Neuromyotonia with Central Nervous System Lesions following Quadrivalent Human Papilloma Virus Vaccination
Abstract
1. Introduction
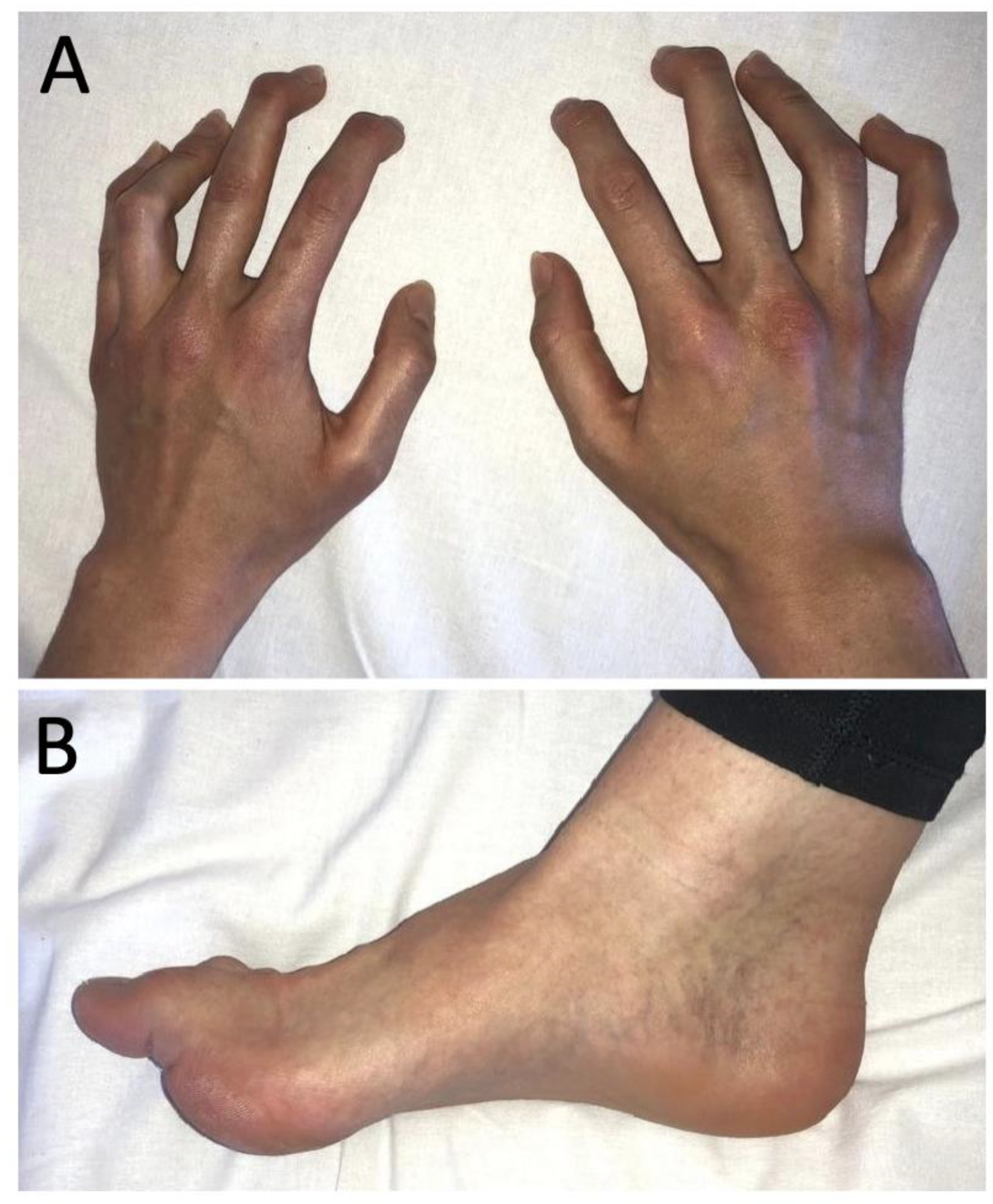
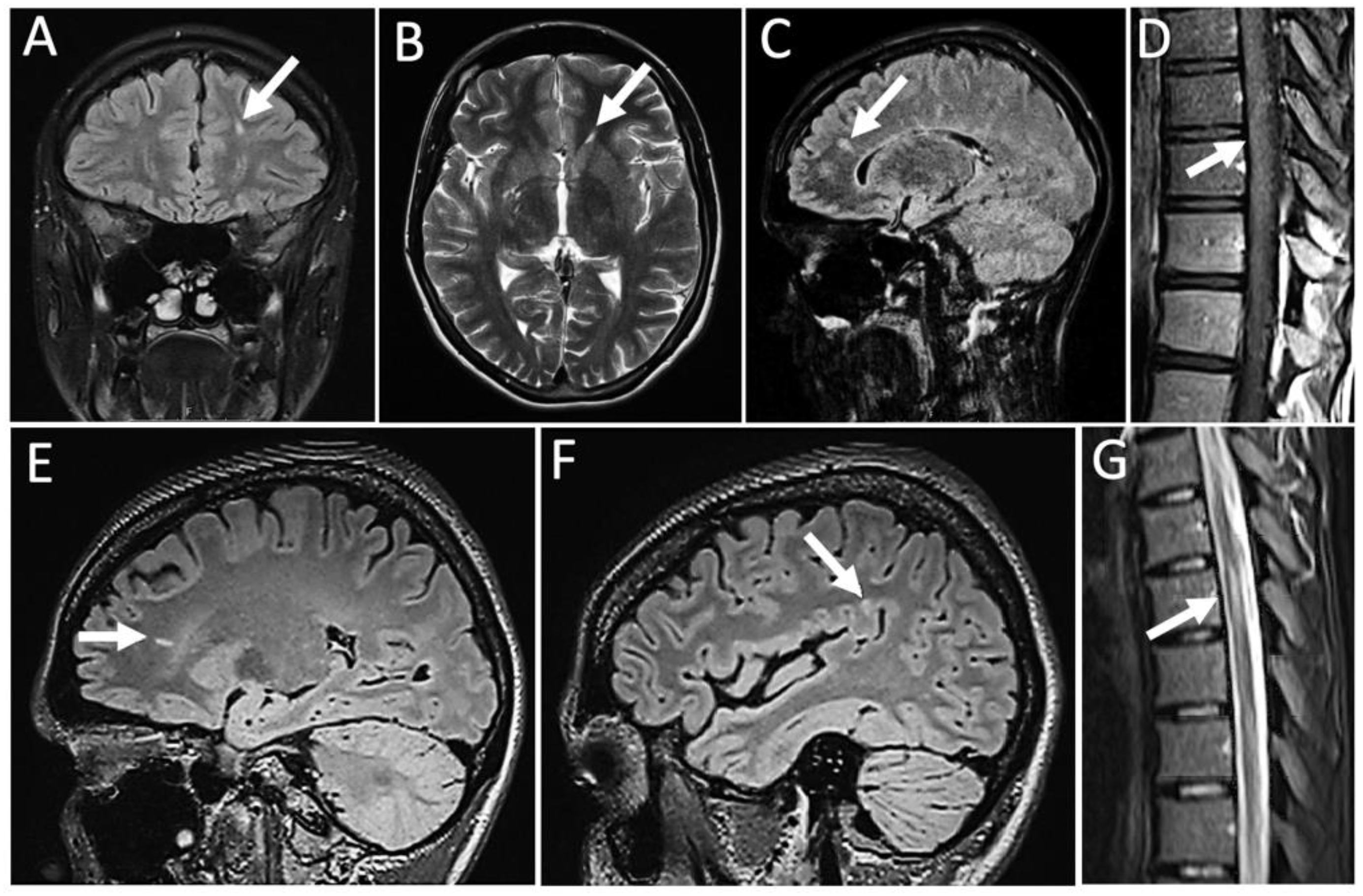
2. Case Report
3. Results
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isaacs, H. A Syndrome of Continuous Muscle-Fibre Activity. J. Neurol. Neurosurg. Psychiatry 1961, 24, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Newsom-Davis, J.; Mills, K.; Byrne, N.; Lang, B.; Vincent, A. Autoimmune Aetiology for Acquired Neuromyotonia (Isaacs’ Syndrome). Lancet 1991, 338, 75–77. [Google Scholar] [CrossRef]
- Park, S.B.; Thurbon, R.; Kiernan, M.C. Isaacs Syndrome: The Frontier of Neurology, Psychiatry, Immunology and Cancer. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1243–1244. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Waters, P.; Irani, S.R. Stop testing for autoantibodies to the VGKC-complex: Only request LGI1 and CASPR2. Pract. Neurol. 2020, 20, 377. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Castrillo, S.; Joubert, B.; Elsensohn, M.-H.; Pinto, A.-L.; Saint-Martin, M.; Vogrig, A.; Picard, G.; Rogemond, V.; Dubois, V.; Tamouza, R.; et al. Anti-CASPR2 Clinical Phenotypes Correlate with HLA and Immunological Features. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1076. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C.; Corbo, M.; Piccolo, G.; Iannaccone, S. Autoimmune Neuromyotonia Following Human Papilloma Virus Vaccination. Muscle Nerve 2013, 47, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Sedarous, M.; Lange, D.J. Can White Matter Changes Occur in Disorders of Peripheral Nerve Hyperexcitability? Mult. Scler. Relat. Disord. 2013, 2, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Srijithesh, P.R. An Unusual Cause of Swan Neck Deformity of the Fingers. JAMA Neurol. 2013, 70, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Pettingill, P.; Pettingill, R.; Lang, B.; Birch, R.; Waters, P.; Irani, S.R.; Buckley, C.; Watanabe, O.; Arimura, K. Association of Leucine-Rich Glioma Inactivated Protein 1, Contactin-associated Protein 2, and Contactin 2 Antibodies with Clinical Features and Patient-reported Pain in Acquired Neuromyotonia. JAMA Neurol. 2018, 75, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Morvan, A.M. De la Chorée Fibrillaire. Gaz. Hebd. Mèdicine.Chir. 1890, 27, 173–200. [Google Scholar]
- Sutton, I.; Lahoria, R.; Tan, I.; Clouston, P.; Barnett, M.H. CNS Demyelination and Quadrivalent HPV Vaccination. Mult. Scler. J. 2009, 15, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Füle, T.; Máthé, M.; Suba, Z.; Csapó, Z.; Szarvas, T.; Tátrai, P.; Paku, S.; Kovalszky, I. The Presence of Human Papillomavirus 16 in Neural Structures and Vascular Endothelial Cells. Virology 2006, 348, 289–296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lenz, P.; Day, P.M.; Pang, Y.-Y.S.; Frye, S.A.; Jensen, P.N.; Lowy, D.R.; Schiller, J.T. Papillomavirus-like Particles Induce Acute Activation of Dendritic Cells. J. Immunol. 2001, 166, 5346–5355. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi-Bensouda, L.; Rossignol, M.; Koné-Paut, I.; Krivitzky, A.; Lebrun-Frenay, C.; Clet, J.; Brassat, D.; Papeix, C.; Nicolino, M.; Benhamou, P.-Y. Risk of Autoimmune Diseases and Human Papilloma Virus (HPV) Vaccines: Six Years of Case-referent Surveillance. J. Autoimmun. 2017, 79, 84–90. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K. Single Vaccine Shot Protects against Cervical Cancer. Nat. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatami, M.; Förster, M.; Weyers, V.; Räuber, S.; Meuth, S.G.; Kremer, D. Neuromyotonia with Central Nervous System Lesions following Quadrivalent Human Papilloma Virus Vaccination. Vaccines 2022, 10, 1132. https://doi.org/10.3390/vaccines10071132
Hatami M, Förster M, Weyers V, Räuber S, Meuth SG, Kremer D. Neuromyotonia with Central Nervous System Lesions following Quadrivalent Human Papilloma Virus Vaccination. Vaccines. 2022; 10(7):1132. https://doi.org/10.3390/vaccines10071132
Chicago/Turabian StyleHatami, Maryam, Moritz Förster, Vivien Weyers, Saskia Räuber, Sven G. Meuth, and David Kremer. 2022. "Neuromyotonia with Central Nervous System Lesions following Quadrivalent Human Papilloma Virus Vaccination" Vaccines 10, no. 7: 1132. https://doi.org/10.3390/vaccines10071132
APA StyleHatami, M., Förster, M., Weyers, V., Räuber, S., Meuth, S. G., & Kremer, D. (2022). Neuromyotonia with Central Nervous System Lesions following Quadrivalent Human Papilloma Virus Vaccination. Vaccines, 10(7), 1132. https://doi.org/10.3390/vaccines10071132





