Immune Correlates of Disseminated BCG Infection in IL12RB1-Deficient Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.1.1. Mice and BCG
2.1.2. BCG Inoculation and Sample Collection
2.1.3. CFU Counts of BCG in Lung and Spleen
2.1.4. Real-Time PCR Detection of Cytokine Transcription in Lung Tissue
2.1.5. Measurement of PPD-Specific IgG in Mouse Serum
2.1.6. IFN-γ ELISPOT Assay
2.1.7. Cytokine Beads Array (CBA) Assay
2.1.8. Statistical Analysis
3. Results
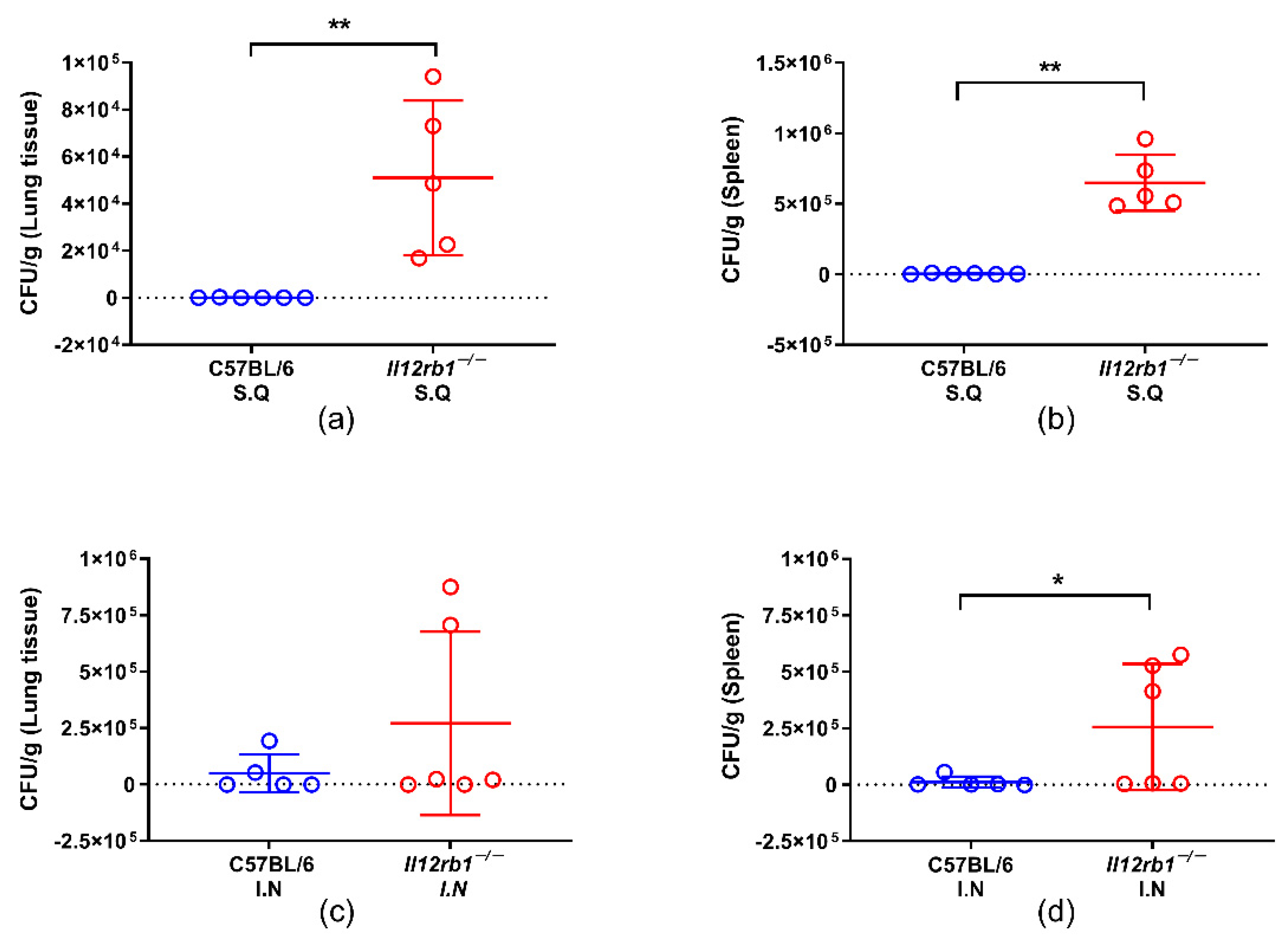
3.1. IL12RB1 Deficiency Compromised the In Vivo Containment of BCG Especially When Inoculated Subcutaneously
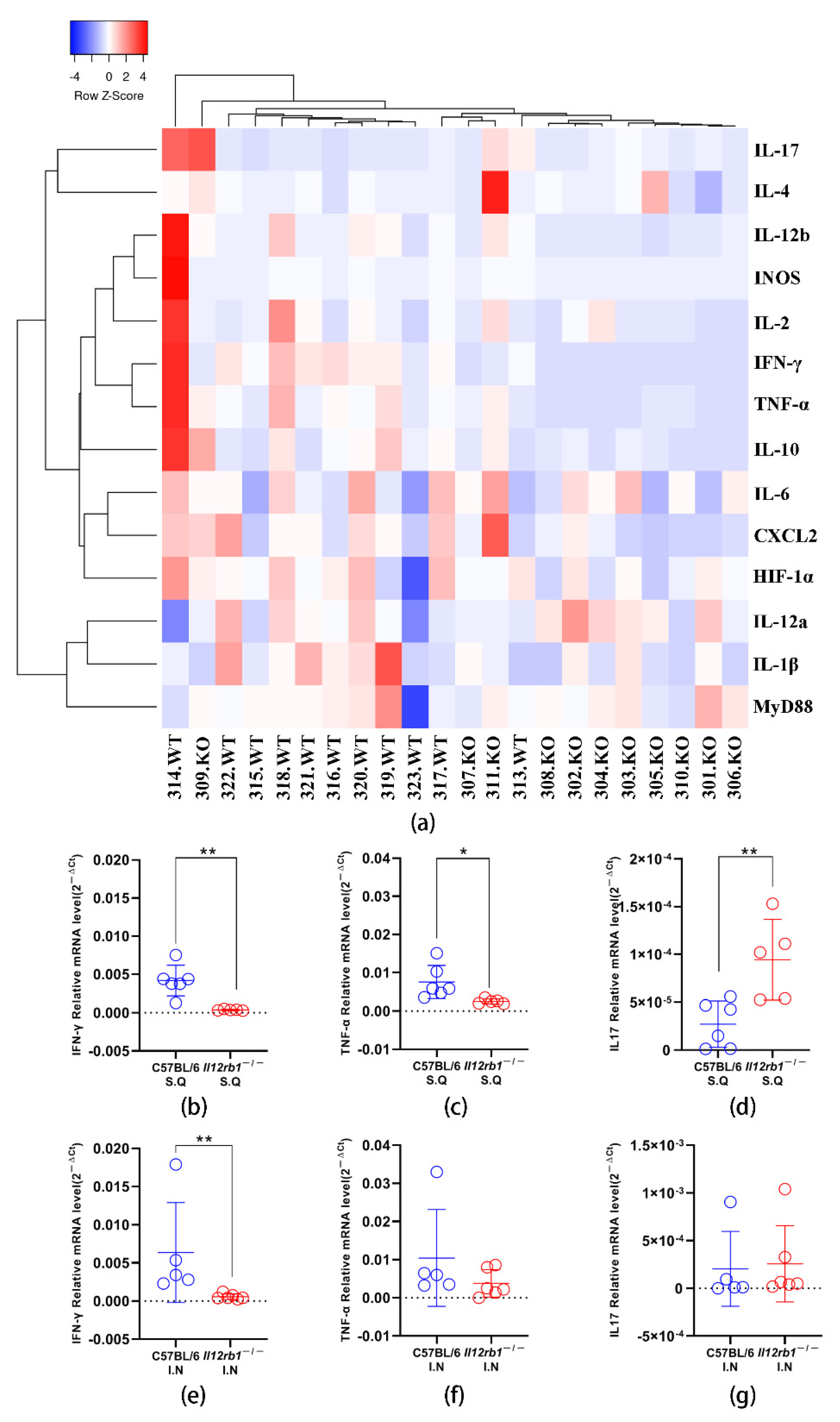
3.2. Profiling of Cytokine Transcription in Lung Tissue Revealed Significantly Altered Innate Responses upon BCG Inoculation in Il12rb1−/− Mice
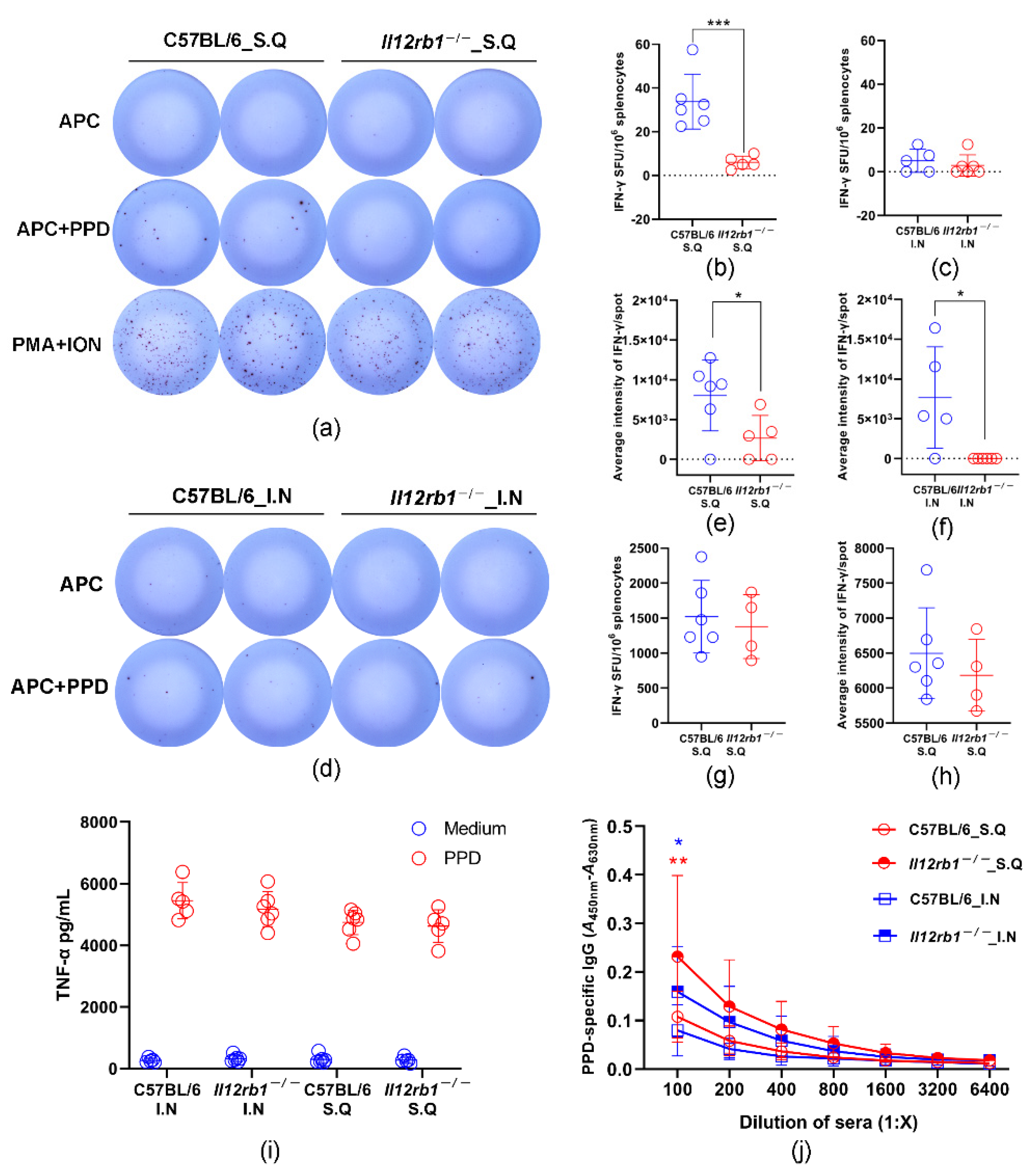
3.3. PPD-Specific T Cell Responses Were Partially Impaired in Il12rb1−/− Mice
3.4. PPD-Specific Antibody Responses Were Significantly Enhanced in Il12rb1−/− Mice
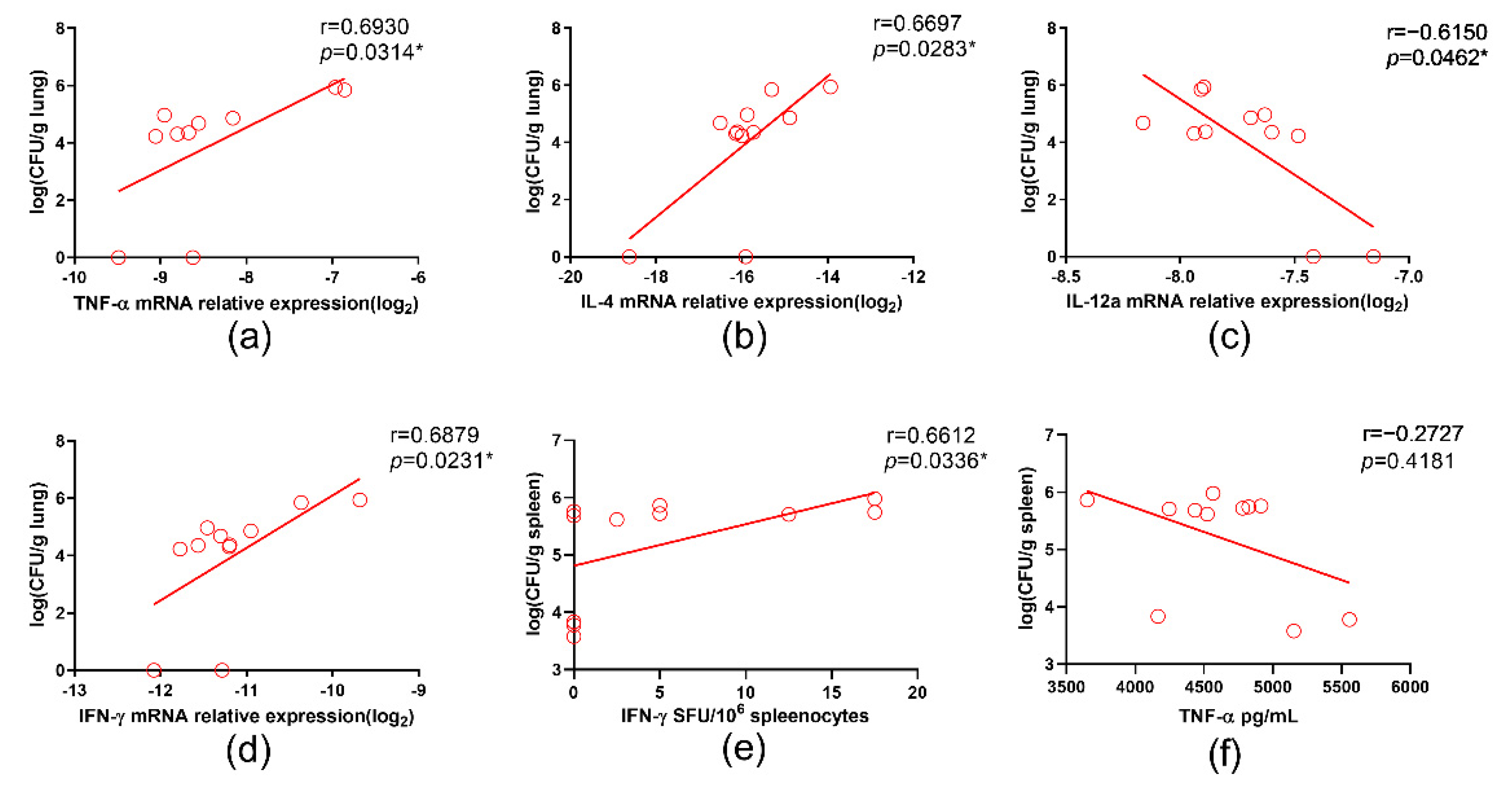
3.5. IL-12a Correlated with Better Containment of BCG in Il12rb1−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.; Bowman, E.P.; McElwee, J.J.; Smyth, M.J.; Casanova, J.L.; Cooper, A.M.; Cua, D.J. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015, 21, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Presky, D.H.; Gubler, U.; Chizzonite, R.A.; Gately, M.K. IL12 receptors and receptor antagonists. Res. Immunol. 1995, 146, 439–445. [Google Scholar] [CrossRef]
- Robinson, R.T. IL12Rβ1: The cytokine receptor that we used to know. Cytokine 2015, 71, 348–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Vosse, E.; Haverkamp, M.H.; Ramirez-Alejo, N.; Martinez-Gallo, M.; Blancas-Galicia, L.; Metin, A.; Garty, B.Z.; Sun-Tan, Ç.; Broides, A.; de Paus, R.A.; et al. IL-12Rβ1 deficiency: Mutation update and description of the IL12RB1 variation database. Hum. Mutat. 2013, 34, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Ferrante, J.; Gately, M.K.; Magram, J. Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J. Immunol. 1997, 159, 1658–1665. [Google Scholar]
- Altare, F.; Durandy, A.; Lammas, D.; Emile, J.F.; Lamhamedi, S.; Le Deist, F.; Drysdale, P.; Jouanguy, E.; Döffinger, R.; Bernaudin, F.; et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 1998, 280, 1432–1435. [Google Scholar] [CrossRef]
- De Jong, R.; Altare, F.; Haagen, I.A.; Elferink, D.G.; Boer, T.; van Breda Vriesman, P.J.; Kabel, P.J.; Draaisma, J.M.; van Dissel, J.T.; Kroon, F.P.; et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 1998, 280, 1435–1438. [Google Scholar] [CrossRef]
- Cooper, A.M.; Dalton, D.K.; Stewart, T.A.; Griffin, J.P.; Russell, D.G.; Orme, I.M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993, 178, 2243–2247. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef] [Green Version]
- Reljic, R. IFN-gamma therapy of tuberculosis and related infections. J. Interferon. Cytokine Res. 2007, 27, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Cui, W.; Guan, L.; Shen, F.; Xu, J.; Zhou, F.; Li, M.; Gao, C.; Jin, Q.; et al. Potential association of pulmonary tuberculosis with genetic polymorphisms of toll-like receptor 9 and interferon-gamma in a Chinese population. BMC Infect. Dis. 2013, 13, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Liu, X.; Wang, Y.; Zhang, M.; Wang, M.; He, J.Q. Genetic polymorphisms of IFNG and IFNGR1 with latent tuberculosis infection. Dis. Markers 2019, 2019, 8410290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Beaucoudrey, L.; Puel, A.; Filipe-Santos, O.; Cobat, A.; Ghandil, P.; Chrabieh, M.; Feinberg, J.; von Bernuth, H.; Samarina, A.; Janniere, L.; et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 2008, 205, 1543–1550. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, N.; Bustamante, J.; Bourdery, L.; Bentebibel, S.E.; Boisson-Dupuis, S.; Hamlin, F.; Tran, M.V.; Blankenship, D.; Pascual, V.; Savino, D.A.; et al. IL-12 receptor β1 deficiency alters in vivo T follicular helper cell response in humans. Blood 2013, 121, 3375–3385. [Google Scholar] [CrossRef] [Green Version]
- De Beaucoudrey, L.; Samarina, A.; Bustamante, J.; Cobat, A.; Boisson-Dupuis, S.; Feinberg, J.; Al-Muhsen, S.; Janniere, L.; Rose, Y.; de Suremain, M.; et al. Revisiting human IL-12Rbeta1 deficiency: A survey of 141 patients from 30 countries. Medicine 2010, 89, 381–402. [Google Scholar] [CrossRef] [Green Version]
- Fieschi, C.; Dupuis, S.; Catherinot, E.; Feinberg, J.; Bustamante, J.; Breiman, A.; Altare, F.; Baretto, R.; Le Deist, F.; Kayal, S.; et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: Medical and immunological implications. J. Exp. Med. 2003, 197, 527–535. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, E.; Wang, J.; Li, R.; Li, G.; Liu, G.; Song, N.; Huang, Q.; Kong, C.; Wang, H. Prime-boost bacillus Calmette-Guérin vaccination with lentivirus-vectored and DNA-based vaccines expressing antigens Ag85B and Rv3425 improves protective efficacy against Mycobacterium tuberculosis in mice. Immunology 2014, 143, 277–286. [Google Scholar] [CrossRef]
- Saxena, R.K.; Weissman, D.; Simpson, J.; Lewis, D.M. Murine model of BCG lung infection: Dynamics of lymphocyte subpopulations in lung interstitium and tracheal lymph nodes. J. Biosci. 2002, 27, 143–153. [Google Scholar] [CrossRef]
- Fulton, S.A.; Martin, T.D.; Redline, R.W.; Henry Boom, W. Pulmonary immune responses during primary mycobacterium bovis-Calmette-Guerin bacillus infection in C57Bl/6 mice. Am. J. Respir. Cell Mol. Biol. 2000, 22, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Kouadjo, K.E.; Nishida, Y.; Cadrin-Girard, J.F.; Yoshioka, M.; St-Amand, J. Housekeeping and tissue-specific genes in mouse tissues. BMC Genom. 2007, 8, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Ren, X.; Ren, Y.; Wang, J.; Hu, Z.; Xie, X.; Xu, J. As a genetic adjuvant, CTA improves the immunogenicity of DNA vaccines in an ADP-ribosyltransferase activity- and IL-6-dependent manner. Vaccine 2014, 32, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wan, Y.; Li, Q. Interleukin-21 enhances the antibody avidity elicited by DNA prime and MVA boost vaccine. Cytokine 2020, 125, 154814. [Google Scholar] [CrossRef]
- Presky, D.H.; Yang, H.; Minetti, L.J.; Chua, A.O.; Nabavi, N.; Wu, C.Y.; Gately, M.K.; Gubler, U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 14002–14007. [Google Scholar] [CrossRef] [Green Version]
- Parham, C.; Chirica, M.; Timans, J.; Vaisberg, E.; Travis, M.; Cheung, J.; Pflanz, S.; Zhang, R.; Singh, K.P.; Vega, F.; et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002, 168, 5699–5708. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Barricarte, R.; Markle, J.G.; Ma, C.S.; Deenick, E.K.; Ramirez-Alejo, N.; Mele, F.; Latorre, D.; Mahdaviani, S.A.; Aytekin, C.; Mansouri, D.; et al. Human IFN-gamma immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 2018, 3, eaau6759. [Google Scholar] [CrossRef]
- Miller, H.E.; Robinson, R.T. Early control of Mycobacterium tuberculosis infection requires il12rb1 expression by rag1-dependent lineages. Infect. Immun. 2012, 80, 3828–3841. [Google Scholar] [CrossRef] [Green Version]
- Lichtenauer-Kaligis, E.G.; de Boer, T.; Verreck, F.A.; van Voorden, S.; Hoeve, M.A.; van de Vosse, E.; Ersoy, F.; Tezcan, I.; van Dissel, J.T.; Sanal, O.; et al. Severe Mycobacterium bovis BCG infections in a large series of novel IL-12 receptor beta1 deficient patients and evidence for the existence of partial IL-12 receptor beta1 deficiency. Eur. J. Immunol. 2003, 33, 59–69. [Google Scholar] [CrossRef]
- Hatipoglu, N.; Güvenç, B.H.; Deswarte, C.; Koksalan, K.; Boisson-Dupuis, S.; Casanova, J.-L.; Bustamante, J. Inherited IL-12Rβ1 deficiency in a child with BCG adenitis and oral candidiasis: A case report. Pediatrics 2017, 140, e20161668. [Google Scholar] [CrossRef] [Green Version]
- Darleguy, A.; Bost-Bru, C.; Pagnier, A.; Plantaz, D.; Piolat, C.; Nugues, F.; Picard, C. Mendelian susceptibility to mycobacterial disease: A case report of disseminated infection due to Mycobacterium avium. Arch. Pediatr. 2013, 20, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Boisson-Dupuis, S.; Abel, L.; Casanova, J.-L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin. Immunol. 2014, 26, 454–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, L.; Cheraghi, T.; Yazdani, R.; Azizi, G.; Rasouli, S.; Zavareh, F.T.; Parvaneh, L.; Parvaneh, N.; Sohani, M.; Delavari, S.; et al. Mendelian susceptibility to mycobacterial disease: Clinical and immunological findings of patients suspected for IL12Rbeta1 deficiency. Allergol. Immunopathol. 2019, 47, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Pourakbari, B.; Hosseinpour Sadeghi, R.; Mahmoudi, S.; Parvaneh, N.; Keshavarz Valian, S.; Mamishi, S. Evaluation of interleukin-12 receptor β1 and interferon gamma receptor 1 deficiency in patients with disseminated BCG infection. Allergol. Immunopathol. 2019, 47, 38–42. [Google Scholar] [CrossRef]
- Dhiman, N.; Ovsyannikova, I.G.; Vierkant, R.A.; Pankratz, V.S.; Jacobson, R.M.; Poland, G.A. Associations between cytokine/cytokine receptor single nucleotide polymorphisms and humoral immunity to measles, mumps and rubella in a Somali population. Tissue Antigens 2008, 72, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Gröndahl-Yli-Hannuksela, K.; Vahlberg, T.; Ilonen, J.; Mertsola, J.; He, Q. Polymorphism of IL-10 gene promoter region: Association with T cell proliferative responses after acellular pertussis vaccination in adults. Immunogenetics 2016, 68, 733–741. [Google Scholar] [CrossRef]
- Puel, A.; Cypowyj, S.; Bustamante, J.; Wright, J.F.; Liu, L.; Lim, H.K.; Migaud, M.; Israel, L.; Chrabieh, M.; Audry, M.; et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011, 332, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Ouederni, M.; Sanal, O.; Ikinciogullari, A.; Tezcan, I.; Dogu, F.; Sologuren, I.; Pedraza-Sánchez, S.; Keser, M.; Tanir, G.; Nieuwhof, C.; et al. Clinical features of Candidiasis in patients with inherited interleukin 12 receptor β1 deficiency. Clin. Infect. Dis. 2014, 58, 204–213. [Google Scholar] [CrossRef]
- Lee, J.S.; Tato, C.M.; Joyce-Shaikh, B.; Gulen, M.F.; Cayatte, C.; Chen, Y.; Blumenschein, W.M.; Judo, M.; Ayanoglu, G.; McClanahan, T.K.; et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015, 43, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Huang, L.; Vergis, A.L.; Ye, H.; Bajwa, A.; Narayan, V.; Strieter, R.M.; Rosin, D.L.; Okusa, M.D. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Investig. 2010, 120, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Katayama, M.; Ohmura, K.; Yukawa, N.; Terao, C.; Hashimoto, M.; Yoshifuji, H.; Kawabata, D.; Fujii, T.; Iwakura, Y.; Mimori, T. Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS ONE 2013, 8, e62231. [Google Scholar] [CrossRef]
- Allen, J.E.; Sutherland, T.E.; Rückerl, D. IL-17 and neutrophils: Unexpected players in the type 2 immune response. Curr. Opin. Immunol. 2015, 34, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Nagao, T.; Nagi-Miura, N.; Ohno, N.; Yasuhara, M.; Yamamoto, K.; Nakayama, T.; Suzuki, K. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J. Autoimmun. 2008, 31, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Schön, M.P.; Erpenbeck, L. The Interleukin-23/Interleukin-17 Axis Links Adaptive and Innate Immunity in Psoriasis. Front. Immunol. 2018, 9, 1323. [Google Scholar] [CrossRef] [Green Version]
- Puerta-Arias, J.D.; Mejía, S.P.; González, Á. The Role of the Interleukin-17 Axis and Neutrophils in the Pathogenesis of Endemic and Systemic Mycoses. Front. Cell Infect. Microbiol. 2020, 10, 595301. [Google Scholar] [CrossRef]
- Moreno, S.E.; Alves-Filho, J.C.; Alfaya, T.M.; da Silva, J.S.; Ferreira, S.H.; Liew, F.Y. IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture. J. Immunol. 2006, 177, 3218–3224. [Google Scholar] [CrossRef]
- Ethuin, F.; Gérard, B.; Benna, J.E.; Boutten, A.; Gougereot-Pocidalo, M.A.; Jacob, L.; Chollet-Martin, S. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab. Investig. 2004, 84, 1363–1371. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.R.; Fernandes, R.K.; Balderramas Hde, A.; Penitenti, M.; Bachiega, T.F.; Calvi, S.A.; Dias-Melicio, L.A.; Ikoma, M.R.; Soares, Â.M. Interferon-gamma production by human neutrophils upon stimulation by IL-12, IL-15 and IL-18 and challenge with Paracoccidioides brasiliensis. Cytokine 2014, 69, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Gee, K.; Guzzo, C.; Che Mat, N.F.; Ma, W.; Kumar, A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. Allergy Drug Targets 2009, 8, 40–52. [Google Scholar] [CrossRef]
- Behzadi, P.; Behzadi, E.; Ranjbar, R. IL-12 family cytokines: General characteristics, pathogenic microorganisms, receptors, and signalling pathways. Acta Microbiol. Immunol. Hung. 2016, 63, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, S.M.; Eisenstein, E.M.; Kuhns, D.B.; Turner, M.L.; Fleisher, T.A.; Strober, W.; Gallin, J.I. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N. Engl. J. Med. 1994, 330, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Alangari, A.A.; Al-Zamil, F.; Al-Mazrou, A.; Al-Muhsen, S.; Boisson-Dupuis, S.; Awadallah, S.; Kambal, A.; Casanova, J.L. Treatment of disseminated mycobacterial infection with high-dose IFN-gamma in a patient with IL-12Rbeta1 deficiency. Clin. Dev. Immunol. 2011, 2011, 691956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Group | Mouse Strain | BCG Dosage (CFUs/Mouse) | Inoculation Route | Schedule | |
|---|---|---|---|---|---|
| Inoculation | Euthanasia | ||||
| 1 | C57BL/6 (n = 5) | 1.5 × 106 | Intranasally | Day 0 | Day 27 |
| 2 | Il12rb1−/− (n = 6) | ||||
| 3 | C57BL/6 (n = 6) | 3 × 106 | Subcutaneously | ||
| 4 | Il12rb1−/− (n = 5) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Jia, L.; Liu, Y.; Wang, J.; Qiu, C.; Li, T.; Zhang, W.; Zhu, Z.; Wu, J.; Wan, Y. Immune Correlates of Disseminated BCG Infection in IL12RB1-Deficient Mice. Vaccines 2022, 10, 1147. https://doi.org/10.3390/vaccines10071147
Wang X, Jia L, Liu Y, Wang J, Qiu C, Li T, Zhang W, Zhu Z, Wu J, Wan Y. Immune Correlates of Disseminated BCG Infection in IL12RB1-Deficient Mice. Vaccines. 2022; 10(7):1147. https://doi.org/10.3390/vaccines10071147
Chicago/Turabian StyleWang, Xuyang, Liqiu Jia, Yang Liu, Jing Wang, Chao Qiu, Tao Li, Wenhong Zhang, Zhaoqin Zhu, Jing Wu, and Yanmin Wan. 2022. "Immune Correlates of Disseminated BCG Infection in IL12RB1-Deficient Mice" Vaccines 10, no. 7: 1147. https://doi.org/10.3390/vaccines10071147
APA StyleWang, X., Jia, L., Liu, Y., Wang, J., Qiu, C., Li, T., Zhang, W., Zhu, Z., Wu, J., & Wan, Y. (2022). Immune Correlates of Disseminated BCG Infection in IL12RB1-Deficient Mice. Vaccines, 10(7), 1147. https://doi.org/10.3390/vaccines10071147







