Assessment of Clinical Profile and Treatment Outcome in Vaccinated and Unvaccinated SARS-CoV-2 Infected Patients
Abstract
:1. Introduction
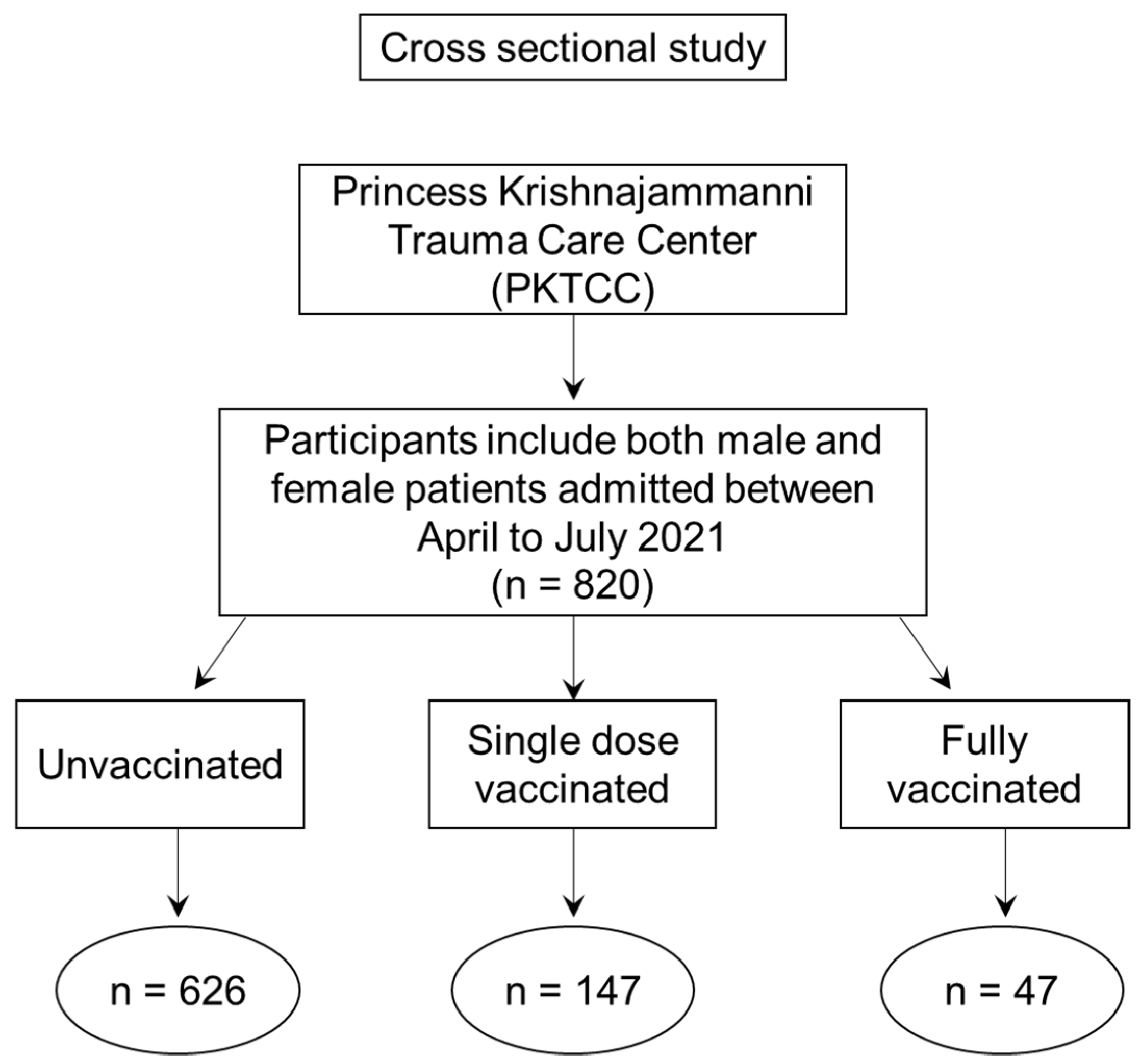
2. Material and Methods
3. Results
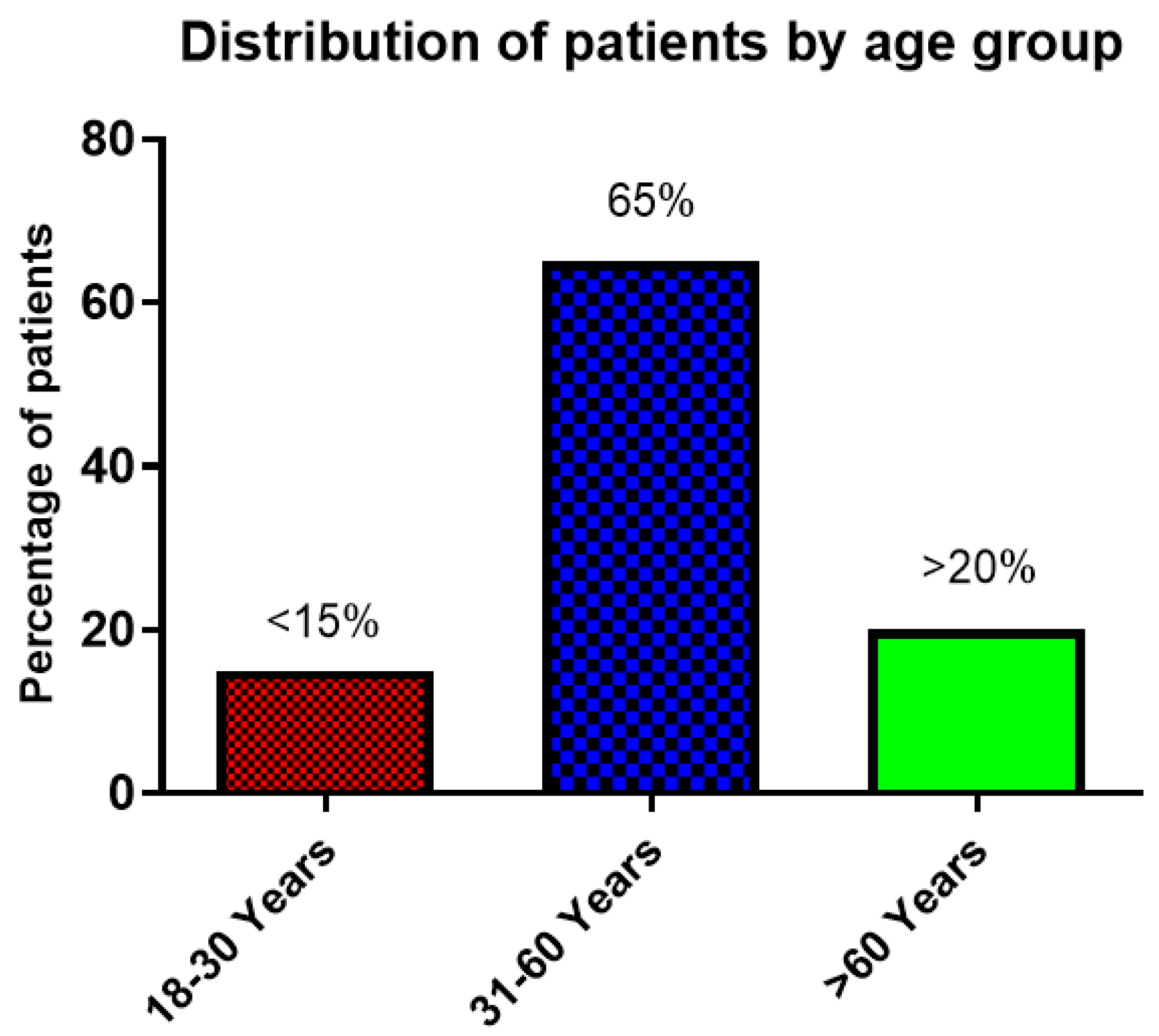
3.1. Gender and Age Distribution
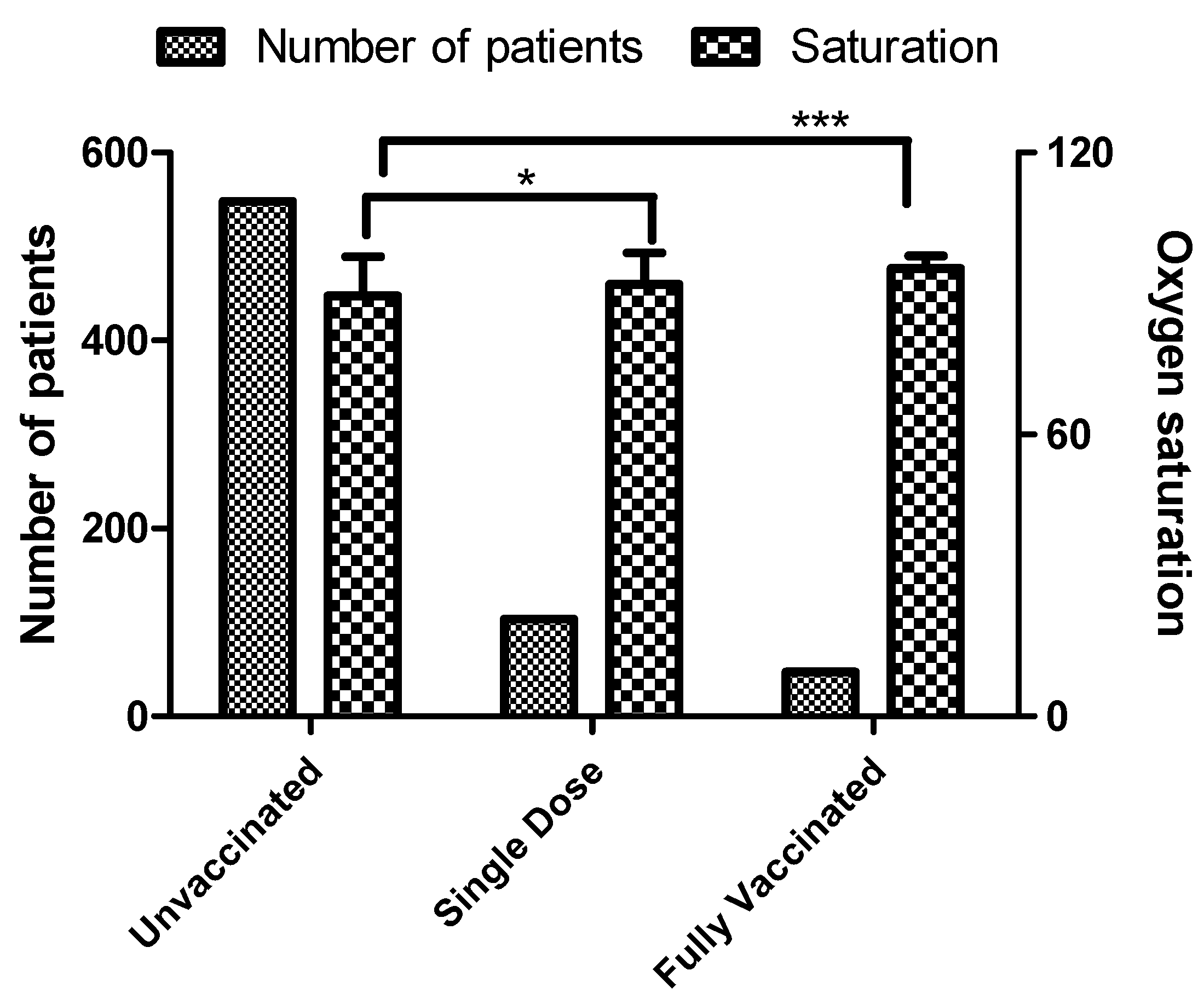
3.2. Oxygen Saturation
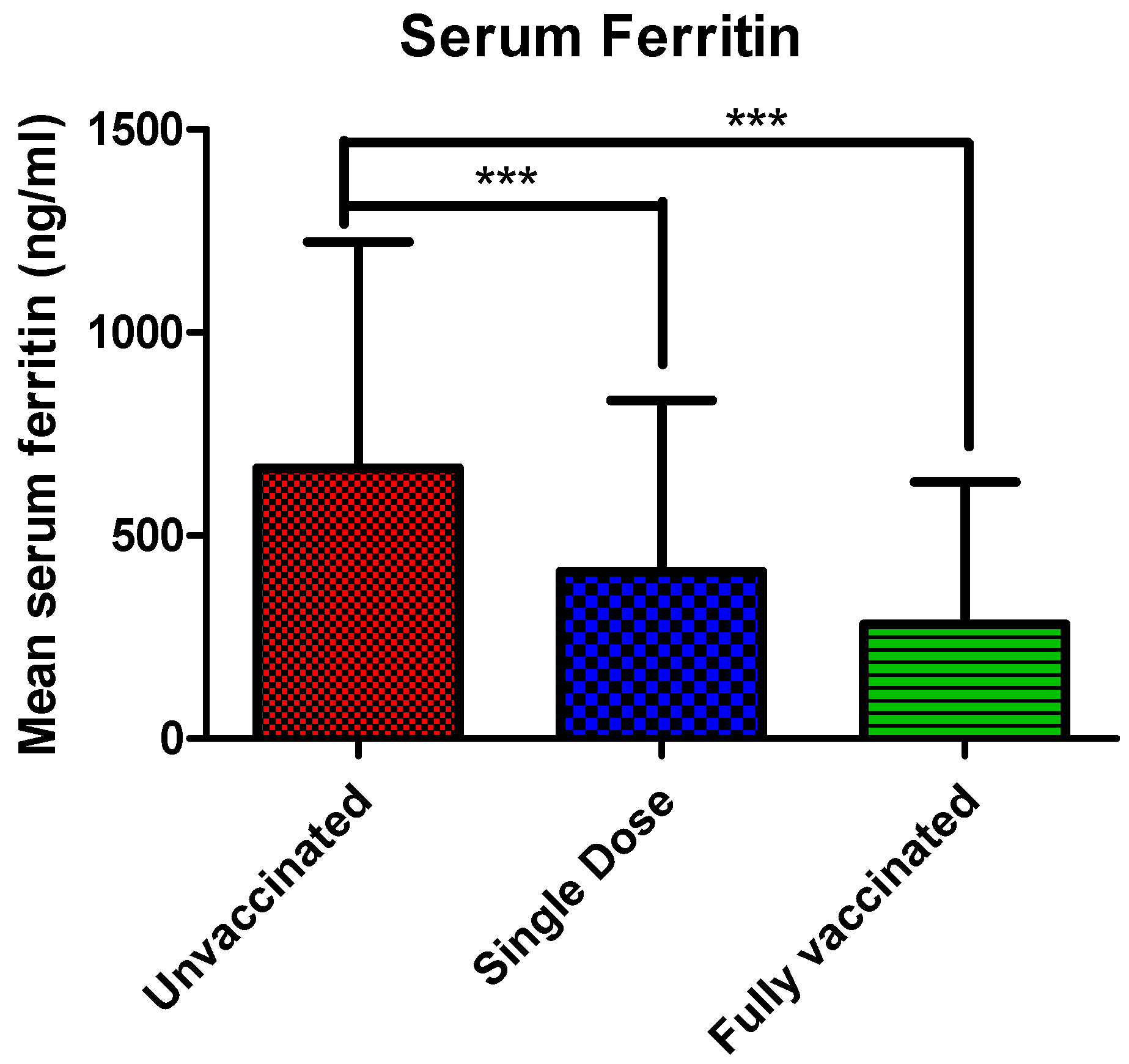
3.3. Serum Ferritin Levels
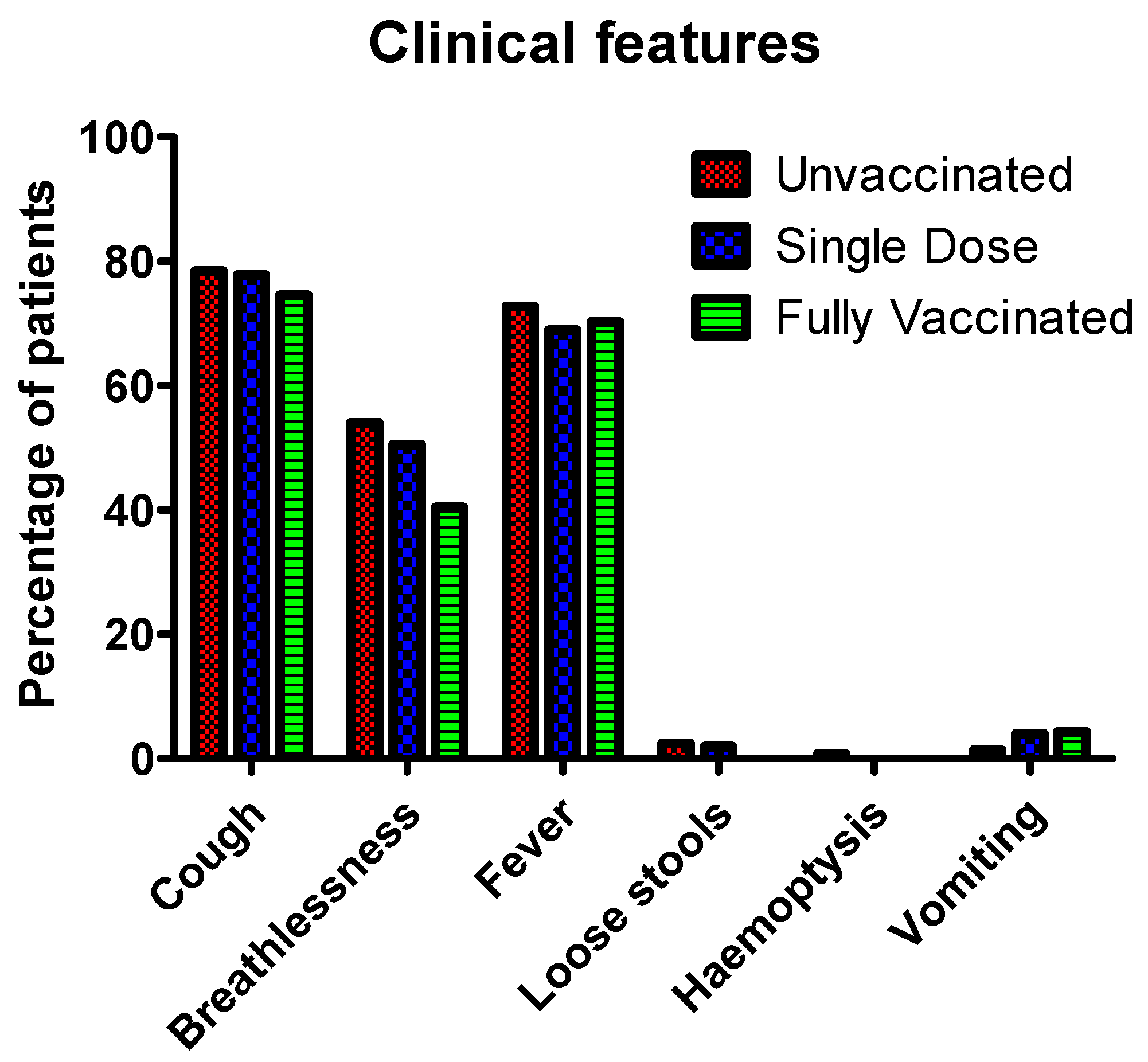
3.4. Clinical Features
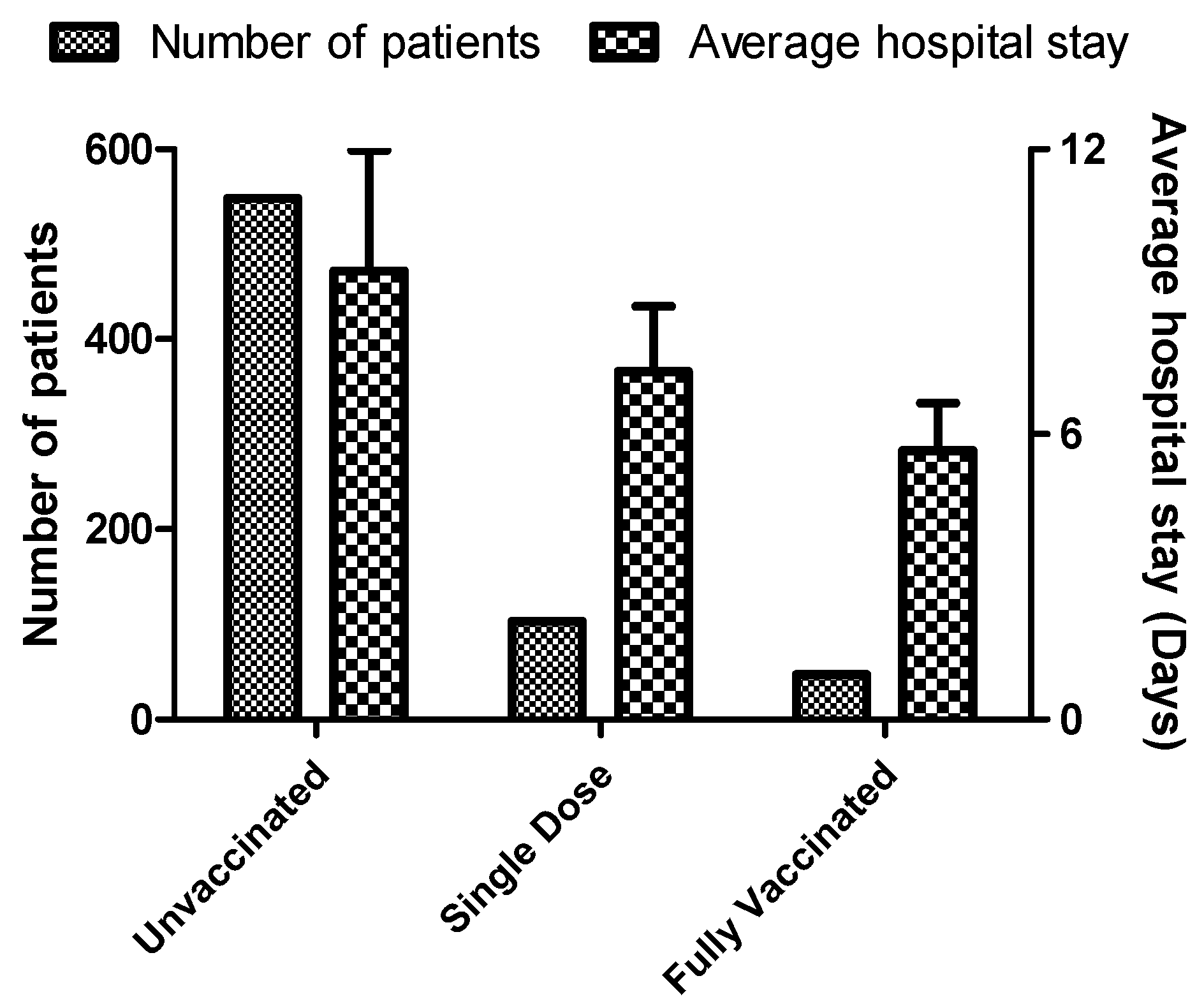
3.5. Average Hospital Stay
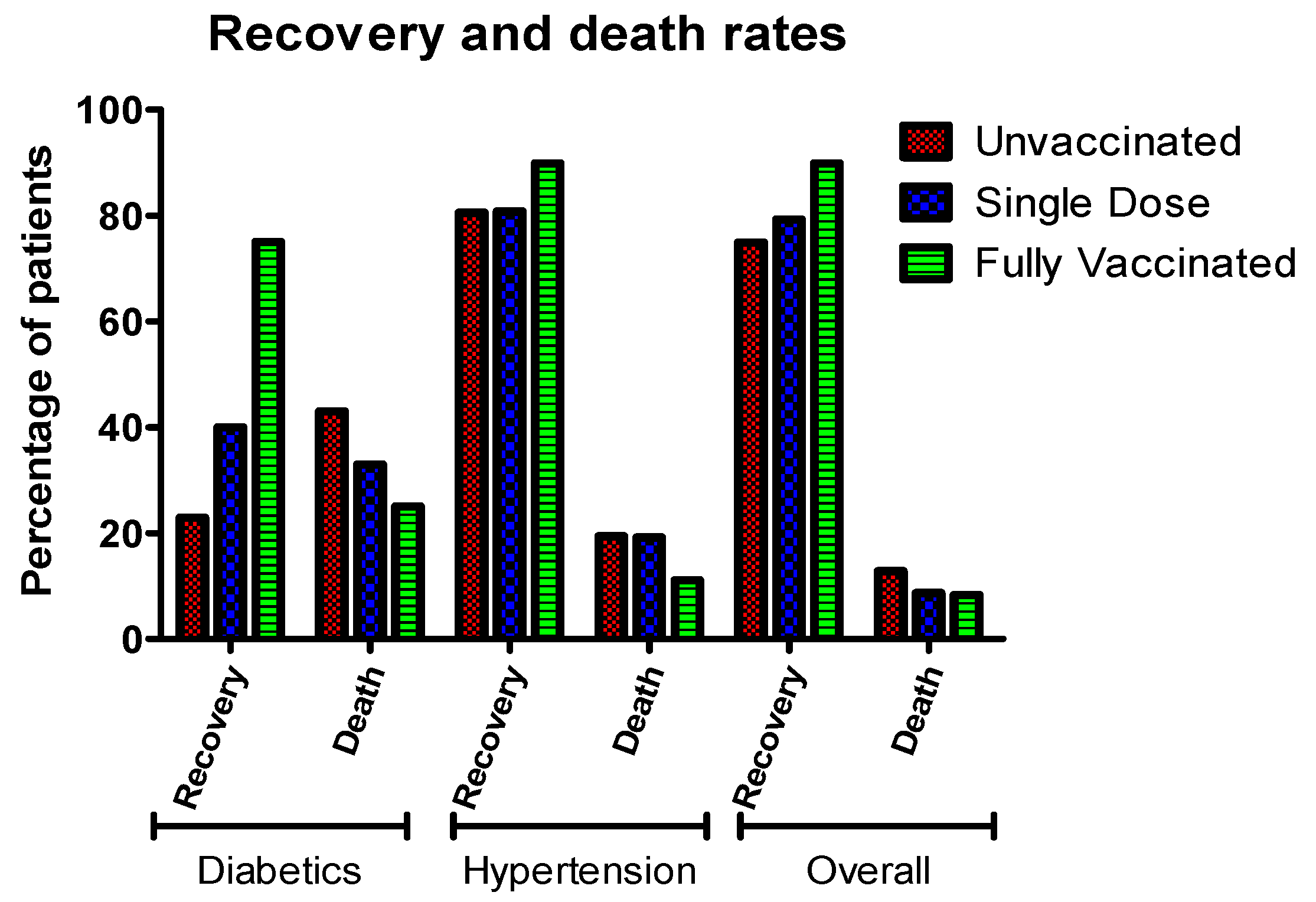
3.6. Comorbidities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/data (accessed on 25 June 2022).
- Pan, A.; Liu, L.; Wang, C.; Guo, H.; Hao, X.; Wang, Q.; Huang, J.; He, N.; Yu, H.; Lin, X. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. J. Am. Med. Assos. 2020, 19, 1915–1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; CMMID COVID-19 working group; Eggo, R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020, 26, 1205–1211. [Google Scholar] [CrossRef]
- Duan, L.; Zheng, Q.; Zhang, H.; Niu, Y.; Lou, Y.; Wang, H. The SARS-CoV-2 spike glycoprotein biosynthesis, structure, function, and antigenicity: Implications for the design of spike-based vaccine immunogens. Front. Immunol. 2020, 11, 576622. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Durmaz, B.; Abdulmajed, O.; Durmaz, R. Mutations observed in the SARS-CoV-2 spike glycoprotein and their effects in the interaction of virus with ACE-2 receptor. Medeni. Med. J. 2020, 35, 253–260. [Google Scholar] [CrossRef]
- Mittal, A.; Manjunath, K.; Ranjan, R.K.; Kaushik, S.; Kumar, S.; Verma, V. COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020, 16, e1008762. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Tortorici, M.A.; Veesler, D. Structural insights into coronavirus entry. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 105, pp. 93–116. [Google Scholar]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin-and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef] [Green Version]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Neagu, M.; Constantin, C.; Surcel, M. Testing Antigens, Antibodies, and Immune Cells in COVID-19 as a Public Health Topic—Experience and Outlines. Int. J. Environ. Res. Public Health 2021, 18, 13173. [Google Scholar] [CrossRef]
- Liu, R.; Han, H.; Liu, F.; Lv, Z.; Wu, K.; Liu, Y.; Feng, Y.; Zhu, C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin. Chim. Acta 2020, 505, 172–175. [Google Scholar] [CrossRef]
- Yu, F.; Yan, L.; Wang, N.; Yang, S.; Wang, L.; Tang, Y.; Gao, G.; Wang, S.; Ma, C.; Xie, R. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin. Infect. Dis. 2020, 71, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Garg, K.; Sharma, K.; Gupta, A.; Chopra, V. Association of cycle threshold values of CBNAAT with severity and outcome in COVID-19. Monaldi Arch. Chest Dis. 2021, 91, 1–19. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Tirupathi, R.; Sule, A.A.; Aldali, J.; Mutair, A.A.; Alhumaid, S.; Gupta, N.; Koritala, T.; Adhikari, R.; Bilal, M. Viral dynamics and real-time RT-PCR Ct values correlation with disease severity in COVID-19. Diagnostics 2021, 11, 1091. [Google Scholar] [CrossRef]
- Shah, S.; Singhal, T.; Davar, N.; Thakkar, P. No correlation between Ct values and severity of disease or mortality in patients with COVID 19 disease. Indian J. Med. Microbiol. 2021, 39, 116–117. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 26 May 2022).
- COVID19 Vaccine Tracker. COVID19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/ (accessed on 13 May 2022).
- Sharma, R.; Tiwari, S.; Dixit, A. Covaxin: An overview of its immunogenicity and safety trials in India. Bioinformation 2021, 17, 840–845. [Google Scholar] [PubMed]
- Sapkal, G.N.; Yadav, P.D.; Ella, R.; Deshpande, G.R.; Sahay, R.R.; Gupta, N.; Vadrevu, K.M.; Abraham, P.; Panda, S.; Bhargava, B. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J. Travel Med. 2021, 28, taab051. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Sakthivel, M.; Kamaraj, P.; Venkataswamy, V.; Thangaraj, J.W.V.; Shete, A.; John, A.; Arjun, J.; CP, G.K.; Yadav, P.D. Effectiveness of ChAdOx1 nCoV-19 Corona Virus Vaccine (CovishieldTM) in preventing SARS-CoV2 infection, Chennai, Tamil Nadu, India, 2021. medRxiv 2022, 10, 970. [Google Scholar] [CrossRef]
- Indian Council of Medical Research. World Health Organisation Approval for COVAXIN—A Path Breaking Moment for India. Available online: https://www.icmr.gov.in/pdf/press_realease_files/Press_Release_ICMR_03_March_2021.pdf (accessed on 25 June 2022).
- Chopra, M.; Jain, A.; Chhabra, S.; Kaundal, S.; Singh, C.; Jandial, A.; Prakash, G.; Khadwal, A.; Das, C.; Singh, M.P. Short Research Communication Anti-Spike Antibody Response to COVISHIELD™(SII-ChAdOx1 nCoV-19) Vaccine in Patients with B-Cell and Plasma Cell Malignancies and Hematopoietic Cell Transplantation Recipients. Indian J. Hematol. Blood Transfus. 2022, 1, 1–5. [Google Scholar] [CrossRef]
- Das, S.; Kar, S.S.; Samanta, S.; Banerjee, J.; Giri, B.; Dash, S.K. Immunogenic and reactogenic efficacy of Covaxin and Covishield: A comparative review. Immunol. Res. 2022, 70, 289–315. [Google Scholar] [CrossRef]
- Andre, F.E.; Booy, R.; Bock, H.L.; Clemens, J.; Datta, S.K.; John, T.J.; Lee, B.W.; Lolekha, S.; Peltola, H.; Ruff, T. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008, 86, 140–146. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Stowe, J.; Robertson, C.; Tessier, E.; Simmons, R.; Cottrell, S.; Robertson, R.; O’Doherty, M. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv 2021, 1, 1–21. [Google Scholar] [CrossRef]
- Yanez, N.D.; Weiss, N.S.; Romand, J.-A.; Treggiari, M.M. COVID-19 mortality risk for older men and women. BMC Public Health 2020, 20, 1742. [Google Scholar] [CrossRef]
- Asirvatham, E.S.; Lakshmanan, J.; Sarman, C.J.; Joy, M. Demystifying the varying case fatality rates (CFR) of COVID-19 in India: Lessons learned and future directions. J. Infect. Dev. Ctries. 2020, 14, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.G.; Palazzo, A.; Rosso, T.; Shbaklo, N.; Mussa, M.; Boglione, L.; Borgogno, E.; Rossati, A.; Mornese Pinna, S.; Scabini, S. Risk factors for mortality in COVID-19 hospitalized patients in Piedmont, Italy: Results from the multicenter, regional, CORACLE registry. J. Clin. Med. 2021, 10, 1951. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.A.; Khan, M.N.; Mustagir, M.G.; Rana, J.; Islam, M.S.; Kabir, M.I. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: A systematic review and meta-analysis. J. Glob. Health 2020, 10, 020503. [Google Scholar] [CrossRef]
- Covid, C.; Team, V.B.C.I.; COVID, C.; Team, V.B.C.I.; COVID, C.; Team, V.B.C.I.; Birhane, M.; Bressler, S.; Chang, G.; Clark, T. COVID-19 vaccine breakthrough infections reported to CDC—United States, January 1–April 30, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 792–793. [Google Scholar]
- Piret, J.; Boivin, G. Pandemics throughout history. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Abd El-Aziz, T.M.; Stockand, J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infect. Genet. Evol. 2020, 83, 104327. [Google Scholar] [CrossRef]
- Zhou, L.; Ayeh, S.K.; Chidambaram, V.; Karakousis, P.C. Modes of transmission of SARS-CoV-2 and evidence for preventive behavioral interventions. BMC Infect. Dis. 2021, 21, 496. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Orenbuch-Harroch, E.; Chowers, M.; Elbaz, M.; Nesher, L.; Stein, M.; Maor, Y.; Cohen, R.; Hussein, K.; Weinberger, M. BNT162b2 vaccine breakthrough: Clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin. Microbiol. Infect. 2021, 27, 1652–1657. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Davis, P.B.; Volkow, N.D.; Xu, R. Increased risk for COVID-19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry 2022, 21, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G. Effectiveness of COVID-19 vaccines against the B. 1.617. 2 (Delta) variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Lord JM: The effect of aging of the immune system on vaccination responses. Hum. Vaccines Immunother. 2013, 9, 1364–1367. [CrossRef] [Green Version]
- AlQahtani, M.; Bhattacharyya, S.; Alawadi, A.; Mahmeed, H.A.; Sayed, J.A.; Justman, J.; El-Sadr, W.M.; Hidary, J.; Mukherjee, S. Morbidity and mortality from COVID-19 post-vaccination breakthrough infections in association with vaccines and the emergence of variants in Bahrain. Icap Glob. Health 2021, 1, 1–2. [Google Scholar]
- El-Sharkawy, Y.H.; Aref, M.H.; Elbasuney, S.; Radwan, S.M.; El-Sayyad, G.S. Oxygen saturation measurements using novel diffused reflectance with hyperspectral imaging: Towards facile COVID-19 diagnosis. Opt. Quantum Electron. 2022, 54, 322. [Google Scholar] [CrossRef]
- Shenoy, N.; Luchtel, R.; Gulani, P. Considerations for target oxygen saturation in COVID-19 patients: Are we under-shooting? BMC Med. 2020, 18, 260. [Google Scholar] [CrossRef]
- Michard, F.; Shelley, K.; L’Her, E. COVID-19: Pulse oximeters in the spotlight. J. Clin. Monitaring Comput. 2021, 35, 11–14. [Google Scholar] [CrossRef]
- Collins, J.-A.; Rudenski, A.; Gibson, J.; Howard, L.; O’Driscoll, R. Relating oxygen partial pressure, saturation and content: The haemoglobin–oxygen dissociation curve. Breathe 2015, 11, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, R.A.; Hansen, B.D. Venous oxygen saturation in critical illness. J. Vet. Emerg. Crit. Care 2018, 1, 387–397. [Google Scholar] [CrossRef]
- Hartog, C.; Bloos, F. Venous oxygen saturation. Best Pract. Res. Clin. Anaesthesiol. 2014, 1, 419–428. [Google Scholar] [CrossRef]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, C.; Jones, S.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ Clin. Res. Educ. 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Puah, S.H.; Young, B.E.; Chia, P.Y.; Ho, V.K.; Loh, J.; Gokhale, R.S.; Tan, S.Y.; Sewa, D.W.; Kalimuddin, S.; Tan, C.K. Clinical features and predictors of severity in COVID-19 patients with critical illness in Singapore. Sci. Rep. 2021, 11, 7477. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Mi, S.; Luo, S.; Wang, Y.; Ren, B.; Cai, L.; Wu, M. Risk factors for mortality in 220 patients with COVID-19 in Wuhan, China: A single-center, retrospective study. Ear Nose Throat J. 2021, 100, 140S–147S. [Google Scholar] [CrossRef]
- Luks, A.M.; Swenson, E.R. Pulse oximetry for monitoring patients with COVID-19 at home. potential pitfalls and practical guidance. Ann. Am. Thorac. Soc. 2020, 1, 1040–1046. [Google Scholar] [CrossRef]
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Sands, K.E.; Wenzel, R.P.; McLean, L.E.; Korwek, K.M.; Roach, J.D.; Miller, K.M.; Poland, R.E.; Burgess, L.H.; Jackson, E.S.; Perlin, J.B. Patient characteristics and admitting vital signs associated with coronavirus disease 2019 (COVID-19)–related mortality among patients admitted with noncritical illness. Infect. Control. Hosp. Epidemiol. 2021, 1, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Mofaz, M.; Oved, S.; Yechezkel, M.; Constantini, K.; Goldstein, N.; Eisenkraft, A.; Shmueli, E.; Yamin, D. Utilizing wearable sensors for continuous and highly-sensitive monitoring of reactions to the BNT162b2 mRNA COVID-19 vaccine. Commun. Med. 2022, 2, 27. [Google Scholar] [CrossRef]
- Wilder-Smith, A. What is the vaccine effect on reducing transmission in the context of the SARS-CoV-2 delta variant? Lancet Infect. Dis. 2022, 22, 152–153. [Google Scholar] [CrossRef]
- Mulchandani, R.; Lyngdoh, T.; Kakkar, A.K. Deciphering the COVID-19 cytokine storm: Systematic review and meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13429. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Lin, Z.; Long, F.; Yang, Y.; Chen, X.; Xu, L.; Yang, M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef] [PubMed]
- Para, O.; Caruso, L.; Pestelli, G.; Tangianu, F.; Carrara, D.; Maddaluni, L.; Tamburello, A.; Castelnovo, L.; Fedi, G.; Guidi, S. Ferritin as prognostic marker in COVID-19: The FerVid study. Postgrad. Med. 2022, 134, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Invernizzi, P.; Arosio, P.; Cairo, G. New functions for an iron storage protein: The role of ferritin in immunity and autoimmunity. J. Autoimmun. 2008, 30, 84–89. [Google Scholar] [CrossRef]
- Vargas-Vargas, M.; Cortés-Rojo, C. Ferritin levels and COVID-19. Rev. Panam. Salud Pública 2020, 44, e72. [Google Scholar] [CrossRef]
- Cao, P.; Wu, Y.; Wu, S.; Wu, T.; Zhang, Q.; Zhang, R.; Wang, Z.; Zhang, Y. Elevated serum ferritin level effectively discriminates severity illness and liver injury of coronavirus disease 2019 pneumonia. Biomarkers 2021, 26, 207–212. [Google Scholar] [CrossRef]
- Gandini, O.; Criniti, A.; Ballesio, L.; Giglio, S.; Galardo, G.; Gianni, W.; Santoro, L.; Angeloni, A.; Lubrano, C. Serum Ferritin is an independent risk factor for Acute Respiratory Distress Syndrome in COVID-19. J. Infect. 2020, 81, 979–997. [Google Scholar] [CrossRef]
- Qeadan, F.; Tingey, B.; Gu, L.Y.; Packard, A.H.; Erdei, E.; Saeed, A.I. Prognostic values of serum ferritin and D-dimer trajectory in patients with COVID-19. Viruses 2021, 13, 419. [Google Scholar] [CrossRef]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak, M.; Kothari, A.; Kumar, S.; Rana, S. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef]
- Carubbi, F.; Salvati, L.; Alunno, A.; Maggi, F.; Borghi, E.; Mariani, R.; Mai, F.; Paoloni, M.; Ferri, C.; Desideri, G. Ferritin is associated with the severity of lung involvement but not with worse prognosis in patients with COVID-19: Data from two Italian COVID-19 units. Sci. Rep. 2021, 11, 4863. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pastora, J.; Weigand, M.; Kim, J.; Wu, X.; Strayer, J.; Palmer, A.F.; Zborowski, M.; Yazer, M.; Chalmers, J.J. Hyperferritinemia in critically ill COVID-19 patients–is ferritin the product of inflammation or a pathogenic mediator? Clin. Chim. Acta Int. J. Clin. Chem. 2020, 509, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.; Moni, M.; Sathyapalan, D.T.; Varghese, P.; Jose, M.P.; Murugan, M.R.; Rajan, C.; Saboo, D.; Nair, S.S.; Varkey, R.A. A comparison of clinical outcomes between vaccinated and vaccine-naive patients of COVID-19, in four tertiary care hospitals of Kerala, South India. Clin. Epidemiol. Glob. Health 2022, 13, 100971. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.R.; Miraglia, J.L.; Malheiros, D.T.; Guozhang, Y.; Teich, V.D.; da Silva Victor, E.; Pinho, J.R.R.; Cypriano, A.; Vieira, L.W.; Polonio, M. Effectiveness of two coronavirus disease 2019 (COVID-19) vaccines (viral vector and inactivated viral vaccine) against severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection in a cohort of healthcare workers. Infect. Control. Hosp. Epidemiol. 2022, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Swift, M.D.; Breeher, L.E.; Tande, A.J.; Tommaso, C.P.; Hainy, C.M.; Chu, H.; Murad, M.H.; Berbari, E.F.; Virk, A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin. Infect. Dis. 2021, 73, 1376–1379. [Google Scholar] [CrossRef]
- Desai, A.; Desai, P.; Mehta, J.; Sachora, W.; Bharti, N.; Patel, T.; Sukhwani, K.; Jain, A.; Sorathiya, D.; Nanda, V. Measuring the impact of a single dose of ChAdOx1 nCoV-19 (recombinant) coronavirus vaccine on hospital stay, ICU requirement, and mortality outcome in a tertiary care centre. Int. J. Infect. Dis. 2021, 113, 282–287. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Subbarayan, P. Impact of vaccination in reducing Hospital expenses, Mortality and Average length of stay among COVID 19 patients. A retrospective cohort study from India. medRxiv 2021, 1, 1–16. [Google Scholar] [CrossRef]
- Whittaker, R.; Kristofferson, A.B.; Salamanca, B.V.; Seppälä, E.; Golestani, K.; Kvåle, R.; Watle, S.V.; Buanes, E.A. Length of hospital stay and risk of intensive care admission and in-hospital death among COVID-19 patients in Norway: A register-based cohort study comparing patients fully vaccinated with an mRNA vaccine to unvaccinated patients. Clin. Microbiol. Infect. 2022, 28, 871–878. [Google Scholar] [CrossRef]
- Baltas, I.; Boshier, F.A.; Williams, C.A.; Bayzid, N.; Cotic, M.; Guerra-Assunção, J.A.; Irish-Tavares, D.; Haque, T.; Hart, J.; Roy, S. Post-vaccination COVID-19: A case-control study and genomic analysis of 119 breakthrough infections in partially vaccinated patients. Clin. Infect. Dis. 2021, 1, 1–18. [Google Scholar] [CrossRef]
- Escobedo-de la Peña, J.; Rascón-Pacheco, R.A.; de Jesús Ascencio-Montiel, I.; González-Figueroa, E.; Fernández-Gárate, J.E.; Medina-Gómez, O.S.; Borja-Bustamante, P.; Santillán-Oropeza, J.A.; Borja-Aburto, V.H. Hypertension, diabetes and obesity, major risk factors for death in patients with COVID-19 in Mexico. Arch. Med. Res. 2021, 52, 443–449. [Google Scholar] [CrossRef]
- Verma, M.; Sharma, S.; Kumar, A.; Hakim, A.; Bhansali, S.; Meena, R. Comorbidities and Vaccination Status of COVID-19 All-Cause Mortality at a Tertiary Care Center of Western India. Cureus 2022, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Yuan, J.; Xiong, X.; Li, M. Mortality rate and characteristics of deaths following COVID-19 vaccination. Front. Med. 2021, 28, 649. [Google Scholar] [CrossRef] [PubMed]
- Epaulard, O.; Abgrall, S.; Lefebvre, M.; Faucher, J.-F.; Michon, J.; Frentiu, E.; Janssen, C.; Charbonnier, G.; Fresse, A.; Laurent, S. Symptoms and severity in vaccinated and unvaccinated patients hospitalised with SARS-CoV-2 delta (B. 1.617. 2) variant infection. medRxiv 2022, 1, 1–21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korishettar, G.; Chikkahonnaiah, P.; Tulimilli, S.V.; Dallavalasa, S.; Byrappa, S.H.; Madhunapantula, S.V.; Veeranna, R.P. Assessment of Clinical Profile and Treatment Outcome in Vaccinated and Unvaccinated SARS-CoV-2 Infected Patients. Vaccines 2022, 10, 1125. https://doi.org/10.3390/vaccines10071125
Korishettar G, Chikkahonnaiah P, Tulimilli SV, Dallavalasa S, Byrappa SH, Madhunapantula SV, Veeranna RP. Assessment of Clinical Profile and Treatment Outcome in Vaccinated and Unvaccinated SARS-CoV-2 Infected Patients. Vaccines. 2022; 10(7):1125. https://doi.org/10.3390/vaccines10071125
Chicago/Turabian StyleKorishettar, Ganesh, Prashanth Chikkahonnaiah, SubbaRao V. Tulimilli, Siva Dallavalasa, Shashidhar H. Byrappa, SubbaRao V. Madhunapantula, and Ravindra P. Veeranna. 2022. "Assessment of Clinical Profile and Treatment Outcome in Vaccinated and Unvaccinated SARS-CoV-2 Infected Patients" Vaccines 10, no. 7: 1125. https://doi.org/10.3390/vaccines10071125
APA StyleKorishettar, G., Chikkahonnaiah, P., Tulimilli, S. V., Dallavalasa, S., Byrappa, S. H., Madhunapantula, S. V., & Veeranna, R. P. (2022). Assessment of Clinical Profile and Treatment Outcome in Vaccinated and Unvaccinated SARS-CoV-2 Infected Patients. Vaccines, 10(7), 1125. https://doi.org/10.3390/vaccines10071125







