Impact of Parental Knowledge and Beliefs on HPV Vaccine Hesitancy in Kenya—Findings and Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Location and Population
2.2. Sample Size Determination
2.3. Eligibility Criteria, Sampling and Participant Recruitment
2.4. Research Instruments and Data Collection
2.5. Data Management
2.6. Variables and Data Analysis
2.7. Ethical Considerations
3. Results
3.1. Recruitment and Baseline Sociodemographic Characteristics of Participants
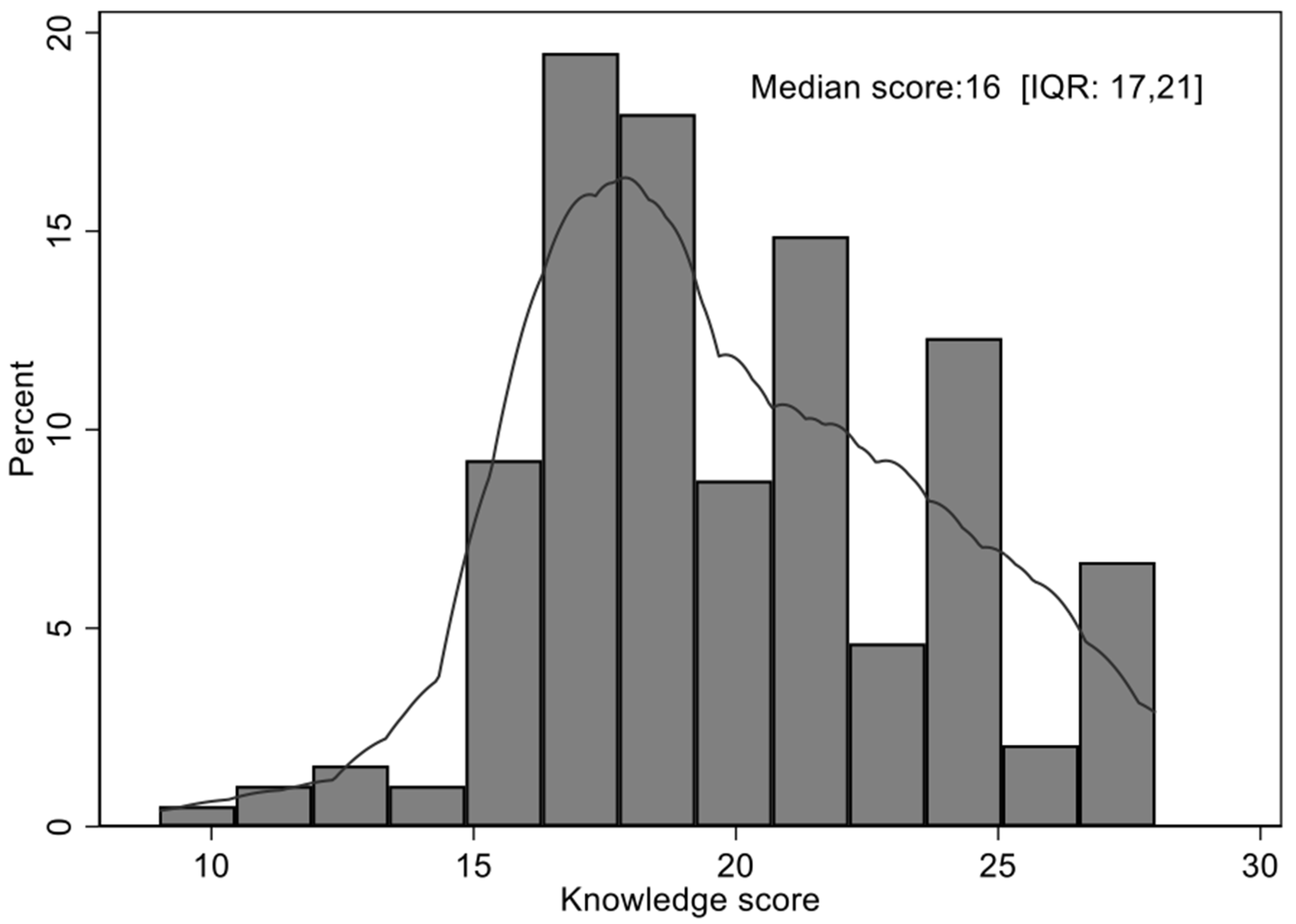
3.2. Overall Knowledge Score
3.3. Sources of Information and Knowledge of HPV Infection, Cervical Cancer and Its Prevention
3.4. Determinants of Knowledge of HPV Infection, Cervical Cancer and HPV Vaccination
3.5. Parental Views on Knowledge Empowerment and Beliefs about the Safety and Effectiveness of the HPV Vaccine
3.6. Determinants of Beliefs about the HPV Vaccine
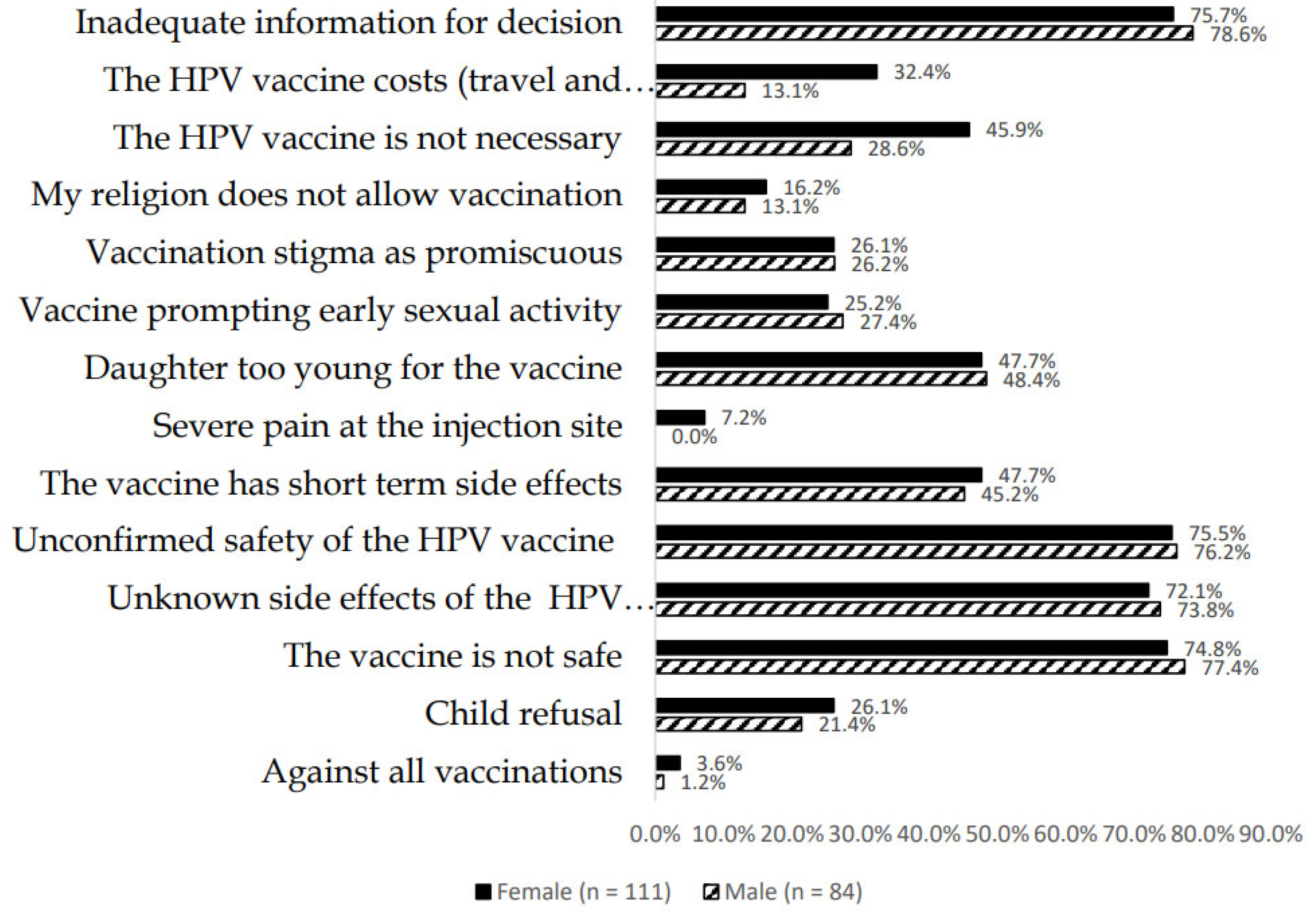
3.7. Parental Willingness to Have Their Children Vaccinated and Reasons for Vaccine Hesitancy
3.8. Logistic Regression Analysis for Determinants of Willingness of Parents to Vaccinate Their Child against HPV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [Green Version]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 22 October 2021. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 1 June 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Beddoe, A.M. Elimination of cervical cancer: Challenges for developing countries. Ecancermedicalscience 2019, 13, 975. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, K.; Maza, M.; Cremer, M.; Masch, R.; Soler, M. Removing global barriers to cervical cancer prevention and moving towards elimination. Nat. Rev. Cancer 2021, 21, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- LaVigne, A.W.; Triedman, S.A.; Randall, T.C.; Trimble, E.L.; Viswanathan, A.N. Cervical cancer in low and middle income countries: Addressing barriers to radiotherapy delivery. Gynecol. Oncol. Rep. 2017, 22, 16–20. [Google Scholar] [CrossRef]
- Canfell, K. Towards the global elimination of cervical cancer. Papillomavirus Res. 2019, 8, 100170. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.Y.; Khatun, F.; Alam, A.; Sultana, F.; Bhuiyan, A.; Alam, N.; Reichenbach, L.; Marions, L.; Rahman, M.; Nahar, Q. Knowledge of cervical cancer and HPV vaccine in Bangladeshi women: A population based, cross-sectional study. BMC Womens Health 2018, 18, 15. [Google Scholar] [CrossRef] [Green Version]
- Becker-Dreps, S.; Otieno, W.A.; Brewer, N.T.; Agot, K.; Smith, J.S. HPV vaccine acceptability among Kenyan women. Vaccine 2010, 28, 4864–4867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.D.; Neupane, D.; Vedsted, P.; Kallestrup, P. Cervical Cancer Prevalence, Incidence and Mortality in Low and Middle Income Countries: A Systematic Review. Asian Pac. J. Cancer Prev. 2018, 19, 319–324. [Google Scholar] [PubMed]
- Friedman, A.L.; Oruko, K.O.; Habel, M.A.; Ford, J.; Kinsey, J.; Odhiambo, F.; Phillips-Howard, P.A.; Wang, S.A.; Collins, T.; Laserson, K.F.; et al. Preparing for human papillomavirus vaccine introduction in Kenya: Implications from focus-group and interview discussions with caregivers and opinion leaders in Western Kenya. BMC Public Health 2014, 14, 855. [Google Scholar] [CrossRef] [Green Version]
- Pimple, S.A.; Mishra, G.A. Optimizing high risk HPV-based primary screening for cervical cancer in low- and middle-income countries: Opportunities and challenges. Minerva Ginecol. 2019, 71, 365–371. [Google Scholar] [CrossRef]
- Sayinzoga, F.; Umulisa, M.C.; Sibomana, H.; Tenet, V.; Baussano, I.; Clifford, G.M. Human papillomavirus vaccine coverage in Rwanda: A population-level analysis by birth cohort. Vaccine 2020, 38, 4001–4005. [Google Scholar] [CrossRef]
- Binagwaho, A.; Wagner, C.M.; Gatera, M.; Karema, C.; Nutt, C.T.; Ngabo, F. Achieving high coverage in Rwanda’s national human papillomavirus vaccination programme. Bull. World Health Organ. 2012, 90, 623–628. [Google Scholar] [CrossRef]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, Cd009069. [Google Scholar] [CrossRef]
- Gamble, H.L.; Klosky, J.L.; Parra, G.R.; Randolph, M.E. Factors influencing familial decision-making regarding human papillomavirus vaccination. J. Pediatr. Psychol. 2010, 35, 704–715. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Risk Factors for Cervical Cancer. 2022. Available online: https://www.cancer.org/cancer/cervical-cancer/causes-risks-prevention/risk-factors.html (accessed on 2 June 2022).
- Masika, M.M.; Ogembo, J.G.; Chabeda, S.V.; Wamai, R.G.; Mugo, N. Knowledge on HPV Vaccine and Cervical Cancer Facilitates Vaccine Acceptability among School Teachers in Kitui County, Kenya. PLoS ONE 2015, 10, e0135563. [Google Scholar] [CrossRef]
- Liu, G.; Sharma, M.; Tan, N.; Barnabas, R.V. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018, 32, 795–808. [Google Scholar] [CrossRef]
- Gallagher, K.E.; Howard, N.; Kabakama, S.; Mounier-Jack, S.; Burchett, H.E.; LaMontagne, D.S.; Watson-Jones, D. Human papillomavirus (HPV) vaccine coverage achievements in low and middle-income countries 2007–2016. Papillomavirus Res. 2017, 4, 72–78. [Google Scholar] [CrossRef]
- Gallagher, K.E.; Howard, N.; Kabakama, S.; Mounier-Jack, S.; Griffiths, U.K.; Feletto, M.; Burchett, H.E.; LaMontagne, D.S.; Watson-Jones, D. Lessons learnt from human papillomavirus (HPV) vaccination in 45 low- and middle-income countries. PLoS ONE 2017, 12, e0177773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mburu, A.; Itsura, P.; Mabeya, H.; Kaaria, A.; Brown, D.R. Knowledge of Cervical Cancer and Acceptability of Prevention Strategies among Human Papillomavirus-Vaccinated and Human Papillomavirus-Unvaccinated Adolescent Women in Eldoret, Kenya. BioResearch Open Access 2019, 8, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, A.; Patton, L.L.; Giuliano, A.R.; Estrich, C.G.; Pahlke, S.C.; O’Brien, K.K.; Lipman, R.D.; Araujo, M.W. Summary of the evidence on the safety, efficacy, and effectiveness of human papillomavirus vaccines: Umbrella review of systematic reviews. J. Am. Dent. Assoc. 2020, 151, 245–254.e24. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, R.V.; Brown, E.R.; Onono, M.; Bukusi, E.A.; Njoroge, B.; Winer, R.L.; Donnell, D.; Galloway, D.; Cherne, S.; Heller, L.; et al. Single-dose HPV vaccination efficacy among adolescent girls and young women in Kenya (the KEN SHE Study): Study protocol for a randomized controlled trial. Trials 2021, 22, 661. [Google Scholar] [CrossRef]
- Black, E.; Richmond, R. Prevention of Cervical Cancer in Sub-Saharan Africa: The Advantages and Challenges of HPV Vaccination. Vaccines 2018, 6, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/336583/9789240014107-eng.pdf?sequence=1&isAllowed=y (accessed on 1 June 2022).
- Njuguna, D.W.; Mahrouseh, N.; Isowamwen, O.V.; Varga, O. Knowledge, Attitude and Practice of Main Stakeholders towards Human Papilloma Virus Infection and Vaccination in Mombasa and Tana-River Counties in Kenya: A Qualitative Study. Vaccines 2021, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Karanja-Chege, C.M. HPV Vaccination in Kenya: The Challenges Faced and Strategies to Increase Uptake. Front. Public Health 2022, 10, 802947. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Santibanez, T.A.; Stokley, S.; Lindley, M.C.; Fisher, A.; Kim, D.; Greby, S.; Srivastav, A.; Singleton, J. Parental vaccine hesitancy and its association with adolescent HPV vaccination. Vaccine 2021, 39, 2416–2423. [Google Scholar] [CrossRef]
- Patel, P.R.; Berenson, A.B. Sources of HPV vaccine hesitancy in parents. Hum. Vaccines Immunother. 2013, 9, 2649–2653. [Google Scholar] [CrossRef] [Green Version]
- Tsui, J.; Vincent, A.; Anuforo, B.; Btoush, R.; Crabtree, B.F. Understanding primary care physician perspectives on recommending HPV vaccination and addressing vaccine hesitancy. Hum. Vaccines Immunother. 2021, 17, 1961–1967. [Google Scholar] [CrossRef]
- Karafillakis, E.; Simas, C.; Jarrett, C.; Verger, P.; Peretti-Watel, P.; Dib, F.; De Angelis, S.; Takacs, J.; Ali, K.A.; Celentano, L.P.; et al. HPV vaccination in a context of public mistrust and uncertainty: A systematic literature review of determinants of HPV vaccine hesitancy in Europe. Hum. Vaccines Immunother. 2019, 15, 1615–1627. [Google Scholar] [CrossRef]
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Vermandere, H.; Naanyu, V.; Mabeya, H.; Vanden Broeck, D.; Michielsen, K.; Degomme, O. Determinants of acceptance and subsequent uptake of the HPV vaccine in a cohort in Eldoret, Kenya. PLoS ONE 2014, 9, e109353. [Google Scholar] [CrossRef] [Green Version]
- Watson-Jones, D.; Mugo, N.; Lees, S.; Mathai, M.; Vusha, S.; Ndirangu, G.; Ross, D.A. Access and Attitudes to HPV Vaccination amongst Hard-To-Reach Populations in Kenya. PLoS ONE 2015, 10, e0123701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odipodev. Vaccination in Kenya has a Fake News Problem; And It Is Not Happening Online. 2019. Available online: https://www.theelephant.info/data-stories/2019/01/17/vaccination-in-kenya-has-a-fake-news-problem-and-its-not-happening-online/ (accessed on 1 June 2022).
- Godman, B.; Basu, D.; Pillay, Y.; Almeida, P.H.; Mwita, J.C.; Rwegerera, G.M.; Anand Paramadhas, B.D.; Tiroyakgosi, C.; Patrick, O.; Niba, L.L.; et al. Ongoing and planned activities to improve the management of patients with Type 1 diabetes across Africa; implications for the future. Hosp. Pract. 2020, 48, 51–67. [Google Scholar] [CrossRef]
- Shannon, G.D.; Haghparast-Bidgoli, H.; Chelagat, W.; Kibachio, J.; Skordis-Worrall, J. Innovating to increase access to diabetes care in Kenya: An evaluation of Novo Nordisk’s base of the pyramid project. Glob. Health Action 2019, 12, 1605704. [Google Scholar] [CrossRef] [Green Version]
- Mbui, J.M.; Oluka, M.N.; Guantai, E.M.; Sinei, K.A.; Achieng, L.; Baker, A.; Jande, M.; Massele, A.; Godman, B. Prescription patterns and adequacy of blood pressure control among adult hypertensive patients in Kenya; findings and implications. Expert Rev. Clin. Pharmacol. 2017, 10, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Atieno, O.M.; Opanga, S.; Martin, A.; Kurdi, A.; Godman, B. Pilot study assessing the direct medical cost of treating patients with cancer in Kenya; findings and implications for the future. J. Med. Econ. 2018, 21, 878–887. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Basu, D.; Mueller, D.; Sneddon, J.; Seaton, R.A.; Yinka-Ogunleye, A.F.; Wamboga, J.; Miljković, N.; Mwita, J.C.; Rwegerera, G.M.; et al. Response to the Novel Corona Virus (COVID-19) Pandemic Across Africa: Successes, Challenges, and Implications for the Future. Front. Pharmacol. 2020, 11, 1205. [Google Scholar] [CrossRef]
- Opanga, S.; Rizvi, N.; Wamaitha, A.; Sefah, I.; Godman, B. Availability of Medicines in Community Pharmacy to Manage Patients with COVID-19 in Kenya; Pilot Study and Implications. Sch. Acad. J. Pharm. 2021, 10, 36–42. [Google Scholar] [CrossRef]
- Charan, J.; Biswas, T. How to calculate sample size for different study designs in medical research? Indian J. Psychol. Med. 2013, 35, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Rothman, K.J.; Gallacher, J.E.; Hatch, E.E. Why representativeness should be avoided. International Journal of Epidemiology. 2013, 42, 1012–1014. [Google Scholar] [CrossRef] [Green Version]
- Santhanes, D.; Yong, C.P.; Yap, Y.Y.; Saw, P.S.; Chaiyakunapruk, N.; Khan, T.M. Factors influencing intention to obtain the HPV vaccine in South East Asian and Western Pacific regions: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 3640. [Google Scholar] [CrossRef] [Green Version]
- Almazrou, S.; Saddik, B.; Jradi, H. Knowledge, attitudes, and practices of Saudi physicians regarding cervical cancer and the human papilloma virus vaccine. J. Infect. Public Health 2020, 13, 584–590. [Google Scholar] [CrossRef]
- Jalani, F.F.M.; Rani, M.D.M.; Isahak, I.; Aris, S.M.; Roslan, N. Knowledge, Attitude and Practice of Human Papillomavirus (HPV) Vaccination among Secondary School Students in Rural Areas of Negeri Sembilan, Malaysia. Public Health 2016, 8, 16. [Google Scholar]
- Rashid, S.; Labani, S.; Das, B.C. Knowledge, Awareness and Attitude on HPV, HPV Vaccine and Cervical Cancer among the College Students in India. PLoS ONE 2016, 11, e0166713. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Kim, D.H.; Kim, Y. Men’s awareness of cervical cancer: A qualitative study. BMC Womens Health 2018, 18, 155. [Google Scholar] [CrossRef]
- Daley, E.M.; Vamos, C.A.; Zimet, G.D.; Rosberger, Z.; Thompson, E.L.; Merrell, L. The Feminization of HPV: Reversing Gender Biases in US Human Papillomavirus Vaccine Policy. Am. J. Public Health 2016, 106, 983–984. [Google Scholar] [CrossRef]
- Wong, L.P. Role of men in promoting the uptake of HPV vaccinations: Focus groups’ finding from a developing country. Int. J. Public Health 2010, 55, 35–42. [Google Scholar] [CrossRef]
- Davis, J.; Vyankandondera, J.; Luchters, S.; Simon, D.; Holmes, W. Male involvement in reproductive, maternal and child health: A qualitative study of policymaker and practitioner perspectives in the Pacific. Reprod. Health 2016, 13, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zu, Z.Y.; Di Jiang, M.; Xu, P.P.; Chen, W.; Ni, Q.Q.; Lu, G.M.; Zhang, L.J. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020, 296, E15–E25. [Google Scholar] [CrossRef] [Green Version]
- Haycox, A. Why Cancer? Pharmacoeconomics 2016, 34, 625–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godman, B.; Hill, A.; Simoens, S.; Selke, G.; Selke Krulichová, I.; Zampirolli Dias, C.; Martin, A.P.; Oortwijn, W.; Timoney, A.; Gustafsson, L.L.; et al. Potential approaches for the pricing of cancer medicines across Europe to enhance the sustainability of healthcare systems and the implications. Expert Rev. Pharm. Outcomes Res. 2021, 21, 527–540. [Google Scholar] [CrossRef]
- Sefah, I.A.; Ogunleye, O.O.; Essah, D.O.; Opanga, S.A.; Butt, N.; Wamaitha, A.; Guantai, A.N.; Chikowe, I.; Khuluza, F.; Kibuule, D.; et al. Rapid assessment of the potential paucity and price increases for suggested medicines and protection equipment for COVID-19 across developing countries with a particular focus on Africa and the implications. Front. Pharmacol. 2021, 11, 588106. [Google Scholar] [CrossRef]
- Abena, P.M.; Decloedt, E.H.; Bottieau, E.; Suleman, F.; Adejumo, P.; Sam-Agudu, N.A.; TamFum, J.J.; Seydi, M.; Eholie, S.P.; Mills, E.J.; et al. Chloroquine and Hydroxychloroquine for the Prevention or Treatment of COVID-19 in Africa: Caution for Inappropriate Off-label Use in Healthcare Settings. Am. J. Trop. Med. Hyg. 2020, 102, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Schellack, N.; Strydom, M.; Pepper, M.S.; Herd, C.L.; Hendricks, C.L.; Bronkhorst, E.; Meyer, J.C.; Padayachee, N.; Bangalee, V.; Truter, I.; et al. Social Media and COVID-19;Perceptions and Public Deceptions of Ivermectin, Colchicine and Hydroxychloroquine: Lessons for Future Pandemics. Antibiotics 2022, 11, 445. [Google Scholar] [CrossRef]
- Gattegno, M.V.; Vertamatti, M.A.F.; Bednarczyk, R.A.; Evans, D.P. A cross-sectional survey of parental attitudes towards Human papillomavirus vaccination exclusion categories in Brazil. BMC Int. Health Hum. Rights 2019, 19, 6. [Google Scholar] [CrossRef]
- Dahlström, L.A.; Tran, T.N.; Lundholm, C.; Young, C.; Sundström, K.; Sparén, P. Attitudes to HPV vaccination among parents of children aged 12-15 years—A population-based survey in Sweden. Int. J. Cancer 2010, 126, 500–507. [Google Scholar] [CrossRef]
- Makwe, C.C.; Anorlu, R.I. Knowledge of and attitude toward human papillomavirus infection and vaccines among female nurses at a tertiary hospital in Nigeria. Int. J. Womens Health 2011, 3, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Vorsters, A.; Bonanni, P.; Maltezou, H.C.; Yarwood, J.; Brewer, N.T.; Bosch, F.X.; Hanley, S.; Cameron, R.; Franco, E.L.; Arbyn, M.; et al. The role of healthcare providers in HPV vaccination programs—A meeting report. Papillomavirus Res. 2019, 8, 100183. [Google Scholar] [CrossRef]
- Binagwaho, A.; Ngabo, F.; Wagner, C.M.; Mugeni, C.; Gatera, M.; Nutt, C.T.; Nsanzimana, S. Integration of comprehensive women’s health programmes into health systems: Cervical cancer prevention, care and control in Rwanda. Bull. World Health Organ. 2013, 91, 697–703. [Google Scholar] [CrossRef]
- Dempsey, A.F.; Zimet, G.D.; Davis, R.L.; Koutsky, L. Factors That Are Associated with Parental Acceptance of Human Papillomavirus Vaccines: A Randomized Intervention Study of Written Information About HPV. Pediatrics 2006, 117, 1486–1493. [Google Scholar] [CrossRef]
- Mansfield, L.; Onsomu, E.; Merwin, E.; Hall, N.; Harper-Harrison, A. Association between Parental HPV Knowledge and Intentions to Have Their Daughters Vaccinated. West. J. Nurs. Res. 2018, 40, 481–501. [Google Scholar] [CrossRef]
- Grandahl, M.; Paek, S.C.; Grisurapong, S.; Sherer, P.; Tydén, T.; Lundberg, P. Parents’ knowledge, beliefs, and acceptance of the HPV vaccination in relation to their socio-demographics and religious beliefs: A cross-sectional study in Thailand. PLoS ONE 2018, 13, e0193054. [Google Scholar]
- Ezenwa, B.N.; Balogun, M.R.; Okafor, I.P. Mothers’ human papilloma virus knowledge and willingness to vaccinate their adolescent daughters in Lagos, Nigeria. Int. J. Womens Health 2013, 5, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Ganczak, M.; Owsianka, B.; Korzeń, M. Factors That Predict Parental Willingness to Have Their Children Vaccinated against HPV in a Country with Low HPV Vaccination Coverage. Int. J. Environ. Res. Public Health 2018, 15, 645. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.E.; Credle, M.; Shapiro, E.D.; Niccolai, L.M. “It all depends”: A qualitative study of parents’ views of human papillomavirus vaccine for their adolescents at ages 11–12 years. J. Cancer Educ. 2016, 31, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Baumgaertner, B.; Ridenhour, B.J.; Justwan, F.; Carlisle, J.E.; Miller, C.R. Risk of disease and willingness to vaccinate in the United States: A population-based survey. PLoS Med. 2020, 17, e1003354. [Google Scholar] [CrossRef]
- Underwood, N.L.; Gargano, L.M.; Jacobs, S.; Seib, K.; Morfaw, C.; Murray, D.; Hughes, J.M.; Sales, J.M. Influence of Sources of Information and Parental Attitudes on Human Papillomavirus Vaccine Uptake among Adolescents. J. Pediatric Adolesc. Gynecol. 2016, 29, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Wamai, R.G.; Bain, P.A.; Welty, T.; Welty, E.; Ogembo, J.G. Knowledge and awareness of HPV vaccine and acceptability to vaccinate in sub-Saharan Africa: A systematic review. PLoS ONE 2014, 9, e90912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, Y.E.; Liu, Y.; Xu, X.Y.; Zhang, X.Y.; Wang, N.; Zheng, L.Q. Knowledge of Cervical Cancer, Human Papilloma Virus (HPV) and HPV Vaccination Among Women in Northeast China. J. Cancer Educ. 2020, 35, 1197–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fokom Domgue, J.; Chido-Amajuoyi, O.G.; Yu, R.K.; Shete, S. Beliefs about HPV Vaccine’s Success at Cervical Cancer Prevention among Adult US Women. JNCI Cancer Spectr. 2019, 3, pkz064. [Google Scholar] [CrossRef] [PubMed]
| Participants (n = 195) | ||
|---|---|---|
| Frequency | Percentage (%) | |
| Age distribution (years) | ||
| 18–30 | 12 | 6.2 |
| 31–40 | 79 | 40.5 |
| >40 | 104 | 53.3 |
| Sex | ||
| Male | 84 | 43.1 |
| Female | 111 | 56.9 |
| Occupation | ||
| Formal employment | 54 | 27.7 |
| Self-employment | 122 | 62.6 |
| Other | 19 | 9.7 |
| Education Level | ||
| No formal education | 1 | 0.5 |
| Primary level | 26 | 13.3 |
| Secondary level | 87 | 44.6 |
| Tertiary | 81 | 41.5 |
| Marital status | ||
| Married | 165 | 84.6 |
| Singlehood * | 30 | 15.4 |
| Religion | ||
| Christian | 192 | 98.5 |
| Muslim | 2 | 1 |
| Other | 1 | 0.5 |
| Age of children | ||
| 9–11 years | 70 | 35.9 |
| 12–14 years | 125 | 64.1 |
| Knowledge of HPV Vaccine (Proportion Responding YES or Correctly) | Males N (%) | Females N (%) | p-Value |
|---|---|---|---|
| Are you aware that all girls aged 10 years are being offered a human papilloma virus (HPV) vaccine? | 61 (72.6%) | 92 (82.9%) | 0.062 |
| What is the HPV vaccine used for? | |||
| 45 (53.6%) | 72 (64.9%) | 0.222 |
| 50 (59.5%) | 79 (71.2%) | 0.04 |
| 30 (35.7%) | 32 (28.8%) | 0.563 |
| What is the age group eligible for the HPV vaccine 9–26 years? | 48 (57.1%) | 84 (75.5%) | 0.021 |
| There is no need for Pap smear screening after receiving HPV vaccination | 24 (28.6%) | 66 (59.5%) | <0.001 |
| Bivariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Crude Beta Co-Efficient (95% CI) | p-Value | Adjusted Beta Co-Efficient (95% CI) | p-Value | |
| Age 18–30 (1) 31–40 (2) Above 40 (3) | −0.035 (0.845, −0.915) | 0.937 | −0.881 (−0.881, −2.609) | 0.127 |
| Sex Male (1) Female (0) | 0.849 (1.927, −0.229) | 0.124 | −3.994 (−8.555, 0.566) | 0.088 |
| Occupation Formal employment (1) Self-employed (2) Other (3) | 0.321 (1.238, −0.596) | 0.493 | - | - |
| Education Have no formal education (1) Primary level (2) Secondary level (3) Tertiary (4) | −0.71 (0.047, −1.467) | 0.067 | - | - |
| Marital status Married (0) Single (divorced/never married/widow/widower) (1) | 0.121 (1.609, 1.786) | 0.873 | - - | - |
| Religion | 1.114 (6.441, −4.213) | 0.682 | - - | - |
| Sex of the children | 0.095 (0.775, −0.585) | 0.783 | - - | - |
| Variables | Bivariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Crude Beta Coefficient (95%CI) | p Value | Adjusted Beta Coefficient (95%CI) | p Value | |
| Age 18–30 (1) 31–40 (2) Above 40 (3) | −0.057 (−0.305, 0.191) | 0.652 | - | - |
| Sex Male (1) Female (0) | 0.192 (−0.154, 0.538) | 0.278 | 0.255 (−0.056, 0.565) | 0.11 |
| Occupation Formal employment (1) Self-employed (2) Other (3) | 0.273 (0.003, 0.544) | 0.049 | 0.256 (−0.001, 0.512) | 0.052 |
| Education No formal education (1) Primary level (2) Secondary level (3) Tertiary (4) | 0.075 (−0.157, 0.308) | 0.526 | - | - |
| Marital status Married (0) Single (divorced/never married/widow/widower) (1) | −0.182 (−0.631, 0.268) | 0.43 | - | - |
| Religion Christian (1) Muslim (2) Others (3) | 0.681 (−0.818, 2.181) | 0.374 | - | - |
| Adequate knowledge on HPV * | 0.057 (0.035, 0.080) | <0.001 | −0.06 (0.039, 0.081) | <0.001 |
| Variable | Crude OR | Adjusted OR | ||
|---|---|---|---|---|
| OR (95% CI) | p-Values | OR (95% CI) | p-Values | |
| Age 18–30 (1) 31–40 (2) Above 40 (3) | 0.512 (0.252, 4.043) | 0.065 | 0.431 (0.194, 0.961) | 0.040 |
| Sex Male (1) Female (0) | 0.96 (0.451,2.078) | 0.933 | 2.369 (0.911, 7.643) | 0.074 |
| Occupation Formal employment (1) Self-employed (2) Other (3) | 0.922 (0.482, 1.762) | 0.806 | - | - |
| Level of education Have no formal education (1) Primary level (2) Secondary level (3) Tertiary (4) | 0.663 (0.310,1.419) | 0.290 | 0.392 (0.188, 0.818) | 0.013 |
| Marital status Married (0) Single (divorced/never married/widow/widower) (1) | 0.587 (.227, 1.512) | 0.270 | - | - |
| Knowledge total score (%) | 0.969 (0.951, 0.988) | 0.002 | 1.133 (1.050, 1.222) | <0.001 |
| Belief total score | 2.673 (1.859, 3.845) | <0.001 | 2.395 (1.604, 3.577) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolek, C.O.; Opanga, S.A.; Okalebo, F.; Birichi, A.; Kurdi, A.; Godman, B.; Meyer, J.C. Impact of Parental Knowledge and Beliefs on HPV Vaccine Hesitancy in Kenya—Findings and Implications. Vaccines 2022, 10, 1185. https://doi.org/10.3390/vaccines10081185
Kolek CO, Opanga SA, Okalebo F, Birichi A, Kurdi A, Godman B, Meyer JC. Impact of Parental Knowledge and Beliefs on HPV Vaccine Hesitancy in Kenya—Findings and Implications. Vaccines. 2022; 10(8):1185. https://doi.org/10.3390/vaccines10081185
Chicago/Turabian StyleKolek, Chester O., Sylvia A. Opanga, Faith Okalebo, Alfred Birichi, Amanj Kurdi, Brian Godman, and Johanna C. Meyer. 2022. "Impact of Parental Knowledge and Beliefs on HPV Vaccine Hesitancy in Kenya—Findings and Implications" Vaccines 10, no. 8: 1185. https://doi.org/10.3390/vaccines10081185
APA StyleKolek, C. O., Opanga, S. A., Okalebo, F., Birichi, A., Kurdi, A., Godman, B., & Meyer, J. C. (2022). Impact of Parental Knowledge and Beliefs on HPV Vaccine Hesitancy in Kenya—Findings and Implications. Vaccines, 10(8), 1185. https://doi.org/10.3390/vaccines10081185







