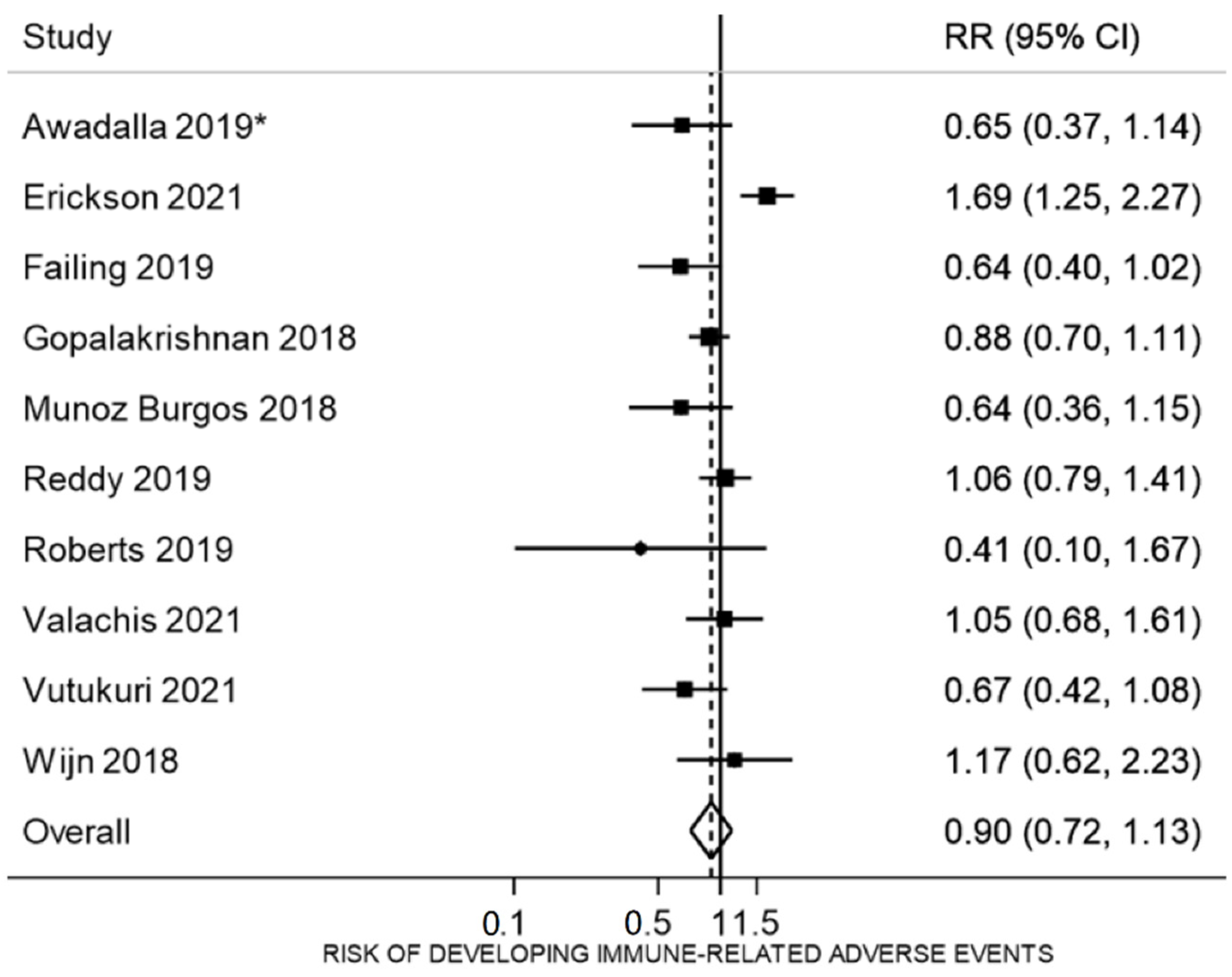
3.5. Proportion of irAEs
3.6. Immunogenicity
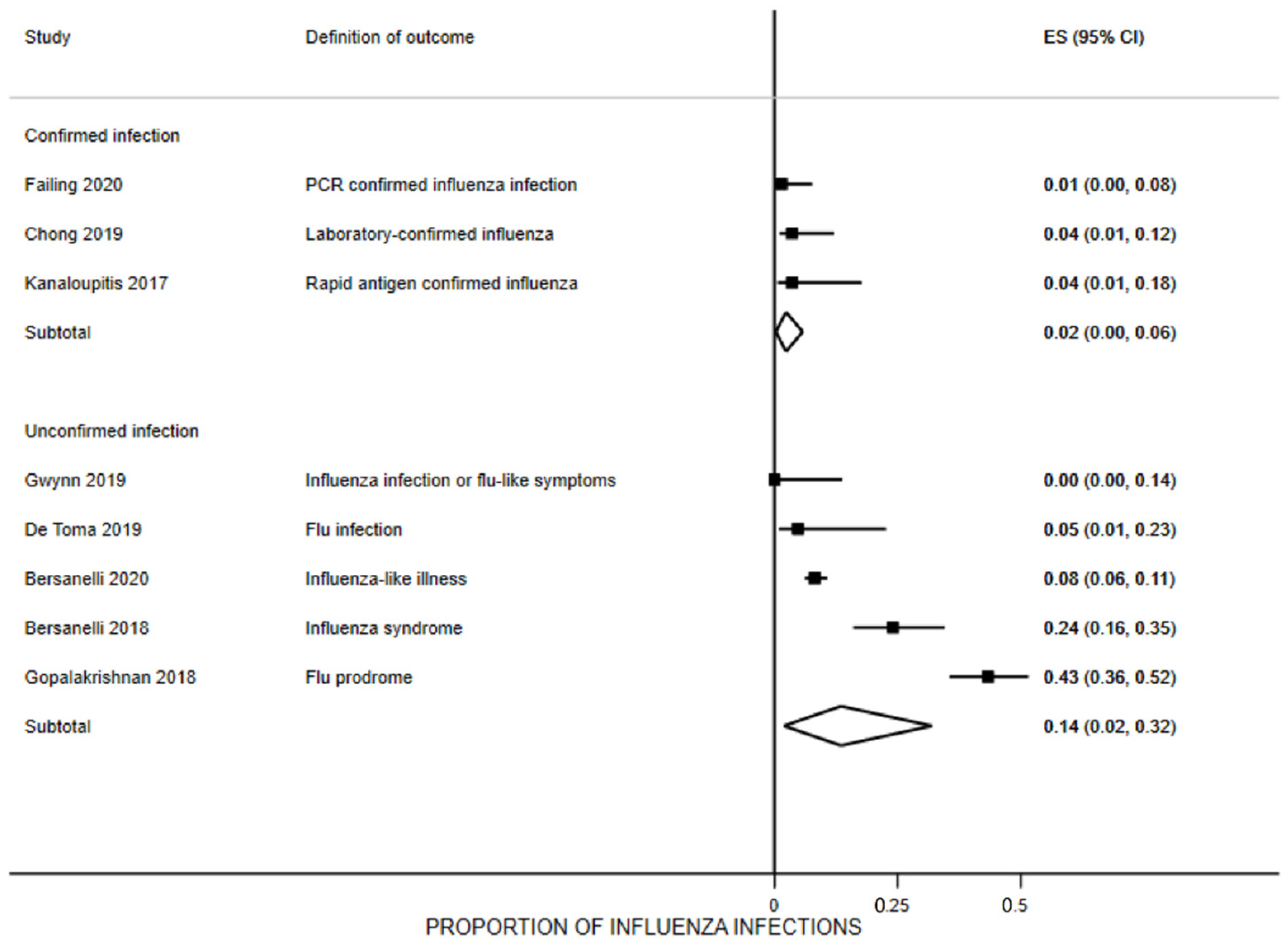
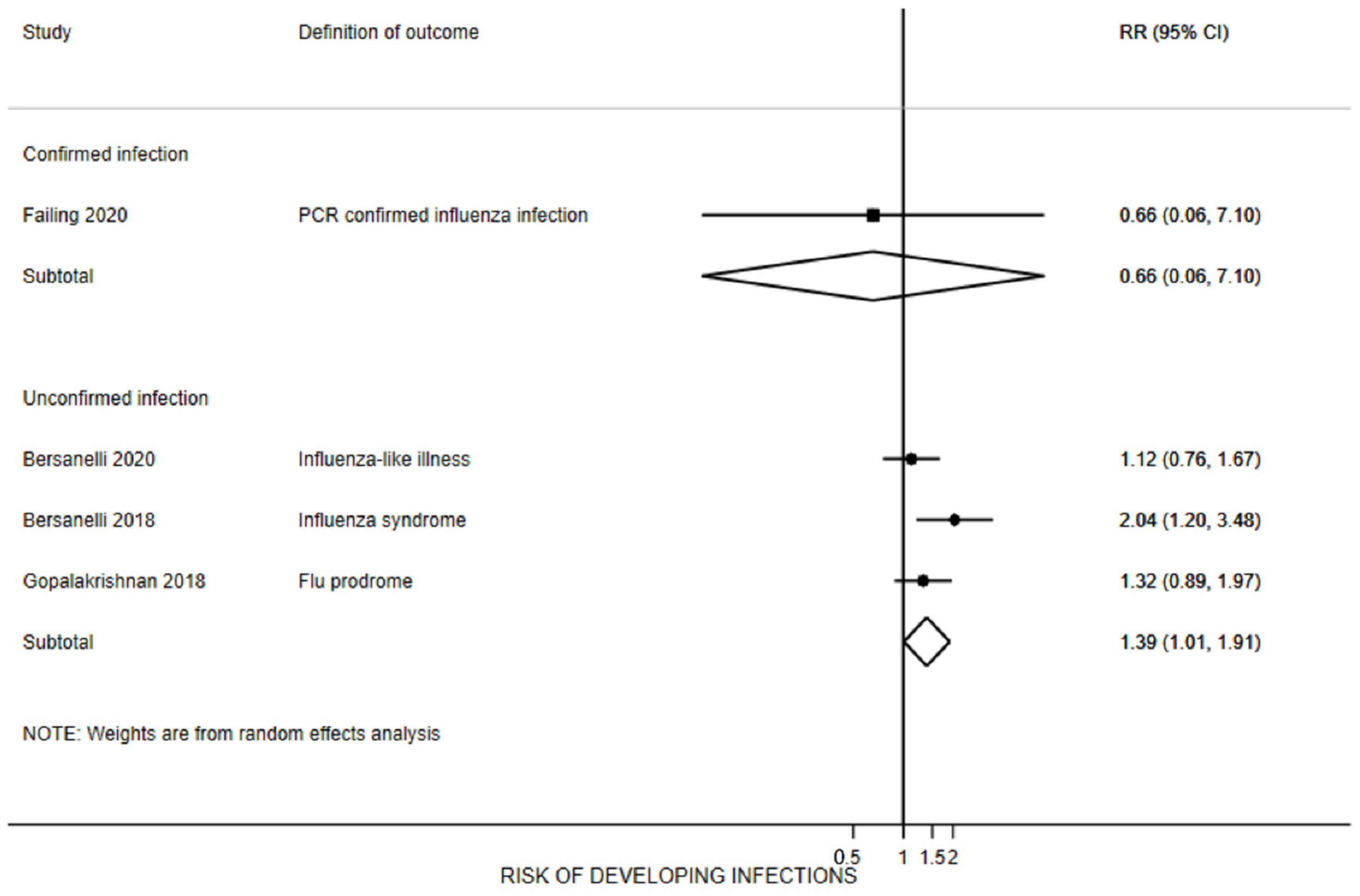
3.7. Influenza Infection Rates
3.8. Cancer-Related Outcomes
3.9. Reporting Biases and Certainty of Evidence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.G.; Chang, H.; Keam, B.; Chun, S.H.; Park, J.; Park, K.U.; Shin, S.H.; An, H.J.; Lee, K.E.; Lee, K.W.; et al. Outcomes and Biomarkers of Immune Checkpoint Inhibitor Therapy in Patients with Refractory Head and Neck Squamous Cell Carcinoma: KCSG HN18-12. Cancer Res. Treat. 2021, 53, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Medina, P.J.; Adams, V.R. PD-1 Pathway Inhibitors: Immuno-Oncology Agents for Restoring Antitumor Immune Responses. Pharmacotherapy 2016, 36, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Nadal, R.; Bellmunt, J. Management of metastatic bladder cancer. Cancer Treat. Rev. 2019, 76, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Hamid, O.; Chasalow, S.D.; Wu, D.Y.; Parker, S.M.; Galbraith, S.; Gnjatic, S.; Berman, D. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J. Immunother. 2012, 35, 89–97. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 20 August 2021).
- Centers for Disease Control and Prevention. Past Seasons Estimated Influenza Disease Burden. Available online: https://www.cdc.gov/flu/about/burden/past-seasons.html (accessed on 20 August 2021).
- Bayle, A.; Khettab, M.; Lucibello, F.; Chamseddine, A.N.; Goldschmidt, V.; Perret, A.; Ropert, S.; Scotte, F.; Loulergue, P.; Mir, O. Immunogenicity and safety of influenza vaccination in cancer patients receiving checkpoint inhibitors targeting PD-1 or PD-L1. Ann. Oncol. 2020, 31, 959–961. [Google Scholar] [CrossRef]
- Cooksley, C.D.; Avritscher, E.B.; Bekele, B.N.; Rolston, K.V.; Geraci, J.M.; Elting, L.S. Epidemiology and outcomes of serious influenza-related infections in the cancer population. Cancer 2005, 104, 618–628. [Google Scholar] [CrossRef]
- Pedrazzoli, P.; Baldanti, F.; Donatelli, I.; Castrucci, M.R.; Puglisi, F.; Silvestris, N.; Cinieri, S.; Italian Society of Medical Oncology. Vaccination for seasonal influenza in patients with cancer: Recommendations of the Italian Society of Medical Oncology (AIOM). Ann. Oncol. 2014, 25, 1243–1247. [Google Scholar] [CrossRef]
- Bitterman, R.; Eliakim-Raz, N.; Vinograd, I.; Zalmanovici Trestioreanu, A.; Leibovici, L.; Paul, M. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst. Rev. 2018, 2, CD008983. [Google Scholar] [CrossRef]
- Bersanelli, M.; Buti, S.; De Giorgi, U.; Di Maio, M.; Giannarelli, D.; Pignata, S.; Banna, G.L. State of the art about influenza vaccination for advanced cancer patients receiving immune checkpoint inhibitors: When common sense is not enough. Crit. Rev. Oncol. Hematol. 2019, 139, 87–90. [Google Scholar] [CrossRef]
- Desage, A.L.; Bouleftour, W.; Rivoirard, R.; Magne, N.; Collard, O.; Fournel, P.; Tissot, C. Vaccination and Immune Checkpoint Inhibitors: Does Vaccination Increase the Risk of Immune-related Adverse Events? A Systematic Review of Literature. Am. J. Clin. Oncol. 2021, 44, 109–113. [Google Scholar] [CrossRef]
- Spagnolo, F.; Boutros, A.; Croce, E.; Cecchi, F.; Arecco, L.; Tanda, E.; Pronzato, P.; Lambertini, M. Influenza vaccination in cancer patients receiving immune checkpoint inhibitors: A systematic review. Eur. J. Clin. Investig. 2021, 51, e13604. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane. 2019. Available online: www.training.cochrane.org/handbook (accessed on 22 April 2022).
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 22 April 2022).
- Allen, I.M.; Murciano-Goroff, Y.R.; Zubiri, L.; Li, Q.; Hughes, M.S.; Velimirovic, M.; Chu, J.N.; Durbin, S.; Klauer, M.J.; Awadalla, M.; et al. Flu vaccination rate of patients with severe immune-related adverse events. J. Clin. Oncol. 2019, 37, e18234. [Google Scholar] [CrossRef]
- Awadalla, M.; Golden, D.L.A.; Mahmood, S.S.; Alvi, R.M.; Mercaldo, N.D.; Hassan, M.Z.O.; Banerji, D.; Rokicki, A.; Mulligan, C.; Murphy, S.P.T.; et al. Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Buti, S.; Banna, G.L.; De Giorgi, U.; Cortellini, A.; Rebuzzi, S.E.; Tiseo, M.; Fornarini, G.; Mazzoni, F.; Panni, S.; et al. Impact of influenza syndrome and flu vaccine on survival of cancer patients during immunotherapy in the INVIDIa study. Immunotherapy 2020, 12, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Giannarelli, D.; Castrignano, P.; Fornarini, G.; Panni, S.; Mazzoni, F.; Tiseo, M.; Rossetti, S.; Gambale, E.; Rossi, E.; et al. INfluenza Vaccine Indication During therapy with Immune checkpoint inhibitors: A transversal challenge. The INVIDIa study. Immunotherapy 2018, 10, 1229–1239. [Google Scholar] [CrossRef]
- Bersanelli, M.; Giannarelli, D.; Verzoni, E.; Buti, S.; De Giorgi, U.; Clemente, A.; Filetti, M.; Di Napoli, M.; Calvetti, L.; Ermacora, P.; et al. Influenza-like illness and SARS-Cov-2 in the multicenter, prospective, observational INVIDIa-2 study (INfluenza Vaccine Indication During therapy with Immune checkpoint inhibitors: A transversal challenge): A FICOG study. Ann. Oncol. 2020, 31, S1209. [Google Scholar] [CrossRef]
- Bersanelli, M.; Giannarelli, D.; De Giorgi, U.; Pignata, S.; Di Maio, M.; Clemente, A.; Verzoni, E.; Giusti, R.; Di Napoli, M.; Aprile, G.; et al. INfluenza Vaccine Indication During therapy with Immune checkpoint inhibitors: A multicenter prospective observational study (INVIDIa-2). J. Immunother. Cancer 2021, 9, e002619. [Google Scholar] [CrossRef]
- Bersanelli, M.; Giannarelli, D.; Verzoni, E.; Procopio, G.; Clemente, A.; Signorelli, D.; Costanzo, R.; De Vivo, R.; Zara, D.; Zacheo, A.; et al. Influenza vaccine indication during therapy with immune checkpoint inhibitors: A transversal challenge. The multicenter, prospective, observational INVIDIA-2 study (a FICOG study). Tumori J. 2020, 106, 1. [Google Scholar]
- Chong, C.R.; Park, V.; Harding, J.J.; Brite, J.; Wolchok, J.D.; Kamboj, M. Safety of influenza vaccination in patients undergoing immunotherapy treatment for advanced cancer. J. Clin. Oncol. 2018, 36, e15073. [Google Scholar] [CrossRef]
- Chong, C.R.; Park, V.J.; Cohen, B.; Postow, M.A.; Wolchok, J.D.; Kamboj, M. Safety of Inactivated Influenza Vaccine in Cancer Patients Receiving Immune Checkpoint Inhibitors. Clin. Infect. Dis. 2020, 70, 193–199. [Google Scholar] [CrossRef] [PubMed]
- De Toma, A.; Lo Russo, G.; Proto, C.; Signorelli, D.; Ferrara, R.; Prelaj, A.; Galli, G.; Pagani, F.; Trevisan, B.; Zilembo, N.; et al. Influenza vaccine in non-small cell lung cancer (NSCLC) patients treated with immune checkpoint inhibitors (ICIs): A single institution experience. Tumori J. 2019, 105, 96. [Google Scholar]
- Erickson, M.; Truong, A.; Boucher, K.; Hyngstrom, J. Associations between influenza vaccine and immunotherapy outcomes in metastatic melanoma patients. J. Investig. Dermatol. 2021, 141, S91. [Google Scholar] [CrossRef]
- Failing, J.J.; Ho, T.P.; Yadav, S.; Majithia, N.; Riaz, I.B.; Shin, J.Y.; Schenk, E.L.; Xie, H. Safety of Influenza Vaccine in Patients With Cancer Receiving Pembrolizumab. JCO Oncol. Pract. 2020, 16, e573–e580. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Johnson, D.B.; York, S.; Neuss, M.N.; Osterman, T.J.; Chism, D.D. Impact of the influenza vaccination on cancer patients undergoing therapy with immune checkpoint inhibitors (ICI). J. Clin. Oncol. 2018, 36, 3053. [Google Scholar] [CrossRef]
- Gwynn, M.E.; DeRemer, D.L.; Saunders, K.M.; Parikh, J.; Bollag, R.J.; Clemmons, A.B. Immune-mediated adverse events following influenza vaccine in cancer patients receiving immune checkpoint inhibitors. J. Oncol. Pharm. Pract. 2020, 26, 647–654. [Google Scholar] [CrossRef]
- Kanaloupitis, D.K.; Chandran, A.; Ralph, A.; Thompson, R.; Richards, J.M.; Hallmeyer, S. Safety and efficacy of concurrent administration of influenza vaccine in patients undergoing anti-PD-1 immunotherapy. J. Clin. Oncol. 2017, 35, e14607. [Google Scholar] [CrossRef]
- Kang, C.K.; Kim, H.R.; Song, K.H.; Keam, B.; Choi, S.J.; Choe, P.G.; Kim, E.S.; Kim, N.J.; Kim, Y.J.; Park, W.B.; et al. Cell-Mediated Immunogenicity of Influenza Vaccination in Patients With Cancer Receiving Immune Checkpoint Inhibitors. J. Infect. Dis. 2020, 222, 1902–1909. [Google Scholar] [CrossRef]
- Keam, B.; Kang, C.K.; Jun, K.I.; Moon, S.M.; Suh, K.J.; Lee, D.W.; Ock, C.Y.; Kim, M.; Choi, Y.; Lim, Y.; et al. Immunogenicity of Influenza Vaccination in Patients with Cancer Receiving Immune Checkpoint Inhibitors. Clin. Infect. Dis. 2020, 71, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Balmelli, C.; Kaufmann, L.; Stanczak, M.; Syedbasha, M.; Vogt, D.; Hertig, A.; Muller, B.; Gautschi, O.; Stenner, F.; et al. Influenza vaccination of cancer patients during PD-1 blockade induces serological protection but may raise the risk for immune-related adverse events. J. Immunother. Cancer 2018, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Laubli, H.P.; Balnnelli, C.; Kaufmann, L.; Stanczak, M.; Syedbasha, M.; Vogt, D.; Mueller, B.; Gautschi, O.; Stenner, F.; Zippelius, A.; et al. Immune response and adverse events to influenza vaccine in cancer patients undergoing PD-1 blockade. J. Clin. Oncol. 2017, 35, e14523. [Google Scholar] [CrossRef]
- Reddy, H.; Weis, T.; Hough, S.; Daignault-Newton, S.; Schneider, B. Immune Related Adverse Events in NSCLC Patients Treated with Immune Checkpoint Therapy Who Received the Influenza Vaccination vs. No Vaccination. J. Thorac. Oncol. 2019, 14, S722. [Google Scholar] [CrossRef]
- Roberts, N.; Dothard, A.; Ahmed, T.; Petty, W.; Ruiz, J.; Lycan, T. Safety and Efficacy of Flu Vaccination After Treatment with Immune Checkpoint Inhibitors: A Retrospective Review. J. Thorac. Oncol. 2019, 14, S1125. [Google Scholar] [CrossRef]
- Rothschild, S.I.; Balmelli, C.; Kaufmann, L.; Stanczak, M.; Syedbasha, M.; Vogt, D.; Gautschi, O.; Egli, A.; Zippelius, A.; Laeubli, H. Immune response and adverse events to influenza vaccine in cancer patients undergoing PD-1 blockade. Ann. Oncol. 2017, 28, ii40–ii41. [Google Scholar] [CrossRef] [Green Version]
- Valachis, A.; Rosen, C.; Koliadi, A.; Digkas, E.; Gustavsson, A.; Nearchou, A.; Ullenhag, G.J. Improved survival without increased toxicity with influenza vaccination in cancer patients treated with checkpoint inhibitors. Oncoimmunology 2021, 10, 1886725. [Google Scholar] [CrossRef]
- Vutukuri, N.M.; Corcoran, C.; Comeau, J.; Stephenson, E.; Beedupalli, K.; Shi, R.H. Effect of influenza vaccination on immune-related adverse events in patients receiving single-agent immune checkpoint inhibitors for the treatment of cancer. J. Clin. Oncol. 2021, 39, e18763. [Google Scholar] [CrossRef]
- Wijn, D.H.; Groeneveld, G.H.; Vollaard, A.M.; Muller, M.; Wallinga, J.; Gelderblom, H.; Smit, E.F. Influenza vaccination in patients with lung cancer receiving anti-programmed death receptor 1 immunotherapy does not induce immune-related adverse events. Eur. J. Cancer 2018, 104, 182–187. [Google Scholar] [CrossRef]
- Yuen, C.; Kamson, D.; Soliven, B.; Kramer, C.; Goldenberg, F.; Rezania, K. Severe Relapse of Vaccine-Induced Guillain-Barre Syndrome After Treatment With Nivolumab. J. Clin. Neuromuscul. Dis. 2019, 20, 194–199. [Google Scholar] [CrossRef]
- Gatti, M.; Raschi, E.; Moretti, U.; Ardizzoni, A.; Poluzzi, E.; Diemberger, I. Influenza Vaccination and Myo-Pericarditis in Patients Receiving Immune Checkpoint Inhibitors: Investigating the Likelihood of Interaction through the Vaccine Adverse Event Reporting System and VigiBase. Vaccines 2021, 9, 19. [Google Scholar] [CrossRef]
- Wulff-Burchfield, E.M.; Kurkowski, A.; Grauer, D.; Ralph, S.; Mahmoudjafari, Z.; Parikh, R.A.; Martin, G. Safety of inactivated vaccines in patients with genitourinary (GU) malignancies receiving immune checkpoint inhibitors (ICI). J. Clin. Oncol. 2020, 38, e17108. [Google Scholar] [CrossRef]
- Schenk, E.L. Clinical outcomes of patients on check point inhibitor therapy who receive routine vaccinations. J. Clin. Oncol. 2017, 35, e14597. [Google Scholar] [CrossRef]
- Buti, S.; Giannarelli, D.; De Giorgi, U.; Pignata, S.; Di Maio, M.; Clemente, A.; Verzoni, E.; Guadalupi, V.; Giusti, R.; Garassino, M.; et al. COVID-19 Analysis from the multicenter, prspective observational INVIDIa-2 study (INfluenza Vaccine Indication During therapy with Immune checkpoint inhibitors: A transversal challenge)—A FICOG study. Tumori J. 2020, 106, 64–65. [Google Scholar]
- Bersanelli, M.; Giannarelli, D.; De Giorgi, U.; Pignata, S.; Di Maio, M.; Verzoni, E.; Clemente, A.; Guadalupi, V.; Signorelli, D.; Tiseo, M.; et al. Symptomatic COVID-19 in advanced-cancer patients treated with immune-checkpoint inhibitors: Prospective analysis from a multicentre observational trial by FICOG. Ther. Adv. Med. Oncol. 2020, 12, 1758835920968463. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Burgos, M.; Perez Velasco, L.; Flores Moreno, S.; Baez Gutierrez, N.; Vega Coca, M.D.; Abdel-Kader Martin, L.; Rodriguez Ramallo, H.; Mejias Trueba, M. Patients treated with PD-1 checkpoint inhibitors: Immune-related adverse events to influenza vaccine. Eur. J. Oncol. Pharm. 2018, 1, 24. [Google Scholar]
- Wan X, Wang W, Liu J, Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res. Methodol. 2014, 14, 135. [CrossRef] [Green Version]
- Trombetta, C.M.; Perini, D.; Mather, S.; Temperton, N.; Montomoli, E. Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future. Vaccines 2014, 2, 707–734. [Google Scholar] [CrossRef] [Green Version]
- Tokars, J.I.; Olsen, S.J.; Reed, C. Seasonal Incidence of Symptomatic Influenza in the United States. Clin. Infect. Dis. 2018, 66, 1511–1518. [Google Scholar] [CrossRef]
- Enamorado, M.; Iborra, S.; Priego, E.; Cueto, F.J.; Quintana, J.A.; Martínez-Cano, S.; Mejías-Pérez, E.; Esteban, M.; Melero, I.; Hidalgo, A.; et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8(+) T cells. Nat. Commun. 2017, 8, 16073. [Google Scholar] [CrossRef]

| Author, Year | Publication Type | Country | # of Centres | Design | Sample Size e | Recruitment Period | Follow-Up | Primary Outcome | Secondary Outcomes | Funding |
|---|---|---|---|---|---|---|---|---|---|---|
| Awadalla 2019 [19,20] a | Full-text | USA, MA | 16 for cases, 1 for controls | Case-control | 641 a | 02/2011–06/2017 and 11/2013–10/2018 | 290 days for controls, 175 for cases b | Vaccine rates | MACE rates | None |
| Bayle 2020 [9] | Full-text | France | 1 | Case series | 30 | 2018–2019 | 6 months | Seroprotection rate, seroconversion | irAEs rates | None |
| Bersanelli 2018 [21,22] | Full-text | Italy | 21 | Retrospective cohort | 300 | 11/2016–05/2017 | 31 months b | Influenza syndrome rates | Lethality rates, cancer outcomes c | NR d |
| Bersanelli 2021 [23,24,25] | Full-text | Italy | 82 | Prospective cohort | 1188 | 10/2019–01/2020 | 16 months | Influenza syndrome rates, COVID-19 rates | Lethality rates, cancer outcomes c | FICOG |
| Chong 2019 [26,27] | Full-text | USA, NY | 1 | Case series | 370 | 09/2014–03/2018 | 512 days b | irAEs rates | Infection rates | NIH |
| De Toma 2019 [28] | Abstract | Italy | 1 | Case series | 75 | 10/2018–01/2019 | NR | irAEs rates, lethality rates | Influenza syndrome rates | NR |
| Erickson 2021 [29] | Abstract | USA, UT | 1 | Retrospective cohort | 176 | 2013–2018 | NR | irAEs rates, PFS, overall survival | ICI treatment discontinuation | |
| Failing 2019 [30] a | Full-text | USA, MN | 1 | Retrospective cohort | 162 | 09/2014–08/2017 | 17.1 months b | irAEs rates | Influenza syndrome, ICI treatment discontinuation | NR d |
| Gopalakrishnan 2018 [31] | Abstract | USA, TN | 1 | Retrospective cohort | 534 | 2010–2017 | NR | Cancer outcomes c | Influenza syndrome rates, lethality rates | NR |
| Gwynn 2019 [32] | Full-text | USA, GA | 1 | Uncontrolled trial | 24 | 10/2017–12/2017 | 60 days | Influenza syndrome rates, irAEs rates | Cytokine levels | None |
| Kanaloupitis 2017 [33] | Abstract | USA, IL | NR | Case series | 28 | NR | 90 days or more | Immunoglobulin levels, infection | Hospitalizations, irAEs rates | NR |
| Keam 2019 [34,35] | Full-text | South Korea | 2 | Uncontrolled trial | 136 | 09/2018–11/2018 | 6 months | Seroprotection rates, seroconversion | irAEs rates | GC Pharma, Seoul National University Hospital Research Fund |
| Laubli 2018 [36,37,40] | Full-text | Switzerland | 2 | Retrospective cohort | 34 | 10/2015–11/2015 | 60 days (37.5 months for overall survival) | Cytokine levels Seroprotection rate, seroconversion | irAEs rates, radiographic and clinical response | Schoenmakers Foundation, Goldschmidt-Jacobson Foundation, Swiss National Foundation |
| Munoz Burgos 2018 [50] | Abstract | Spain | NR | Retrospective cohort | 42 | 10/2017–01/2018 | NR | irAEs rates | ICI treatment discontinuation | NR |
| Reddy 2019 [38] | Abstract | USA, MI | NR | Retrospective cohort | 117 | 2014–2019 | NR | irAEs rates | ICI treatment discontinuation | NR |
| Roberts 2019 [39] | Abstract | USA, MA | 1 | Retrospective cohort | 285 | 01/2014–05/2018 | NR | irAEs rates | NA | NR |
| Valachis 2021 [41] | Full-text | Sweden | 3 | Retrospective cohort | 303 | 01/2016–05/2019 | 15 months b | PFS, overall survival | irAEs rates | None |
| Vutukuri 2021 [42] | Abstract | USA, LA | 1 | Retrospective cohort | 133 | 08/2015–08/2019 | NR | irAEs rates | NA | NR |
| Wijn 2018 [43] | Full-text | Netherlands | 1 | Retrospective cohort | 127 | 09/2015–01/2016 and 09/2016–01/2017 | 107 days for cases, 118 days for controls | irAEs rates | ICI treatment discontinuation Tumor response, deaths | NR |
| Study | Age, Mean Years (SD) a | Males | ICIs Considered | Cancer Type | Vaccine Timing | Cases | Controls | ||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Description | Sample | Description | ||||||
| Case-Control | |||||||||
| Awadalla 2019 [19,20] | 65 (15.6) b | 72.0% | Ipilimumab, pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab, or combination | Advanced solid tumors including melanoma, NSCLC, SCCHN | Anytime from 6 months prior to ICI to receiving the vaccine during ICI therapy | 151 | irAEs [19] | 389 | No irAEs [19] |
| 101 | Myocarditis [20] | 201 | No Myocarditis [20] | ||||||
| Case series and uncontrolled trials (prospective studies with 1 group) | |||||||||
| Intervention group | |||||||||
| Sample | Description | ||||||||
| Bayle 2020 [9] | 63 (7.6) | 83.0% | Nivolumab, pembrolizumab, atezolizumab | NSCLC, urothelial | 7 (±2) days after the last administration of ICI | 30 | 1 standard dose of the French National Health authorities-approved subcutaneous vaccine | ||
| Chong 2019 [26,27] | 63 (13.8) | 54.0% | Ipilimumab, pembrolizumab, nivolumab or combination | Lung, melanoma, others (NS) | 2 months before or after ICI administration c | 370 | Trivalent or quadrivalent vaccines d at high or standard doses | ||
| De Toma 2019 [28] | NR | NR | Pembrolizumab, atelozolizumab, nivolumab, durvalumab | NSCLC | Before or within 30 days after ICI start | 21 | NS inactivate influenza vaccine | ||
| Gwynn 2019 [32] | 61 (11.8) | 42.0% | Nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab | NSCLC, melanoma, urothelial, RCC, colon, hepatocellular, head/neck | Vaccine administered in patients with at least 1 cycle of ICI | 24 | 0.5 mL intramuscular IIV Fluarix® or Fluzone® quadrivalent | ||
| Kanaloupitis 2017 [33] | NR | NR | Anti-PD-1 (NS) | NR | NR | 28 | Afluria (Seqirus) | ||
| Keam 2019 [34,35] | 63 (9.0) | 79.0% | Nivolumab, pembrolizumab, atezolizumab | Lung, kidney, melanoma, others e | Concomitantly on day 1 of ICI | 46 | 0.5 mL GCFLU quadrivalent pre-filled syringe injection; GC Pharma f | ||
| Prospective and retrospective cohorts (studies with 2 groups) | |||||||||
| Intervention group | Control group | ||||||||
| Sample | Description | Sample | Description | ||||||
| Bersanelli 2018 [21,22] | 64.3 (8.5) | 69.0% | Ipilimumab, pembrolizumab, nivolumab, atezolizumab, avelumab, combinations, or chemo-immunotherapy | NSCLC, RCC, melanoma, head/neck, urothelial, gastric, colon | NR | 79 | Trivalent or quadrivalent vaccines d | 221 | No vaccine |
| Bersanelli 2020 [23,24,25] | 65.6 (11.1) | 69.9% | NS | NSCLC, RCC, melanoma, urothelial, head & neck, other (NS) | During ICI therapy | 429 g | Trivalent or quadrivalent vaccines | 402 | No vaccine |
| Erickson 2021 [29] | 64.6 (NR) | NR | NR | Metastatic melanoma | Anytime during the observation (51% before starting ICI) | 90 | NS | 86 | No vaccine |
| Failing 2019 [30] | 63.5 (NR) | 56.2% | Pembrolizumab or combined with chemo or radiation | NSCLC, melanoma, other (NS) | Within 30 days before initiation or during ICI therapy | 70 | High dose trivalent, quadrivalent, or NS type vaccines | 92 | No vaccine |
| Gopalakrishnan 2018 [31] | 54 (NR) | 76.0% | NS | Lung, melanoma, GU, breast, lymphoma | NR | 385 | NS | 149 | No vaccine |
| Laubli 2018 [36,37,40] | 62 (10.0) | 69.6% | Nivolumab, pembrolizumab | NSCLC, RCC, melanoma | Median time from ICI initiation to vaccination was 74 days (range 4 to 457 days) | 23 | Trivalent intramuscular (Agrippal, Novartis) vaccine h | 11 | Healthy controls |
| 40 | No vaccine h | ||||||||
| Munoz Burgos 2018 [50] | 64.2 (NR) | 64.3% | Nivolumab, pembrolizumab | NSCLC, melanoma, RCC, head/neck, breast | NR | 21 | NS inactive influenza vaccine | 21 | No vaccine |
| Reddy 2019 [38] | NR | NR | Anti-PD-1 or PD-L1 (NS) | NSCLC | During ICI therapy | 33 | 19 received quadrivalent, 13 trivalent, 1 NS | 53 | Not vaccinated during ICI |
| Roberts 2019 [39] | NR | NR | NS | NSCLC | NR | 45 | NS influenza vaccine | 240 | No vaccine |
| Valachis 2021 [41] | 67 (13) | 56.4% | Nivolumab, pembrolizumab, atezolizumab | Melanoma, NSCLC, RCC | 2 months before or after ICI initiation | 67 | NS influenza vaccine | 236 | No vaccine |
| Vutukuri 2021 [42] | NR | NR | Pembrolizumab, nivolumab, atezolizumab, durvalumab | NS lung, melanoma | NR | 53 | NS influenza vaccine | 80 | No vaccine |
| Wijn 2018 [43] | 62.6 (3.9) | 48.0% | Nivolumab | NS advanced lung | After starting ICI or 30 days before | 42 | NS influenza vaccine | 85 | No vaccine i |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Olivo, M.A.; Valerio, V.; Karpes Matusevich, A.R.; Brizio, M.; Kwok, M.; Geng, Y.; Suarez-Almazor, M.E.; Colmegna, I. Safety and Efficacy of Influenza Vaccination in Patients Receiving Immune Checkpoint Inhibitors. Systematic Review with Meta-Analysis. Vaccines 2022, 10, 1195. https://doi.org/10.3390/vaccines10081195
Lopez-Olivo MA, Valerio V, Karpes Matusevich AR, Brizio M, Kwok M, Geng Y, Suarez-Almazor ME, Colmegna I. Safety and Efficacy of Influenza Vaccination in Patients Receiving Immune Checkpoint Inhibitors. Systematic Review with Meta-Analysis. Vaccines. 2022; 10(8):1195. https://doi.org/10.3390/vaccines10081195
Chicago/Turabian StyleLopez-Olivo, Maria A., Valeria Valerio, Aliza R. Karpes Matusevich, Marianela Brizio, Michelle Kwok, Yimin Geng, Maria E. Suarez-Almazor, and Ines Colmegna. 2022. "Safety and Efficacy of Influenza Vaccination in Patients Receiving Immune Checkpoint Inhibitors. Systematic Review with Meta-Analysis" Vaccines 10, no. 8: 1195. https://doi.org/10.3390/vaccines10081195
APA StyleLopez-Olivo, M. A., Valerio, V., Karpes Matusevich, A. R., Brizio, M., Kwok, M., Geng, Y., Suarez-Almazor, M. E., & Colmegna, I. (2022). Safety and Efficacy of Influenza Vaccination in Patients Receiving Immune Checkpoint Inhibitors. Systematic Review with Meta-Analysis. Vaccines, 10(8), 1195. https://doi.org/10.3390/vaccines10081195






