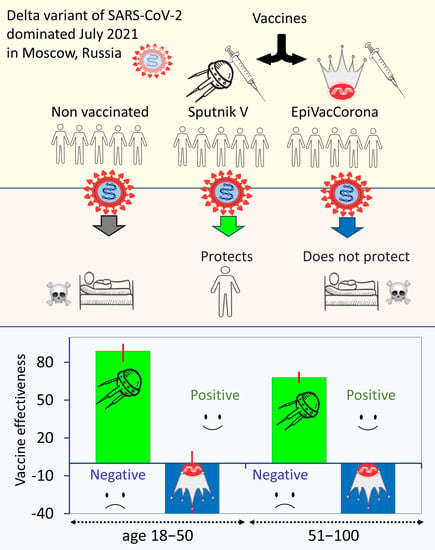
Retrospective Cohort Study of the Effectiveness of the Sputnik V and EpiVacCorona Vaccines against the SARS-CoV-2 Delta Variant in Moscow (June–July 2021)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset of COVID-19-Infected Individuals
2.2. Estimation of the Percentage of Moscow Residents with Antibodies
2.3. Method of VE Calculation
- number of COVID-19 cases among vaccinated people in Moscow
- number of vaccinated in Moscow
- number of COVID-19 cases among unvaccinated people in Moscow
- number of Moscow residents in the control group. To calculate the number of individuals in the control group, two variants of numerical estimation were used for each age group: (1) the number of unvaccinated Moscow residents and (2) the number of seronegative city residents.
- b.
- d.
- A numerical estimate of the number of individuals in each age group of the control group was made by subtracting the number of vaccinated or seropositive citizens from the number of Moscow residents in each age group.
2.4. Confidence Intervals for Vaccine Effectiveness Estimates
3. Results
3.1. The Effectiveness of Sputnik V and EpiVacCorona against Severe COVID-19 and Death
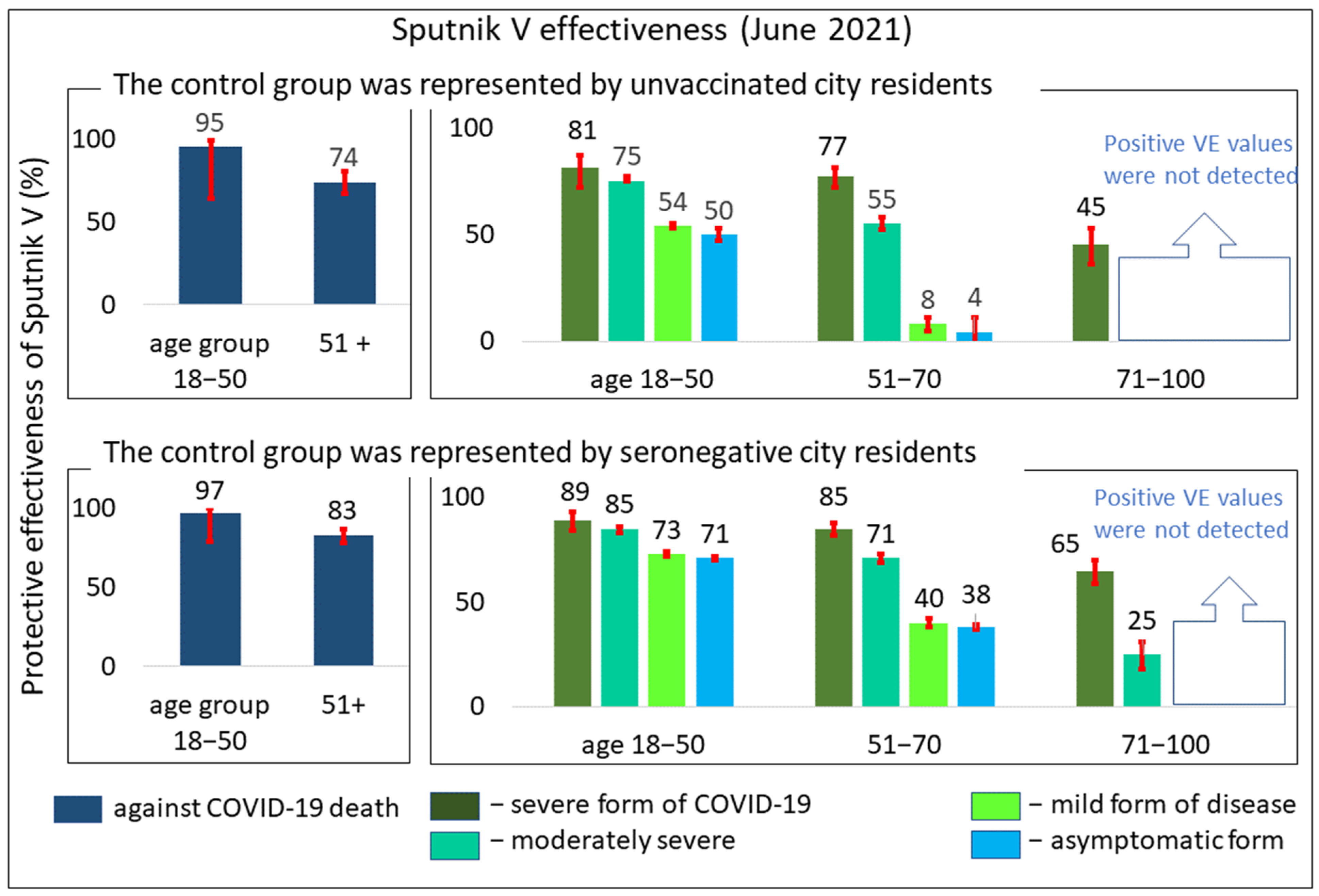
3.2. Comparative Effectiveness of the Sputnik V Vaccine in Preventing COVID-19 of Varying Severity in Different Age Groups
4. Discussion
4.1. Limitations of Our Study
4.2. EpiVacCorona Is an Inefficient Vaccine
4.3. Perhaps There Are More People with Comorbid Chronic Conditions in the Vaccinated Elderly Groups
4.4. Biological Factors May Contribute to the Decrease in VE in Older Age Groups
4.4.1. Protection against SARS-CoV-2 Reinfection Is Lower in the Older Age Group
4.4.2. The Rate of Decrease in the Level of Antibodies Is Higher in the Older Age Group
4.5. Comparison with Retrospective Studies of Vaccine Effectiveness Estimates in Different Countries
4.6. Perspectives. How Long Does Vaccine Protection Last?
5. Concluding Remarks
6. Conclusions
- The EpiVacCorona vaccine does not protect against COVID-19.
- The failure of EpiVacCorona to protect those who have been vaccinated demonstrates that no vaccine should be introduced to the public until strong evidence shows that it is safe and effective.
- The Sputnik V vaccine appears to confer high (significant) protection against the Delta variant.
- The more severe the COVID-19 disease, the better the Sputnik V vaccine protects against it.
- The estimated VE of Sputnik V was lower in the elderly than in the young.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Marfe, G.; Perna, S.; Shukla, A.K. Effectiveness of COVID-19 vaccines and their challenges (Review). Exp. Ther. Med. 2021, 22, 1407. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Voko, Z.; Kiss, Z.; Surjan, G.; Surjan, O.; Barcza, Z.; Palyi, B.; Formanek-Balku, E.; Molnar, G.A.; Herczeg, R.; Gyenesei, A.; et al. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary-the HUN-VE study. Clin. Microbiol. Infect. 2022, 28, 398–404. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, M.; Bhattacharyya, S.; Alawadi, A.; Mahmeed, H.A.; Sayed, J.A.; Justman, J.; El-Sadr, W.M.; Hidary, J.; Mukherjee, S. Morbidity and mortality from COVID-19 post-vaccination breakthrough infections in association with vaccines and the emergence of variants in Bahrain. Res. Sq. 2021, preprint. [Google Scholar] [CrossRef]
- Ryzhikov, A.B.; Ryzhikov, E.A.; Bogryantseva, M.P.; Danilenko, E.D.; Imatdinov, I.R.; Nechaeva, E.A.; Pyankov, O.V.; Pyankova, O.G.; Susloparov, I.M.; Taranov, O.S.; et al. Immunogenicity and Protectivity of the Peptide Vaccine against SARS-CoV-2. Ann. Russ. Acad. Med. Sci. 2021, 76, 5–19. [Google Scholar] [CrossRef]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020, 370, eabd4250. [Google Scholar] [CrossRef]
- Farrera-Soler, L.; Daguer, J.P.; Barluenga, S.; Vadas, O.; Cohen, P.; Pagano, S.; Yerly, S.; Kaiser, L.; Vuilleumier, N.; Winssinger, N. Identification of immunodominant linear epitopes from SARS-CoV-2 patient plasma. PLoS ONE 2020, 15, e0238089. [Google Scholar] [CrossRef]
- Yi, Z.; Ling, Y.; Zhang, X.; Chen, J.; Hu, K.; Wang, Y.; Song, W.; Ying, T.; Zhang, R.; Lu, H.; et al. Functional mapping of B-cell linear epitopes of SARS-CoV-2 in COVID-19 convalescent population. Emerg. Microbes Infect. 2020, 9, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.M.; Carissimo, G.; Wang, B.; Amrun, S.N.; Lee, C.Y.; Chee, R.S.; Fong, S.W.; Yeo, N.K.; Lee, W.H.; Torres-Ruesta, A.; et al. Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nat. Commun. 2020, 11, 2806. [Google Scholar] [CrossRef]
- Li, Y.; Lai, D.Y.; Zhang, H.N.; Jiang, H.W.; Tian, X.; Ma, M.L.; Qi, H.; Meng, Q.F.; Guo, S.J.; Wu, Y.; et al. Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, M.L.; Lei, Q.; Wang, F.; Hong, W.; Lai, D.Y.; Hou, H.; Xu, Z.W.; Zhang, B.; Chen, H.; et al. Linear epitope landscape of the SARS-CoV-2 Spike protein constructed from 1051 COVID-19 patients. Cell Rep. 2021, 34, 108915. [Google Scholar] [CrossRef] [PubMed]
- Vladimir Putin Held a Regular Meeting with Government Members, via Videoconference. The Kremlin, Moscow. 2020. Available online: http://en.kremlin.ru/events/president/news/64203 (accessed on 20 January 2021).
- Pichugina, T. What to Expect from EpiVacCorona. All About the Peptide Vaccine Against COVID-19 RIA-Novosti. Available online: https://ria.ru/20210122/epivakkorona-1594051697.html (accessed on 22 January 2021).
- Ryzhikov, A.B.; Ryzhikov, E.A.; Bogryantseva, M.P.; Usova, S.V.; Danilenko, E.D.; Nechaeva, E.A.; Pyankov, O.V.; Pyankova, O.G.; Gudymo, A.S.; Bodnev, S.A.; et al. A single blind, placebo-controlled randomized study of the safety, reactogenicity and immunogenicity of the “EpiVacCorona” Vaccine for the prevention of COVID-19, in volunteers aged 18–60 years (phase I–II). Russ. J. Infect. Immun. 2021, 11, 283–296. [Google Scholar] [CrossRef]
- Matveeva, O. The Problem of Selecting Peptides for EpiVacCorona and a Review of the Article by the Vaccine Developers on Phase I and Phase II Clinical Trials BioMolecule (in Russian). An Informative Online Publication about Modern Biology. 2021. Available online: https://biomolecula.ru/articles/problema-vybora-peptidov-dlia-epivakkorony (accessed on 17 June 2021).
- Krinitsky, A.A. Study of immunogenicity and potential protectiveness of EpiVacCorona vaccine. COVID19 PREPRINTS MICROBE.RU, 2021; preprint. [Google Scholar] [CrossRef]
- ClinicalTrialNCT04780035. Study of the Tolerability, Safety, Immunogenicity and Preventive Efficacy of the EpiVacCorona Vaccine for the Prevention of COVID-19 ClinicalTrials.gov Identifier NCT04780035. 3 March 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04780035 (accessed on 30 April 2021).
- Mishra, S.; Mindermann, S.; Sharma, M.; Whittaker, C.; Mellan, T.A.; Wilton, T.; Klapsa, D.; Mate, R.; Fritzsche, M.; Zambon, M.; et al. Changing composition of SARS-CoV-2 lineages and rise of Delta variant in England. EClinicalMedicine 2021, 39, 101064. [Google Scholar] [CrossRef]
- Rella, S.A.; Kulikova, Y.A.; Dermitzakis, E.T.; Kondrashov, F.A. Rates of SARS-CoV-2 transmission and vaccination impact the fate of vaccine-resistant strains. Sci. Rep. 2021, 11, 15729. [Google Scholar] [CrossRef]
- Brown, C.M.; Vostok, J.; Johnson, H.; Burns, M.; Gharpure, R.; Sami, S.; Sabo, R.T.; Hall, N.; Foreman, A.; Schubert, P.L.; et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Chavatte, J.M.; Mak, T.M.; Cui, L.; Kalimuddin, S.; Chia, W.N.; Tan, C.W.; et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: A multicentre cohort study. Clin. Microbiol. Infect. 2022, 28, 612.E1–612.E7. [Google Scholar] [CrossRef]
- Bian, L.; Gao, Q.; Gao, F.; Wang, Q.; He, Q.; Wu, X.; Mao, Q.; Xu, M.; Liang, Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 1201–1209. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Harder, T.; Kulper-Schiek, W.; Reda, S.; Treskova-Schwarzbach, M.; Koch, J.; Vygen-Bonnet, S.; Wichmann, O. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: Second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Eurosurveillance 2021, 26, 2100920. [Google Scholar] [CrossRef]
- Dolzhikova, I.V.; Gushchin, V.A.; Shcheblyakov, D.V.; Tsybin, A.N.; Shchetinin, A.M.; Pochtovyi, A.A.; Komissarov, A.B.; Kleymenov, D.A.; Kuznetsova, N.A.; Tukhvatulin, A.I.; et al. One-shot immunization with Sputnik Light (the first component of Sputnik V vaccine) is effective against SARS-CoV-2 Delta variant: Efficacy data on the use of the vaccine in civil circulation in Moscow. medRxiv 2021. [Google Scholar] [CrossRef]
- Mullen, J.L.; Tsueng, G.; Latif, A.A. Center for Viral Systems Biology. 2021. Available online: https://outbreak.info/ (accessed on 28 August 2021).
- Knorre, D.; Nabiyeva, E.; Garushyants, S.K.; Bazykin, G.A. Russian Consortium for Coronavirus Genome Sequencing (CORGI). 2021. Available online: https://taxameter.ru/ (accessed on 15 September 2021).
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Telegram Group. COVID-19 Vaccine News: 2021. This Group Is Created for Medical Professionals and Enthusiasts Who Have Something to Share about the Status of the Development and Clinical/Preclinical Trials of the COVID-19 Vaccines Candidates Worldwide. Available online: https://t.me/COVID19VaccinesNews/9479 (accessed on 24 August 2021).
- State Services Portal Ministry of Communications of Russia. 2021. Available online: https://www.gosuslugi.ru/covid-cert/ (accessed on 28 August 2021).
- Population of the Russian Federation by Gender and Age. Federal State Statistics Service. 2021. Available online: https://rosstat.gov.ru/compendium/document/13284 (accessed on 30 September 2021).
- Coronavirus Vaccination Statistics. Number of People Vaccinated against Coronavirus in Moscow. 2021. Available online: https://gogov.ru/covid-v-stats/msk (accessed on 20 September 2021).
- Statistics on the Spread of Coronavirus in Moscow. 2021. Available online: https://coronavirus-monitor.info/ (accessed on 20 September 2021).
- Weinberg, G.A.; Szilagyi, P.G. Vaccine epidemiology: Efficacy, effectiveness, and the translational research roadmap. J. Infect. Dis. 2010, 201, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Tenny, S.; Hoffman, M.R. Odds Ratio. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- McHugh, M.L. The chi-square test of independence. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Barchuk, A.; Bulina, A.; Cherkashin, M.; Berezina, N.; Rakova, T.; Kuplevatskaya, D.; Stanevich, O.; Skougarevskiy, D.; Okhotin, A. COVID-19 vaccines effectiveness against symptomatic SARS-CoV-2 Delta variant infection: A population-based case-control study in St. Petersburg, Russia. medRxiv 2022. [Google Scholar] [CrossRef]
- Hansen, C.H.; Michlmayr, D.; Gubbels, S.M.; Molbak, K.; Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet 2021, 397, 1204–1212. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef]
- Sjaarda, C.P.; Moslinger, E.; Tozer, K.; Colautti, R.I.; Kheitan, S.; Meurant, R.; Cleaf, S.V.; Ardakani, A.; Bosnjak, O.; Ghaffari, A.; et al. Distinct age-specific SARS-CoV-2 IgG decay kinetics following natural infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y.; et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef]
- Mwimanzi, F.; Lapointe, H.R.; Cheung, P.K.; Sang, Y.; Yaseen, F.; Umviligihozo, G.; Kalikawe, R.; Datwani, S.; Omondi, F.H.; Burns, L.; et al. Older adults mount less durable humoral responses to two doses of COVID-19 mRNA vaccine, but strong initial responses to a third dose. J. Infect. Dis. 2022, jiac199. [Google Scholar] [CrossRef]
- Keshavarz, B.; Richards, N.E.; Workman, L.J.; Patel, J.; Muehling, L.M.; Canderan, G.; Murphy, D.D.; Brovero, S.G.; Ailsworth, S.M.; Eschenbacher, W.H.; et al. Trajectory of IgG to SARS-CoV-2 After Vaccination With BNT162b2 or mRNA-1273 in an Employee Cohort and Comparison With Natural Infection. Front. Immunol. 2022, 13, 850987. [Google Scholar] [CrossRef] [PubMed]
- Grange, Z.; Buelo, A.; Sullivan, C.; Moore, E.; Agrawal, U.; Boukhari, K.; McLaughlan, I.; Stockton, D.; McCowan, C.; Robertson, C.; et al. Characteristics and risk of COVID-19-related death in fully vaccinated people in Scotland. Lancet 2021, 398, 1799–1800. [Google Scholar] [CrossRef]
- Nordstrom, P.; Ballin, M.; Nordstrom, A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic COVID-19 infection in Sweden: A nationwide cohort study. Lancet Reg. Health Eur. 2021, 11, 100249. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Dercon, Q.; Harrison, P.J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immun. 2022, 103, 154–162. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Israel, A.; Merzon, E.; Schaffer, A.A.; Shenhar, Y.; Green, I.; Golan-Cohen, A.; Ruppin, E.; Magen, E.; Vinker, S. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: Test negative design study. BMJ 2021, 375, e067873. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Hasan, M.R.; Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; AlMukdad, S.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat. Med. 2021, 27, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Delbrück, M.; Toptan, H.; Schenk, S.G.B.; Grikscheit, K.; Metzler, M.; Ciesek, E.H.S. Low But Recoverable Markers of Humoral Immune Response to BNT162b2 in Elderly LTCF Residents Five to Seven Months After Two-Dose Vaccination. Front. Aging 2022, 3, 883724. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef]
- Epivaccorona_effectiveness_moscow. 2022. Available online: https://github.com/anershov/epivaccorona_effectiveness_moscow (accessed on 26 January 2022).
- Matveeva, O.; Ershov, A. Retrospective cohort study of the effectiveness of the Sputnik V and EpiVacCorona vaccines against the SARS-CoV-2 Delta variant in Moscow (June–July 2021). Version 1. Res. Sq. 2022; preprint. [Google Scholar] [CrossRef]
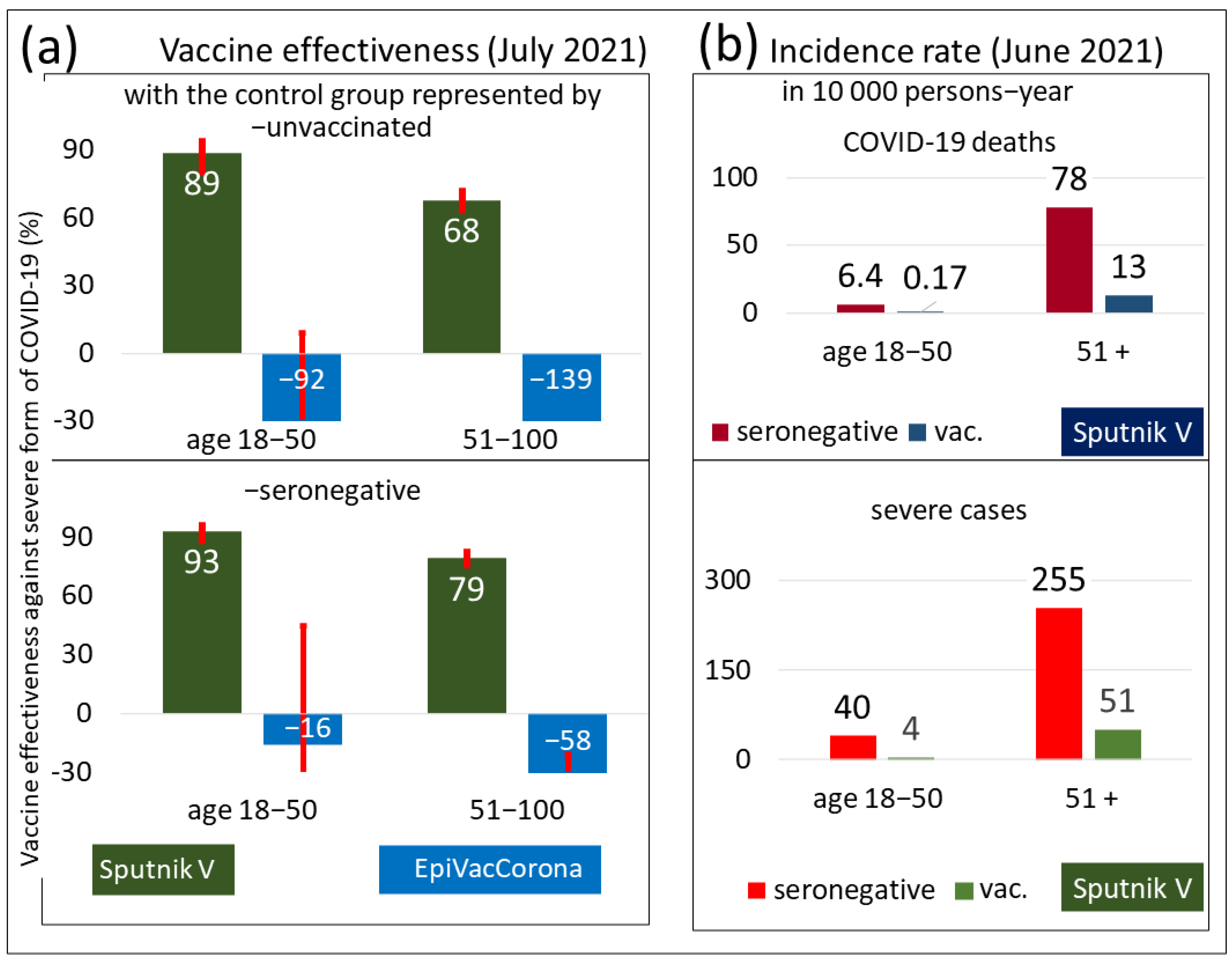
| Age, Years | Control Group—Unvaccinated | Control Group—Seronegative | CI 95% of Both Control Groups | |||||
|---|---|---|---|---|---|---|---|---|
| VE% | CI 95% | VE% | CI 95% | |||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||
| 18–50 | 89 | 81 | 94 | 93 | 88 | 96 | 81 | 96 |
| 51–70 | 76 | 70 | 81 | 84 | 80 | 87 | 70 | 87 |
| 70+ | 42 | 32 | 51 | 63 | 56 | 69 | 32 | 69 |
| Age | Normalized Data (10,000 Persons per Year) | Odds Ratio | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | Severe Form of COVID-19 | Deaths | Severe Form of COVID-19 | |||||||
| Unvac. | Vac. | Unvac. | Vac. | Deaths | Severe Form | Lower | Upper | Lower | Upper | |
| 18–50 | 6.4 | 0.17 | 40 | 4 | 0.03 | 0.1 | 0 | 0.21 | 0.07 | 0.15 |
| 51+ | 78 | 13 | 255 | 51 | 0.17 | 0.2 | 0.13 | 0.22 | 0.18 | 0.23 |
| Country of Data Origin | Type of Research | Vaccines | Outcomes of COVID-19 | Age Dependence of VE | Virus Variant | Type of Publication | Ref. |
|---|---|---|---|---|---|---|---|
| Scotland | retrospective case control | BNT162b2, ChAdOx1 nCoV-19 | death | not detected | mainly Delta | article | [48] |
| Sweden | retrospective cohort | BNT162b2, ChAdOx1 nCoV-19, mRNA-1273 | death, hospitalizations, symptomatic and asymptomatic | detected | not specified | article | [49] |
| Hungary | retrospective cohort | BNT162b2, mRNA-1273, Sputnik V, Sinofarm, ChAdOx1 nCoV-19 | infection | not detected | not specified | article | [7] |
| UK | case-control study | BNT162b2, ChAdOx1 nCoV-19 | mild and severe | detected | Delta | article | [50] |
| UK | case-control study | BNT162b2, ChAdOx1 nCoV-20 | PCR-positive | detected | Delta | article | [51] |
| USA | retrospective cohort, controlled by those who got flu vaccine | is not specified | death, ICU hospitalizations, and many other outcomes | detected | not specified | article | [52] |
| USA | retrospective cohort | BNT162b2 | PCR-positive and hospitalization | detected, but minor | Delta | article | [53] |
| Israel | case-negative control | BNT162b2 | PCR-positive or severe form of COVID-19 | not detected for PCR+ and detected for severe COVID-19 | not specified | article | [54] |
| Israel | case-negative control | BNT162b2, mRNA-1273 | infection | detected but positive | Delta | article | [55] |
| Qatar | case-negative control | BNT162b2, mRNA-1273 | symptomatic or asymptomatic infection | detected | Delta | article | [56] |
| Russia | retrospective cohort | Sputnik Light | symptomatic infection | detected | Delta | preprint | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveeva, O.; Ershov, A. Retrospective Cohort Study of the Effectiveness of the Sputnik V and EpiVacCorona Vaccines against the SARS-CoV-2 Delta Variant in Moscow (June–July 2021). Vaccines 2022, 10, 984. https://doi.org/10.3390/vaccines10070984
Matveeva O, Ershov A. Retrospective Cohort Study of the Effectiveness of the Sputnik V and EpiVacCorona Vaccines against the SARS-CoV-2 Delta Variant in Moscow (June–July 2021). Vaccines. 2022; 10(7):984. https://doi.org/10.3390/vaccines10070984
Chicago/Turabian StyleMatveeva, Olga, and Alexander Ershov. 2022. "Retrospective Cohort Study of the Effectiveness of the Sputnik V and EpiVacCorona Vaccines against the SARS-CoV-2 Delta Variant in Moscow (June–July 2021)" Vaccines 10, no. 7: 984. https://doi.org/10.3390/vaccines10070984
APA StyleMatveeva, O., & Ershov, A. (2022). Retrospective Cohort Study of the Effectiveness of the Sputnik V and EpiVacCorona Vaccines against the SARS-CoV-2 Delta Variant in Moscow (June–July 2021). Vaccines, 10(7), 984. https://doi.org/10.3390/vaccines10070984